Abstract
The presented work deals with continuous UV-B irradiation of riboflavin in MeOH solution, leading to its degradation under anaerobic as well as aerobic conditions (faster in the former case), which is related to riboflavin photosensitizing properties (type I photosensitizer in the first case, and type II in the other one). Addition of quercetin, a well-known antioxidant in the system causes a decrease of the (riboflavin) degradation in both cases. In anaerobic conditions it might be a consequence of quercetin antioxidant scavenging activity, while under aerobic conditions it could be related to singlet oxygen formation. The degradation dynamics—in both systems, in the presence and in the absence of quercetin—is well synchronized with dynamics formation of the two major products, lumiflavin and lumichrome.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Solar energy reaching the Earth’s surface consists of photons from UV (100–400 nm), visible (400–800 nm), and infrared (800 nm to ~1 mm) range. UV light makes about 5 % in the total sunlight energy flux, it is conventionally divided into UV-A (>315–400 nm), UV-B (>280–315 nm), and UV-C (>100–280 nm) [1, 2]. The most dangerous part of UV radiation is UV-B (representing 4–5 % of total UV flux reaching the Earth). Although it stimulates vitamin D synthesis it may also induce a lot of harmful effects including sunburns, inflammation, immunosuppression, premature aging, and skin cancer [3]. Such detrimental effects are undoubtedly related to sunlight absorption by certain molecules, called “endogenous photosensitizers” located on various depths of skin layers. These molecules, among others, include melanin and its precursors, NADH/NADPH, tryptophan, trans-urocanic acid, and flavins (riboflavin) [4, 5]. They are responsible for the generation of light-driven Reactive Oxygen Species (ROS), free radicals and singlet oxygen.
Riboflavin (vitamin B2; 7,8-dimethyl-10-ribityl-isoalloxazine) is one of the most important members of the huge family of flavins—prosthetic group of flavoproteins—characterized by the tricyclic heterocycle isoalloxazine (Fig. 1). It belongs to a group of hydrosoluble vitamins being an essential nutrient for humans. Because of humans’ inability to synthesize vitamin B2, it has been obtained through diet. Riboflavin is predominantly found in milk, eggs, meat products, cereals, fatty fish, dark-green vegetables, and fermented beverages [6]. It exists in three forms: free riboflavin and two cofactor forms, flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN). It is used as a component of liquid preparations and parenteral nutrition solutions [7]. Riboflavin (RFL) is known for its photosensitizing abilities in biological processes, acting both as type I (free radical-) and type II sensitizer [8]. One of the most important medical uses of RFL photosensitizing property is in the novel therapy of keratoconus called Corneal Collagen Crosslinking (CXL). ROS generated during RFL photosensitization mediate production of new covalent bonds between collagen molecules, thus increasing biomechanical strength and stiffness of the corneal stroma [9].
Major (a) and minor (b) products formed as a result of continual UV-B irradiation of riboflavin in methanol (according to the scheme given by Insinska-Rak et al. [27])
Due to its strong absorbance in the ultraviolet and visible region with absorbance maxima at 223, 267 (ε = 32,500 M−1 cm−1), 373 (ε = 10600 M−1 cm−1), and 444 nm (ε = 12,500 M−1 cm−1) [10] its photoexcitation may potentially occur in the organs and tissues most exposed to light such as the skin (riboflavin is located in the basal cells and dermis) and eyes (in the lens, with concentration of 4.5 µM) [11, 12], provoking damage to other cell components. Riboflavin is capable of oxidizing a variety of biomolecules including proteins (i.e., amino acids, such as tryptophan) [13], carbohydrates [14], lipids [15, 16], nucleic acids [17], as well as other vitamins [18, 19], through mixed type I and type II photosensitizing mechanisms [20, 21]. In direct chemical quenching of its singlet or triplet state (both states are reactive) [21] by various substrates (such as phenolic compounds) RFL donates or accepts hydrogen or electron, thus producing free radicals (type I mechanism) which undergo further reactions with oxygen or other organic molecules. Non-radical type II mechanism includes physical quenching (i.e., energy transfer) from the triplet-state RFL to molecular oxygen in its ground state (3O2) yielding very reactive singlet oxygen (1O2). (Pre)domination of one or the other sensitizing mechanism depends upon the type of substrate and reaction conditions such as pH, solvent, concentration of oxygen, concentration of evolving compounds, etc. [22]. Generally, low oxygen concentration favors type I mechanism [23].
