Abstract
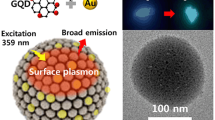
We have demonstrated a green and facile approach to prepare the gold nanoparticles-graphene oxide (Au NPs-GO) film using the electrostatic self-assembly method. Cubic Au NPs with positive charge were firstly synthesized by a seed-growth method. By alternating deposition of the negatively charged GO sheets and Au NPs on the quartz substrate, the Au NPs-GO film can be achieved. AFM images show that GO sheets fully covered the quartz substrate after twice deposition. Meanwhile, Au NPs scattered on the surface of film can be found. The Au NPs-GO film exhibits the desirable surface-enhanced Raman scattering (SERS) properties against Rhodamine 6G molecules, including the high enhancement factor of 5.1 × 105 and high detection limit of 10−10 M. Besides the strong electromagnetic effect of Au NPs, GO sheets play a key in improvement of SERS properties through the strong adsorption of molecules, favorable fluorescence quenching, and chemical enhancement effect.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Graphene (GE), a monolayer of sp2 hybridized carbon atoms from graphite, packed into a two-dimensional (2D) honeycomb lattice [1, 2]. Ling and co-workers reported that GE showed a surface-enhanced Raman scattering (SERS) property based on a chemical enhancement mechanism (CM) [3]. Interestingly, they found that the SERS sensitivity of GE film is strongly influenced by the numbers of GE layers. In addition, the film with GE monolayer is most sensitive. Recently, Graphene oxide (GO), one of GE derivatives has attracted tremendous attention because of its fascinating properties such as water dispersibility and biocompatibility [4, 5]. Besides, GO was also discovered having an obvious Raman enhancing effect for molecules detection due to its high specific surface area, powerful adsorption ability and well fluorescence quenching property [6–8]. Yu et al. have proved that GO is one of the superior candidates for SERS substrates [8]. The challenge of using GO as the SERS substrate is its low Raman sensitivity. In order to address this problem, a new hybrid consisting GO and metal nanoparticles (NPs) (e.g., Au, Ag.) is necessary.
The enhancement mechanism of typical metal NPs substrates is the electromagnetic mechanism (EM), which relies on the interaction between the appropriate morphology of metal substrates and the investigated molecules [9, 10]. Recent reports showed that Au NPs or Ag NPs-GO hybrids have significant enhancement SERS properties [11, 12]. Hu et al. reported that GO-Au NPs exhibited an enhancement in Raman effects on detection of cationic dyes [7]. However, the SERS sensitivity of such substrate still needs to be improved. Lu et al. reported a SERS substrate with high enhancement by depositing the Au NPs on single graphene layer prepared by chemical vapor deposition (CVD) technique [13]. However, the experiment condition of this approach is harsh because of the CVD technique. Finding a facile method to fabricate a SERS substrate with high sensitivity is very important.
In our previous work, the electrostatic self-assembly approach has been employed to fabricate the highly dispersive Ag NPs in thin GO films [14]. Herein, in order to construct a sensitive SERS substrate that can be used for detecting ultralow concentration of molecules; we designed a SERS substrate with positively charged Au NPs and negatively charged GO on alkylated quartz substrate by electrostatic self-assemble method. The Au NPs-GO film exhibits desirable SERS properties against Rhodamine 6G (R6G) molecules. The successful synthesis of Au NPs-GO film paves a way to explore the GO in SERS application.
Results and discussion
Characterization of Au NPs
As is known to all of us, CTAB is a quaternary ammonium surfactant, which is usually used as a stabilizing agent [15]. Au NPs was packaged with CTAB to form positively charged particles, which can be well dispersed in colloidal solution. Therefore, this positively charged Au NPs can play the polycation solution substitute in electrostatic self-assembly process [16]. Figure 1a shows the UV–vis absorbance spectrum of prepared Au colloid. The typical absorbance peak of Au NPs can be seen at 557 nm. Figure 1b shows the XRD pattern of the prepared Au NPs. Five typical peaks are observed, which could be indexed 38.3°, 44.5°, 64.2°, 77.7°, and 82.4° correspondingly to the (111), (200), (220), (311), and (222) reflections of a face-centered cubic crystal structure (JCPDS card No.004-0784) [17].
The morphologies of Au NPs were analyzed by TEM. As shown in Fig. 2a, the Au NPs have the cube-like morphology. In addition, the average particle size of Au NPs is 54.0 ± 3.4 nm with the narrow particle distribution (Fig. 2b). Figure 2b also presents that the color of Au NPs colloid is purplish red. It is well known that the particle size and morphology are keys for enhancement of SERS properties [18]. A relatively larger particle size is superior to little one, and Au NPs with some edges and corners are better than nanosphere-like morphology. In this work, as-prepared Au NPs with the desirable size of ~54 nm and cube-like morphology show the potential in development of good SERS substrates.
