Abstract
Objective
Epstein-Barr virus (EBV)-associated gastric cancer (EBVaGC) is a distinct molecular subtype of gastric cancer (GC). At present, the clinical characteristics and prognostic implications of EBV infection and the potential clinical benefits of immune checkpoint blockade in GC remain to be clarified. Hence, this study was designed to analyze the clinical and pathological characteristics of GC patients with varying EBV infection states and compare their overall survival (OS).
Methods
A retrospective study was performed on 1031 consecutive GC patients who underwent gastrectomy at the Affiliated Hospital of Xuzhou Medical University from February 2018 to November 2022. EBV-encoded RNA (EBER) in situ hybridization (ISH) was used for EBV assessment, and immunohistochemical staining was used for evaluation of human epidermal growth factor receptor 2 (HER2), programmed death ligand 1 (PD-L1), and Ki67 expression. EBVaGC was defined as tumors with EBV positivity. In addition, EBV-negative GC (EBVnGC) patients were matched with EBVaGC patients based on seven clinicopathological parameters (age, gender, anatomic subsite, tumor size, Lauren classification, degree of differentiation, and tumor-node-metastasis [TNM] stage). The correlations of clinical features with HER2, PD-L1, and Ki67 expression were evaluated statistically. The survival of patients was assessed through medical records, telephone, or WeChat communication, and prognostic analysis was performed using the logrank test as well as univariable and multivariable regression analysis.
Results
Out of 1031 GC patients tested, 35 (3.4%) were diagnosed with EBVaGC. Notably, the EBVaGC group exhibited a distinct predominance of males and younger patients, significantly higher Ki67 and PD-L1 expression levels, and a lower prevalence of pericancerous nerve invasion than the EBVnGC group (P < 0.01). In the 35 EBVaGC cases, Ki67 expression was negatively correlated with age (P < 0.05), suggesting that a younger onset age was associated with higher Ki67 expression. In addition, PD-L1 expression was correlated with the degree of differentiation, T-stage, and clinical stage of the patient. Furthermore, PD-L1 expression was elevated in tumors with lower differentiation or at later stages (P < 0.05). Using univariate analysis, Ki67, PD-L1, and clinical stage were identified as significant factors influencing the overall survival (OS) of EBVaGC patients (P < 0.05). Moreover, multivariate survival analysis revealed that clinical stage and Ki67 expression were independent risk factors for the OS of the patients (P < 0.05), and the three-year OS rate of EBVaGC patients was 64.2%.
Conclusion
EBV-ISH is a practical and valuable method to identify EBVaGC. Owing to its unique etiological, pathological, and clinical characteristics, patients with EBVaGC might benefit from immune checkpoint blockade therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC), which is the sixth-most-common cancer and the third leading cause of cancer-related death worldwide, is a significant public health concern, particularly in East Asia [1]. Thus, the role of Epstein-Barr virus (EBV) and EBV-encoded RNA (EBER) in GC is an important topic in cancer research [2, 3].
EBV, a prevalent gammaherpesvirus, has a double-stranded DNA genome of about 170 kb and infects over 95% of the population of the world. Unlike oncogenic viruses such as hepatitis B virus (HBV) and human papillomavirus (HPV), elements of the EBV genome usually do not integrate into the host genome but instead persist as independently replicating extrachromosomal structures called episomes [4]. The initial EBV infection is often asymptomatic and is transmitted through oral secretions during childhood. EBV then establishes a long-term latent infection in a small subset of human memory B cells (MemB), which can last throughout the lifetime of a healthy individual [5]. MemB cells express three latent proteins as a part of EBV’s latency transcription program, with Epstein–Barr nuclear antigen 1 (EBNA1) being the only consistently expressed protein in all EBV-associated malignancies [6, 7]. EBNA1 plays a crucial role in EBV episome replication and stable persistence and has been shown to possess the potential to modify the cellular environment. Moreover, EBNA1 promotes genomic instability and potentially acts as an oncogene [5], which might make cytotoxic T lymphocytes unable to effectively recognize and respond to infected cells, thereby allowing EBV-infected cells to evade immune surveillance [8]. Understanding these mechanisms may provide valuable insights that help in identifying effective drug targets, developing promising therapeutic strategies, and minimizing the toxicity associated with individual drugs.