Due to the ability to accept and/or donate a pair of hydrogen atoms and thus become reduced and/or oxidized [10], riboflavin photochemistry is complex and can be separated in three categories: intra- and intermolecular photoreduction, intra- and intermolecular photoaddition, and intramolecular photodealkylation. Some or all of the photoreactions may occur simultaneously yielding cyclodehydroriboflavin (CDRF), formylmethylflavin (FMF), lumichrome (LC), lumiflavin (LF), and many other degradation products, depending on reaction conditions, especially on solution pH value [24–26].
A recent study dealing with UV-A continuous irradiation of riboflavin and its derivatives in aerobic and argon-saturated methanol solution suggests that flavin triplet states have been included in flavin degradation and products formation [27]; the authors postulated two different mechanism, one including free radicals (in which flavins react as type I sensitizer) and the other, non-radical type involving singlet oxygen formation (permitted through the flavins type II sensitization). They have also recorded LF- and LC-type of compounds among the major products. Overall they concluded that RFL photolysis was more efficient under anaerobic conditions. This study presents a step ahead since it deals with UV-B irradiation of riboflavin in methanol solution in the presence of quercetin (QC), well known for its antioxidant properties and behavior; however, it can also act as a quencher of riboflavin triplet state by electron transfer, and as a radical scavenger [28, 29].
Both RFL and QC are present in epidermis skin layer and both are excellent UV-B absorbers (e.g., endogenous photosensitizers) [5], reducing therefore the amount of the most dangerous sunlight fraction that hits the skin and consequently the potential damage as well. However, the governing mechanisms are still very little known; so the picture of their potential interaction—mediated by UV-B—is still pretty much obscure and barely more than a speculation. This work provides a basic data set for RFL-QC interaction, in much simpler environment (MeOH solution), under continuous UV-B irradiation by using ultra high-performance liquid chromatography–electrospray ionization–mass spectrometry (UHPLC–ESI–MS) method, and—the authors hope—could be of some relevance for the analog picture in vivo.
Results and discussion
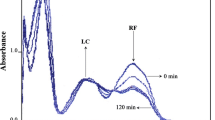
The effect of UV-B continuous irradiation on mixture of riboflavin and quercetin in methanol solution is shown on UHPLC chromatograms in Fig. 2a. The traces were recorded at λ det = 385 nm. The top trace belongs to the non-irradiated mixture, while the middle and the lower trace belong to 6 min irradiated mixture under aerobic and anaerobic conditions, respectively. The detection wavelength of 385 nm was chosen based on absorption spectra of RFL (peak No. 1, t ret = 2.41 min), LF (peak No. 3, t ret = 3.26 min) and LC (peak No. 7, t ret = 6.56 min), shown in Fig. 2c; evidently the all three species have a strong absorption at 385 nm. The peaks No. 2, 4, and 6 (from Fig. 2a) have not been identified, though the latter two have a LF-type of structure (based on the absorption spectra). The peak No. 5 belongs to formyl methyl flavin, FMF (Fig. 1b). Part of the chromatogram from t ret = 4–8 min was recorded at λ det = 300 nm (Fig. 2b). The intention was to record possible QC degradation products found also in the other UV-irradiation studies done in MeOH solution—with absorption maximum at 300 nm [30–32]. Evidently, the two new peaks appeared, No. 9 and 10; based on the absorption spectra, peak 10 is very similar to the one proposed in the earlier work [31], resulting from the opening from the QC B-ring.