Characterization of Au NPs-GO film
As above-mentioned that the SERS sensitivity of GO sheets is decreasing with increase of numbers of GO layers. Our previous work showed that layer-by-layer assembly method is superior approach to fabricate the thin GO films with controlled GO layers [14]. Figure 3 illustrates our strategy of the Au NPs-GO film preparation. The pretreated and hydroxylated quartz substrate was firstly immersed in GO aqueous solution. A few of negatively charged GO sheets can be adsorbed onto the positive quartz substrate. Then, this substrate with adsorbed GO sheets was immersed in Au colloid for another 20 min. The positively charged Au NPs can be adsorbed onto the surface of GO sheets. According to our experience, two cycles of assembly is favorable to fabricate such NPs-GO thin film.
The surface morphologies of the films were performed by AFM. Figure 4a, a* show the AFM images of GO thin film without Au NPs. The typical two-dimensional GO sheets and GO wrinkles can be clearly seen, which confirmed the GO sheets adsorbed onto the quartz substrate successfully. The thickness of adsorbed GO sheet on the substrate measured from the height profile were around 1.1 nm, indicating that few layers of GO were uniformly assembled onto substrate (Fig. 4a). Figure 4b, b* show the AFM images of Au NPs-GO film. The Au NPs can be seen clearly on to the surface of film. Oxygen-containing functional groups such as hydroxyl, epoxy, carbonyl, and carboxyl groups exiting on GO sheets cause GO sheets exhibit the negative charged, which is not only important for deposition of Au NPs, but also important for good dispersion of Au NPs on the surface of film [19].
Raman spectroscopy is an effective way to analyze the carbon materials such as carbon nanotubes, graphite and GE. Figure 5 shows the typical Raman spectra of GO and Au NPs-GO films. Both of the films have prominent D and G bands. The G mode and D mode correspond to the first-order scattering of E2g phonon of C sp2 atoms and a breathing mode of k-point phonons of A1g symmetry, respectively [20]. The intensity ratios of D/G (I D/I G) are approximate 0.99 for GO and 0.97 for Au NPs-GO film, which are consistent with the references. More importantly, the Raman intensity of GO in Au NPs-GO film is 2.3 times higher than that of GO film without Au NPs, implying the obvious Raman enhancement effect of Au NPs in Au NPs-GO film. The Raman result reveals that such Au NPs-GO film is probably a good SERS substrate for detecting molecules.
SERS performances of Au NPs-GO film
To further investigate the SERS performance of the Au NPs-GO film, R6G molecules were used as detection probes. Figure 6a shows the Raman spectra of the pure R6G solid and the R6G molecules adsorbed on the Au NPs-GO film. It is difficult to get the useful information of R6G molecules by the Raman spectrum due to the R6G’s high fluorescence, which can be seen in Fig. 6a. Compared with the weak Raman signals of pure R6G solid, the Raman intensities of R6G molecules were significantly enhanced by the Au NPs-GO film, indicates the obvious SERS property of this film (Fig. 6a). In detail, the bands at 612 and 771 cm−1 in the Fig. 6a are assigned to the C–C–C ring in-plane vibration mode and the C-H out-of-plane bend mode, respectively [7].
The bands of 1130 and 1180 cm−1 are assigned to C-H in-plane bend modes. The bands at 1310 and 1570 cm−1 are assigned to the N–H in-plane bend modes. The other bands of 1380, 1510 and 1650 cm−1 are assigned to the C–C stretching modes. The vibrational band presented at 241 cm−1 indicated that a chemisorptive bond has been formed (Au–N) between Au NPs and R6G via the ethylamino group [20]. It is clearly seen that R6G solid have high fluorescence and low Raman intensity (in Fig. 6a) while the Raman signals of R6G adsorbed on Au NPs-GO film are enhanced remarkably and presents excellent fluorescence quenching property.
The SERS property of the Au NPs-GO film can be assessed by the enhancement factor (EF). EF can be estimated as follows [21]:
where I SERS represents the intensity of a vibrational mode in the surface-enhanced spectrum in the presence of different substrates, I bulk represents the intensity of the same mode in the Raman spectrum from the pure R6G solid. The N ads is the number of molecules adsorbed on the Au NPs under the laser spot area. We assume that the surface of Au NPs has been fully surrounded with R6G molecules after soaking the substrate for 12 h in the R6G aqueous solution. The surface area of single R6G molecule is 2 × 10−18 m2. Therefore, the maximum number of R6G adsorbed onto the surface of Au NPs under laser spot size (ca 2.1 μm in diameter) is 6 × 104. The N bulk is the number of molecules used in the bulk of the R6G sample. The Raman spectrum of R6G solid was used for the EF as the “bulk” values. The area of the laser spot is a circle with a diameter of 100 μm. Thus, the estimated value for N bulk is ca.3.9 × 109. The ratios of intensity (I SERS/I bulk) of bands at 612, 774, 1380, and 1650 cm−1 can be obtained (in Fig. 6a). Taking the band at 612 cm−1 of Au NPs-GO film for example, the I SERS /I bulk is about 7.9 under identical experimental conditions. Consequently, the EF is estimated to be 5.1 × 105 (Fig. 6b) which can be used to estimate other EF values of the substrate at different bands, 774 cm−1 for 4.1 × 105, 1380 cm−1 for 3.7 × 105, 1650 cm−1 for 3.5 × 105.