Since its initial discovery in Burkitt lymphoma, EBV has been firmly linked to various tumors, including Hodgkin’s lymphoma, NK/T-cell lymphoma, nasopharyngeal carcinoma, GC, intrahepatic cholangiocarcinoma, and leiomyosarcoma [9]. In 1990, Burke et al. first reported the detection of the EBV genome in GC using polymerase chain reaction (PCR) techniques [10]. Subsequently, in 1993, Tokunaga et al. detected EBERs in gastric tissue using in situ hybridization (ISH) techniques [11]. Although PCR can be highly sensitive, it may lead to false-positive results [11]. In contrast, EBER-ISH offers a more objective method to confirm the presence of EBV [12, 13]. This is primarily due to the direct demonstration that nearly all carcinoma cells contain specific EBV DNA sequences and EBV terminal repeat sequences of a particular length. As a result, EBER-ISH has become the gold standard for defining EBV-associated GC (EBVaGC). A large-scale study conducted in Japan reported EBERs in 6.6% (122 out of 1849) of GC cases [14]. Similarly, Chen et al. observed an EBVaGC prevalence of 6.7% (45 out of 676) [15]. A recent study in China found an EBVaGC prevalence of 5.1% (140 out of 2760) [16]. In a meta-analysis encompassing 9,738 individuals across 48 trials, Lee et al. reported an average EBVaGC prevalence of 8.8% [17]. Collectively, the reported prevalence of EBVaGC varies significantly, ranging from 1.3–20.1% across different geographic regions [18].
As our understanding of the molecular mechanisms underlying GC advances, several classification approaches have emerged. In 2014, the Cancer Genome Atlas (TCGA) introduced a molecular classification system that categorized GC into four distinct subtypes: EBV-associated (EBVa), microsatellite instability (MSI), genomically stable (GS), and chromosomal instability (CIN) [19]. These subtypes exhibit divergent clinical courses, impacting overall survival (OS) and recurrence-free survival (RFS). EBVaGC, characterized by microsatellite stability and extensive promoter DNA hypermethylation, displays robust lymphocyte infiltration triggered by EBV infection and pronounced tumor heterogeneity. However, whether patients with EBVaGC have a better prognosis than those with the three EBV-negative subtypes remains debatable [18]. To address this, we designed a paired trial to explore the correlation of patient prognosis with their clinicopathological characteristics, as well as the expression of human epidermal growth factor receptor 2 (HER2), programmed death ligand 1 (PD-L1), and Ki67, in EBVaGC and EBVnGC tissues.
Materials and methods
Patients and data
Patients were considered eligible for this study if GC was confirmed through pathological examination and adequate clinical samples were available for histological analysis. Based on these criteria, 1031 consecutive GC patients who underwent gastrectomy at the Affiliated Hospital of Xuzhou Medical University between February 2018 and November 2022 were identified, and their clinicopathological characteristics were extracted from hospital records.
Among these eligible cases, we identified 35 (3.4%) EBVaGC cases with complete clinicopathological data that were independently confirmed by two senior pathologists. Ethical approval for this retrospective study was obtained from the Institutional Ethics Committee (Approval ID: XYFY2021-KL063-01). The clinicopathological characteristics examined included age, gender, anatomic subsite, Lauren classification, histological type, degree of differentiation, and the American Joint Committee on Cancer (AJCC) TNM staging (8th Edition). EBVaGC was defined as a GC case with positive tumor EBV status. To ensure comparability, we matched EBV-negative GC (EBVnGC) patients with EBVaGC patients based on the seven clinicopathological features listed above, and a consistent median follow-up time was maintained for all patients.
EBV-encoded RNA (EBER) in situ hybridization (ISH)
All GC specimens underwent standard formalin fixation and paraffin embedding (FFPE) procedures. Next, 4-µm serial sections were prepared for hematoxylin and eosin (H&E) staining and subsequent testing.
EBERs in the GC specimen slides were detected automatically using an EBV Probe ISH Kit (ISH-6021, Beijing Zhongshan Golden Bridge Biotechnology) according to the manufacturer’s protocol and a BenchMark XT in situ hybridizer. Both positive and negative controls were included. EBV positivity was defined as the presence of a tan signal in tumor cells, with EBER levels equal to or exceeding 20% [20].
Immunohistochemical (IHC) staining
Immunohistochemical (IHC) analysis was performed on all 35 available EBVaGC specimens using the EnVision immunohistochemical procedure. Ventana anti-HER2/neu rabbit monoclonal antibody (4B5, Ventana) and anti-Ki67 antibody (Beijing Zhongshan Golden Bridge Biotechnology) were used for staining in a Ventana BenchMark XT fully automated immunostainer. Anti-PD-L1 was detected using a Link48 semi-automatic immunostainer. PD-L1 rabbit monoclonal antibody (22C3 pharmDX) was purchased from Agilent Technologies Co., Ltd.