Riboflavin (RFL)–quercetin (QC) mixture in methanol, irradiated with continuous UV-B irradiation. The UHPLC chromatograms for the non-irradiated and 6 min irradiated solution (at aerobic and anaerobic conditions), recorded (wavelength detection) at 385 nm (a). Part of the chromatogram recorded at 300 nm: 10 min UV-B irradiated RFL-QC mixture under aerobic conditions (b). Absorption spectra of riboflavin (peak No. 1, t ret = 2.41 min, from a) and UV-B-induced products, 3 and 7, e.g., lumiflavin (LF, peak No. 3, t ret = 3.26 min) and lumichrome (LC, peak No. 7, t ret = 6.56 min) (c) from the chromatogram shown in the (a)
The MS/MS spectra (obtained by using tandem mass spectrometry with collision induced dissociation of the molecular ions) shown in Fig. 3 confirm the structure of the two major products, lumiflavin (Fig. 3b—related to the peak No. 3 on chromatogram in Fig. 2a), and lumichrome (Fig. 3c—the peak No. 7 in Fig. 2a). Riboflavin itself yields LC as the major fragmentation product (Fig. 3a); the following fragmentation involves cleavage of the isoalloxazine entity third ring—through a release of the CONHCO neutral fragment, and that is the same pattern seen in the MS/MS spectra of LC itself (Fig. 3c). On the other hand, according to LF MS/MS spectra the CONHCO elimination occurs in two consecutive steps yielding fragments at m/z = 214 ([MH-CONH]+) and m/z = 186 (the additional loss of neutral CO fragment) (Table 1). Ahmad and coworkers [33] studied the effect of light intensity and irradiation wavelengths on RFL photodegradation reactions (photoaddition and photoreduction) in solution and in the presence of phosphate buffer, using UV and visible light sources. They have also found LC and LF among the major products and reported that the ratio LF/LC increased with the change from UV to VIS radiation. That is in good correspondence with the data presented in this report: it is clear that LC dominates over LF after 6 min of UV-B irradiation under anaerobic conditions (LF is not even recorded after the analog irradiation under aerobic conditions; Fig. 2a).
The MS/MS spectra of riboflavin—RFL (peak No. 1 from UHPLC chromatograms shown in Fig. 2a) (a) and the products, lumiflavin (LF) and lumichrome (LC), the peaks No. 3 and 7 (b) and (c), respectively
Time dynamics of riboflavin degradation in MeOH during UV-B irradiation regime, in the presence and in the absence of quercetin, under anaerobic and aerobic conditions, is shown in Fig. 4a; to be able to better understand quercetin influence, QC degradation was traced alone as well in the mixture with RFL—both under anaerobic and aerobic conditions (Fig. 4b).
Degradation of riboflavin and quercetin in both aerobic and anaerobic conditions under continuos UV-B irradiation regime (particularly, and in the mixture), expressed as the changes in percents of RFL and QC (compared to non-irradiation) for different irradiation time periods (t irr)—e.g., the dynamic plots (a and b, respectively). The plots are based on the data from DAD-recorded chromatograms (the peaks No. 1 and 8, at t ret = 2.41 and 7.80 min, respectively, shown in the Fig. 2a), at 446 and 372 nm, e.g., absorption maximums for RFL and QC, respectively. The percents of RFL and Querc. were calculated by using equation: Content (%) = (A t × 100)/A 0, where A 0 and A t representing the peak area values of RFL and QC at the corresponding t ret values, for t irr = 0, and different irradiation periods, respectively
During first 15 min of UV-B irradiation QC appears to be much more resistant than RFL in aerobic conditions; RFL itself degrades much faster in anaerobic conditions (Fig. 4a, b). It is clear that QC acts as a RFL protective factor toward the applied UV-B irradiation, e.g., the order in dynamics of RFL degradation looks as follows: RFL-anaer. > (RFL+QC)-anaer. > RFL–aer. > (RFL+QC)-aer. (Figure 4a). Synchronously, dynamics ordering in QC decrease with increasing irradiation periods is: QC < (RFL+QC)-anaer. < (RFL+QC)-aer. (Fig. 4b).
The different RFL degradation under anaerobic and aerobic conditions (in the absence of QC) is clearly dictated by RFL photosensitizing properties [20, 21]. The fastest RFL degradation in the absence of quercetin and under anaerobic conditions is clearly related to its type I photosensitizing activity that takes place via its triplet state and involves formation of semiquinone RFL-radical [27]. In the presence of QC—and also under anaerobic conditions—RFL degrades a bit slower. It is well known that QC expresses its antioxidant ability toward UV-induced effects in two different ways, (1) through a strong UV absorption [34, 35], and/or (2) chain-breaking activities, i.e., by scavenging present radicals, for example by preventing oxidative skin damage [36–38]. Though one may reasonably speculate that the both ways could contribute to decrease of RFL degradation (both RFL and QC are excellent UV-B absorbers) [5], the latter one—through possible scavenging of UV-created RFL radicals—would certainly affect rate of RFL degradation, e.g., decrease it, which is shown in Fig. 4a. In the same time a clear decrease of QC concentration was seen under anaerobic conditions in the presence of RFL, compared to the RFL-absence case (Fig. 4b).