The SERS results exhibit that such Au NPs-GO film has the desirable SERS property, which might be attributed to the synergistic effects of the GO and Au NPs. The mechanism of SERS property of Au NPs-GO film against R6G molecules is shown in Fig. 7. GO film without Au NPs shows the obvious SERS property against R6G molecules because of the CM effect (Fig. 6c), which is a great agreement with the previous work. The abundant oxygen-containing functional groups, combining with the high specific surface area of GO make the Au NPs-GO film has the strong adsorption ability towards the molecules, which is useful for collection of Raman signals of molecules (Fig. 7). At the same time, the excellent fluorescence quenching property of GO can significantly weaken the fluorescence of R6G molecules, and enhance the SERS property of the film. Besides the effect of GO, Au NPs in the film contribute the most SERS property against R6G. It is well known that Au NPs have a good SERS property because of EM effect, which can be seen clearly in Fig. 6c. Therefore, combining effects of GO and Au NPs, the Au NPs-GO film shows much better SERS performance against R6G compared with that of pure GO film and Au NPs (Fig. 6d).
Besides high Raman enhancement effect, the Raman sensitivity is another crucial importance for the SERS substrate. In order to know the Raman sensitivity of Au NPs-GO film, we tested the detection limit of this film against R6G molecules under a series of concentrations in range of 10−5–10−10 M. As shown in Fig. 8, the typical Raman signals of R6G molecules adsorbed onto the surface of Au NPs-GO film are weakening with decrease of R6G concentration. Even though the R6G concentration lowers to 10−10 M, we still can identify the Raman signals of R6G molecules. This result reveals that the detection limit of Au NPs-GO film against R6G should lower that 10−10 M. IIiut et al. reported that a new green, ascorbic acid-assisted method for synthesizing the Au NPs-RGO hybrids to detect Nile Blue A (NBA), and it’s detection limit is about 10−9 M [10]. Du et al. reported that a CVD-assisted method to fabricate GO-Au NPs on detecting R6G molecules, and the detection limit is about 10−8 M [9]. Therefore, the SERS results suggest that our Au NPs-GO film has the desirable SERS properties.
Conclusion
In summary, we have demonstrated a green and facile approach for preparation of the Au NPs-GO film using the electrostatic self-assembly method. After two cycles of assembly, a few of GO sheets can fully cover the quartz substrate, meanwhile, Au NPs can be found on the surface of GO film. The Au NPs-GO film exhibited much better SERS properties against R6G molecules compared with those of pure GO film and Au NPs. The EF of Au NPs-GO film reached to 5.1 × 105 at band of 612 cm−1. The detection limit of Au NPs-GO film lowered to 10−10 M. The good SERS properties of Au NPs-GO film are attributed to the strong electromagnetic effect of Au NPs, and high adsorption ability, favorable fluorescence quenching and chemical enhancement effect of GO sheets.
Experimental
Synthesis of positively charged Au colloid
The Au colloid was synthesized by a seed-growth method. Seed solution was prepared by adding 0.6 cm3 ice-cold sodium borohydride (NaBH4, 0.01 M) into the mixture of 0.3 cm3 chloroauric acid (HAuCl4, 0.01 M) and 7.5 cm3 cetyltrimethylammonium bromide (CTAB, 0.1 M). After that, seed solution was obtained after stirring for 2 h at room temperature. As-prepared seed solution was diluted ten times by deionized water. Growth solution was prepared by mixture of 15 cm3 H2O, 3 cm3 CTAB (0.1 M), 0.4 cm3 HAuCl4 (0.01 M), and 2 cm3 ascorbic acid (AA, 0.1 M). Then, the upper seed solution (0.2 cm3) was added into growth solution, and put the vial inversion rapidly for 10 s. Then, the vial was moved in an oven with 30 °C and kept undisturbed for 10 h. Finally, after washing with deionized water for five times, the Au colloid was obtained.