The protective effect of QC (toward RFL) is also seen under aerobic conditions. First of all, it is evident that RFL degradation itself under aerobic conditions is slower compared to the anaerobic conditions (in the absence and in the presence of QC, as well). The addition of QC in the aerobic system makes RFL degradation even more slower (Fig. 4a). Clearly, QC contributes to decrease of RFL degradation both under anaerobic and aerobic conditions, but more in the latter case (Fig. 4a, b). The same conclusion is drawn by tracing change of QC concentration in the presence of RFL, under anaerobic and aerobic conditions (including change of QC concentration under aerobic conditions in the absence of RFL; Fig. 4b). There, RFL certainly acts as the type II photosensitizer, so the protection must include involvement of singlet oxygen formation [27].
The plots tracing dynamics of appearance of the two major products, lumiflavin and lumichrome (Fig. 5a, b) are generally in coherence with the above presented data, though it looks a bit more consistent with lumiflavin (Fig. 5a). Lumiflavin structure is more similar to RFL than lumichrome (Fig. 1), though in both cases the isoalloxazine entity has been survived the applied UV-B irradiation regime. The RFL anaerobic degradation in 8 min (irradiation) period (Fig. 4a, the lowest trace) is matched by a synchronous exponential-shape type of LF rise in the same (anaerobic) conditions (Fig. 5a, the upper trace). The same type of rise but with earlier achieved plateau has been seen when QC was added in the system (Fig. 5a, the middle trace)—and this well matches with the RFL anaerobic degradation in the presence of QC (see Fig. 4a). Finally, in the aerobic conditions LF expresses the slowest, almost linear type of rise (Fig. 5a, the lowest trace), which is nicely matched with RFL aerobic degradation pattern (Fig. 4a). Only one thing makes the exception from this synchronicity between RFL degradation and LF production: when QC is added to RFL under aerobic conditions there is no any trace of LF presence (see Fig. 2a, the middle trace).
The changes in peak areas for LF and LC (the peaks No. 3 and 7 at t ret = 3.26 and 6.56 min, respectively, from chromatogram shown in Fig. 2a) for different irradiation time periods (t irr)—e.g., dynamic plots (a and b, respectively). The plots are based on the data from DAD-recorded chromatograms at 445 and 385 nm, e.g., absorption maximums for LF and LC, respectively
Lumichrome dynamics plots (Fig. 5b) expressed very similar behavior to lumiflavin (Fig. 5a). The same pattern has been seen under anaerobic conditions during the same 8-min irradiation period, while in the aerobic conditions (on a bit longer irradiation scale) the undoubtedly proven QC preventive effect on RFL degradation (Fig. 4a, b) is once again confirmed: more LC is formed in the absence than in the presence of QC (Fig. 5b). Still, a question emerges: why in the same (RFL+QC)-aerobic system, and under the same UV-B irradiation regime, one of the two major products (LC) is being formed, while the other one (LF) is absent (Fig. 2a, the middle and the lowest traces)? The question is quite relevant having in mind that the both products have very similar structures, preserving basic isoalloxazine entity following the irradiation (Fig. 1); it is hard to see how the minor structural differences, presence (LF) or absence (LC) of internal conjugation inside it—though leading to different spectral behavior (Fig. 2c)—may such differently favor formation of the two products. Still, what looks undoubtful is that this behavior must include QC action, and most probably involvement of singlet oxygen, certainly created in the system by RFL type II photosensitizing activity [27]: the plots in Fig. 4b support this explanation.
To conclude, this work confirms that continuous UV-B irradiation of riboflavin in MeOH solution leads to its degradation both under anaerobic and aerobic conditions (faster in the former case) which is related to RFL sensitizing properties (type I photosensitizer in the first case, and type II in the other one). Addition of quercetin in the system leads to decrease of the (RFL) degradation in both cases; in anaerobic conditions it certainly might include QC antioxidant scavenging activity while under aerobic conditions the degradation decrease should be related to the involvement of singlet oxygen formation. The degradation dynamics—in both systems, in the presence and in the absence of QC—is well synchronized with dynamics formation of the two major products, lumiflavin and lumichrome. The unappearance of LF in the aerated (RFL+QC) system still needs to be explained.