Preparation of the Au NPs-GO film
GO was fabricated by oxidation of purified natural graphite bought from Shanghai Yifan Company with a mean particle size of 1.5 μm according to modified Hummers method [22]. The pretreatment and alkylation of quartz substrates is carried out according to our previous work [18]. Typically, the pretreated and hydroxylated quartz substrate was immersed in GO (0.1 mg/cm3) dispersion for 20 min. After that, the substrate was rinsed by deionized water to remove the unabsorbed GO sheets, and dried in flowing nitrogen. Then, the substrate was immersed in Au colloid 20 min, and rinsed by deionized water, dried in flowing nitrogen. After two cycles of deposition, the Au NPs-GO film was obtained. Furthermore, the GO film without NPs was prepared with the same process and condition, which is used as a comparison.
Characterization
The optical property of Au colloid was characterized by UV-6100 spectrophotometer. The X-ray diffraction (XRD) analyses of Au NPs was performed on a Philips 1730 powder X-ray diffractometer with Cu KR radiation (λ = 1.5406 Å). The morphology of Au NPs was characterized by JEOL 2011 TEM at an accelerating voltage of 200 kV. The surface morphology of the film was performed using atomic force microscopy (AFM) with a MFP-3D (0.803 Hz). The Raman spectra were recorded on a Thermofisher (USA) Raman system with an argon ion laser of 532 nm at 10 mW.
Preparation of SERS measurement sample
The Au NPs-GO film was immersed in R6G aqueous solution with certain concentration for 12 h, and washed by deionized water for several times to remove the unabsorbed molecules. The SERS measurement sample can be obtained after drying with nitrogen. The SERS measurement is processed with an argon ion laser of 532 nm at the power of 1 mW. The acquisition time for each measurement was 10 s. Three times on different areas were made to verify the veracity of each experiment.
References
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV (2004) Science 306:666
Geim AK, Novoselov SK (2007) Nat Mater 6:183
Ling X, Xie L, Fang Y, Xu H, Zhang HL, Kong J, Dresselhaus MS, Zhang J, Liu ZF (2010) Nano Lett 10:553
Ren W, Fang YX, Wang EK (2011) ACS Nano 5:6425
Yu XX, Cai HB, Zhang WH, Li XJ, Pan N, Luo Y, X. Wang P, Hou JG (2011) ACS Nano 5:952
Fu WL, Zhen SJ, Huang CZ (2014) RSC Adv 4:16327
Hu CF, Rong JH, Cui JH, Yang YH, Yang LF, Wang YL, Liu YL (2013) Carbon 51:255
Fan Z, Kanchanapally R, Ray PC (2013) J Phys Chem Lett 4:3813
Du YX, Zhao Y, Qu Y, Chen CH, Chen CM, Chuang CH, Zhu YW (2014) J Mater Chem C 2:4683
Iliut M, Leordean C, Canpean V, Teodorescu CM, Astilean S (2013) J Mater Chem C 1:4094
Liu XJ, Cao LY, Song W, Ai KL, Lu LH (2011) ACS Appl Mater Interfaces 3:2944
Jiao SJ, Wang YK, Chen C, Wu XD, Bei FL (2014) J Mol Struct 1062:48
Lu RT, Konzelmann A, Xu F, Gong YP, Liu JW, Liu QF, Xin M, Hui RQ, Wu JZ (2015) Carbon 86:78
Zhou YZ, Yang J, Cheng XN, Zhao N, Sun L, Sun HB, Li D (2012) Carbon 50:4343
Sau TK, Murphy CJ (2004) J Am Chem Soc 126:8648
Li N, Zhao PX, Astruc D (2014) Angew Chem Int Ed 53:1756
Liu ZM, Hu CF, Li SX, Zhang W, Guo ZY (2012) Anal Chem 84:10338
Zhou YZ, Yang J, Sun L, Zhao N, Cheng XN (2012) Chin J Inorg Chem 28:137
Zhou YZ, Cheng XN, Du D, Yang J, Zhao N, Ma SB, Zhong T, Lin YH (2014) J Mater Chem C 2:6850
Fan W, Lee YH, Pedireddy S, Zhang Q, Liu TX, Ling XY (2014) Nanoscale 6:4843
Zhou YZ, Yang J, Ma SB, Zhao N, Cheng XN, Zhong T (2014) Monatsh Chem 145:11
Hummers WS, Offeman RE (1958) J Am Chem Soc 80:1339
Acknowledgments
This work was supported by National Science Foundation of China (51572114) and the Fundamental Research Funds for the Central Universities (No. 30920140122003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Yang, J., Zhou, Yz. et al. Facile synthesis of gold nanoparticles-graphene oxide films and their excellent surface-enhanced Raman scattering activity. Monatsh Chem 147, 677–683 (2016). https://doi.org/10.1007/s00706-015-1576-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-015-1576-7