Experimental
Sample preparation
Riboflavin and quercetin stock solutions were prepared by dissolving 36.7 mg RFL (98 %, Alfa Aesar, Karlsruhe, Germany) in 25 cm3 of 0.01 M NaOH (AnalaR NORMAPUR, Leuven, Belgium) in redestilated water, and 3.02 mg of quercetin in 10 cm3 of methanol (J.T. Baker, BAKER ANALYZED, LC–MS reagent, Deventer, The Netherlands). The sample solutions were made by diluting the stock solution with methanol; they are stored at 4 °C until their use, protected from light with aluminum foil (to avoid any photochemical changes). The concentration of riboflavin as well as quercetin were 0.1 mM in the final methanol solutions with pH = 8.69. All experiments were done at room temperature.
UV-irradiation treatment
Continuous UV-irradiations of the riboflavin samples (under aerobic and anaerobic conditions) in methanol were performed in cylindrical photochemical reactor “Rayonet”, with 10 symmetrically placed lamps with emission maximum at 300 nm (UV-B). The samples were irradiated in quartz closed cuvettes (1 × 1 × 4.5 cm) placed on rotating circular holder. The total measured energy flux was 15.0 W/m2 at 10 cm distance. The anaerobic conditions were achieved by bubbling the solutions with nitrogen for at least 10 min prior to the irradiation.
Ultra high-performance liquid chromatography-diode array-electrospray ionization mass spectrometry analysis
The chromatography runs were carried out using a Dionex Ultimate 3000 UHPLC+ system equipped with a diode array (DAD) detector and connected to LCQ Fleet Ion Trap Mass Spectrometer, Thermo Fisher Scientific, USA. The separations were performed on a Hypersil gold aQ C18 column (150 × 3 mm, 3 µm) at 25 °C.
The mobile phase consisted of (A) 0.1 % formic acid in water and (B) 0.1 % formic acid in methanol. The next linear gradient program at flow rate of 0.5 cm3/min has been applied to RFL in methanol only: 20–50 % (B) for the first 6.5 min, followed by isocratic 50 % (B) from 6.5 to 10th min, 50–20 % (B) from 10 to 10.1 min, and finally isocratic run with 20 % (B) to 20th min. Regarding RFL-QC and QC solutions in methanol the next linear gradient program has been applied: 40 % (B) for the first 2.5 min, 40–95 % (B) from 2.5 to 5.5 min, isocratic run with 95 % (B) for the next 1.5 min, 95 to 40 % (B) from 7 to 7.1 min, and finally isocratic run with 40 % (B) to 13th min. The injection volume was 0.5 mm3. Absorption UV–vis spectra were recorded on DAD-detector (200–800 nm), set at four detection wavelengths, λ det. (for RFL solution): 445 nm (RFL absorption maximum, A max), 410 nm (for CDRF detection), 385 nm (for FMF detection) and 356 nm (for LC detection). For RFL-QC solution λ det. was set at: 445 nm, 410 nm, 385 nm (for the same reasons as in the former case) and 372 nm (QC absorption maximum, A max); finally, for QC sample only: 255 nm (QC A max—band II), 295 nm and 300 nm (QC degradation products A max) and 372 (QC A max—band I), simultaneously. The MS-analysis was performed using a LCQ 3D-ion trap mass spectrometer with electrospray ionization (ESI) in both positive and negative ion mode. For positive ion mode, the ESI-source parameters were set as follows: source voltage 4.5 kV, capillary voltage 22 V, tube lens voltage 65 V, capillary temperature 350 °C, sheath and auxiliary gas flow (N2) 60 and 5 (arbitrary units), respectively. For negative ion mode, source voltage was set to 4.5 kV, capillary voltage −60 V, tube lens voltage −95 V, capillary temperature 350 °C, sheath and auxiliary gas flow 42 and 7, respectively. MS spectra were obtained by full range acquisition of m/z = 150–1000 and 100–650. For fragmentation study (MS/MS), a data dependent scan was performed by deploying the collision–induced dissociation (CID). The normalized collision energy of the CID cell was set at 15 and 17 eV.
References
Young A (2006) Prog Biophys Mol Biol 92:80
International Agency for Research on Cancer (2006) Exposure to artificial UV radiation and skin cancer. IARC working group reports No 1. IARC
Schade N, Esser C, Krutmann J (2005) Photochem Photobiol Sci 3:699
Longstreth J, de Gruijl FR, Kripke ML, Abseck S, Arnold F, Slaper HI, Velders G, Takizawa Y, van der Leun JC (1998) J Photochem Photobiol B Biol 46:20
Wondrak GT, Jacobson MK, Jacobson EL (2006) Photochem Photobiol Sci 5:215
Powers HJ (2003) Am J Clin Nutr 77:1352
Silva E, González T, Edwards AM, Zuloaga F (1998) Nutr Biochem 9:149
Girotti AW (2001) J Photochem Photobiol 63:103
Makdoumi K (2011) Ultraviolet light A (UVA) photoactivation of riboflavin as a potential therapy for infectious keratitis. Ph.D. Thesis, Örebro University, Örebro
Choe E, Huang R, Min DB (2005) J Food Sci 70:R28
Kino K, Kobayashi T, Arima E, Komori R, Kobayashi T, Miyazawa H (2009) Bioorg Med Chem Lett 19:2070
de La Rochette A, Silva E, Birlouez-Aragon I, Mancini M, Edwards A-M, Morlière P (2000) Photochem Photobiol 72:815
Silva E, Ugarte R, Andrade A, Edwards AM (1994) J Photochem Photobiol B Biol 23:43
Silva E, Edwards AM, Pacheco D (1999) J Nutr Biochem 10:181
Chien JT, Lu YF, Hu PC, Chen BH (2003) Food Chem 81:421
Huvaere K, Cardoso DR, Homem-de-Mello P, Westermann S, Skibsted LH (2010) J Phys Chem B 114:5583
Joshi PC, Keane TC (2010) Biochem Biophys Res Commun 400:729
Akhtar MJ, Khan MA, Ahmad I (2000) J Pharm Biomed Anal 23:1039
King JM, Min DB (2002) J Am Oil Chem Soc 79:983
Sikorska E, Khmelinskii I, Komasa A, Koput J, Ferreira LFV, Herance JR, Bourdelande JL, Williams SL, Worrall DR, Insińska-Rak M, Sikorski M (2005) Chem Phys 314:239
Cardoso DR, Libardia SH, Skibsted LH (2012) Food Funct 3:487
Heelis F (1982) Chem Soc Rev 11:15
Min DB, Boff JM (2002) Compr Rev Food Sci Food Saf 1:58
Ahmad I, Fasihullah Q, Vaid FHM (2004) J Photochem Photobiol B Biol 75:13
Ahmad I, Fasihullah Q, Vaid FHM (2005) J Photochem Photobiol B Biol 78:229
Ahmad I, Ahmed S, Sheraz MA, Vaid FHM, Ansari IA (2010) Int J Pharm 390:174
Insinska-Rak M, Golczak A, Sikorski M (2012) J Phys Chem A 116:1199
Huvaere K, Olsen K, Skibsted LH (2009) J Org Chem 74:7283
Becker EM, Cardoso DR, Skibsted LH (2005) Eur Food Res Technol 221:382
Fahlman BM, Krol ES (2009) J Photochem Photobiol B Biol 97:123
Zvezdanović JB, Stanojević JS, Marković DZ, Cvetković DJ (2012) J Serb Chem Soc 77:297
Zvezdanović JB, Marković DZ, Cvetković DJ, Stanojević JS (2012) J Serb Chem Soc 77:1571
Ahmad I, Fasihullah Q, Vaid FHM (2006) J Photochem Photobiol B Biol 82:21
Strid A, Porra RJ (1992) Plant Cell Phys 33:1015
Strid A, Chow WS, Anderson JM (1994) Photosynth Res 39:475
Schoemaker JH, Schoemaker MT, Zijlstra H, van der Horst FA (1995) Dermatology 191:36
Casagrande R, Georgetti SR, Verri WA Jr, Dorta DJ, dos Santos AC, Fonseca MJV (2006) J Photochem Photobiol B: Biol 84:21
Choquenet B, Couteau C, Paparis E, Coiffard LJM (2008) J Nat Prod 71:1117
Acknowledgments
This work was supported under Projects of the Ministry of Education, Science and Technological development of the Republic of Serbia, Projects No.TR-34012 and OI-172044.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stanojević, J.S., Zvezdanović, J.B. & Marković, D.Z. Riboflavin degradation in the presence of quercetin in methanol under continuous UV-B irradiation: the ESI–MS–UHPLC analysis. Monatsh Chem 146, 1787–1794 (2015). https://doi.org/10.1007/s00706-015-1561-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-015-1561-1