Evaluation of immunohistochemical expression of HER2
The Ruschoff/Hofmann method was used for HER2 IHC scoring [21]. Briefly, tumor cells demonstrating strong membranous reactivity received a score of 3+, while those with moderate reactivity were scored as 2+. Specifically, cells with an IHC score of 3 + were immediately classified as HER2 positive, whereas cells with scores of 1 + or 0 were considered HER2 negative. For cases with an IHC score of 2+, further testing was conducted using fluorescence in situ hybridization (FISH) with a PathVysion HER2 DNA Probe Kit (Abbott, IL, USA) to determine the HER2 gene amplification status. If the ratio of the HER2 signal to the chromosome 17 centromere signal was at least 2.0, the gene was considered amplified. HER2 positivity was defined as IHC 2 + with FISH positivity, whereas IHC 2 + cells that were FISH negative were considered HER2 negative.
Evaluation of PD-L1 status
PD-L1 (22C3) positivity on both tumor cells and immune cells was evaluated using the combined positive score (CPS). The CPS was calculated using the following formula: [the number of live tumor cells displaying partial or complete membrane staining (≥ 1+) + the number of lymphocytes and macrophages with membrane/cytoplasmic staining (≥ 1+)] / the number of viable tumor cells (at least 100 viable tumor cells) × 100. The resulting score ranged from 0 to 100, with 100 being the maximum score, although theoretically, it could exceed 100 [22].
Evaluation of Ki67 values
Five fields of view at high magnification (×400) were selected randomly, and 100 cells in each field were examined, resulting in a total count of 500 cells. Cells were judged to be positive for Ki67 by the presence of brownish-yellow granular staining within the nucleus.
Follow-up
Patients’ disease status, including the presence of cancer recurrence or metastasis, and patients’ vital status (survival or death) were determined using hospital records and follow-up information obtained via telephone or WeChat. OS data were collected and analyzed up until January 31, 2023.
Statistical analysis
Data analysis was performed using SPSS version 27.0 and R version 4.3.2 statistical software. The degree of nerve and vascular invasion and the immunohistochemical outcomes between EBVaGC and EBVnGC patients were compared using the χ² test. Differences in Ki67, PD-L1, and HER2 expression between EBVaGC and EBVnGC were assessed using the Wilcoxon test. Fisher’s exact test was employed to examine the correlation of HER2, Ki67, and PD-L1 expression with clinicopathological characteristics of EBVaGC patients. Survival rates were compared using the logrank test, and survival curves were constructed using the Kaplan-Meier method. Prognostic analysis was performed using univariable and multivariable regression. P < 0.05 indicated statistical significance.
Results
EBV infection status of GC and prevalence of EBVaGC
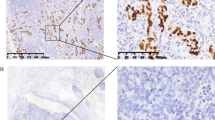
The distribution of EBV-positive signals was observed exclusively within the nuclei of tumor cells, with no detection of EBV in the surrounding uninfected epithelium or adjacent lymphocytes (Fig. 1A2, B2, and C2). In our cohort, EBVaGC accounted for 3.4% (35/1031) of all GC cases. In other investigations, the prevalence of EBVaGC varied considerably, ranging from approximately 1.3–20.1% [18].
Clinicopathological characteristics of EBVaGC
The distribution trends of 35 cases of EBVaGC and 35 cases of EBVnGC were analyzed using IHC, and their clinicopathological parameters were matched. The clinicopathological characteristics of these patients are presented in Table 1. The median age of EBVaGC patients was 59 years (range, 27–79 years). The EBVaGC patients were predominantly male (91.4%, 32/35), tended to have tumors proximal to the stomach (65.7%, 23/35), diffuse Lauren classification (74.3%, 26/35), and a low degree of differentiation (71.4%, 25/35). EBVaGC was also more frequently observed in advanced GC cases (40.0% [14/35] in clinical stage II and 42.9% [15/35] in stage III) than in early GC cases (17.1% [6/35] in stage I). Other characteristics were more likely to manifest in tumors smaller than 5 cm in size (68.6%, 24/35) and with fewer instances of vascular invasion (62.9%, 22/35).
Furthermore, we found that three male patients (8.6%, 3/35) with remnant EBVaGC had a history of prior partial gastrectomy. One of them, a 65-year-old patient with a Billroth II anastomosis for duodenal bulb ulcer 30 years previously presented with acute hematemesis, vertigo, and black stool without clear etiology. The other two patients, aged 58 and 74, had undergone distal gastrectomy for pyloric obstruction 20 and 40 years previously, respectively.
Histological features
Generally, EBVaGC is often manifested as ulcerated masses, accompanied by significant thickening of the gastric wall, medullary tumor cut surfaces, and distinct tumor borders upon macroscopic examination. Histologically, a “lace pattern” is a common early characteristic of EBVaGC. The mucosa surrounding the tumor typically exhibits moderate-to-severe atrophy and intestinal metaplasia. Notably, we observed a more pronounced depletion of mural cells in EBVaGC compared to EBVnGC, which may make gastric epithelial cells more susceptible to EBV infection. EBVaGC can be further subclassified into lymphoepithelioma-like carcinoma (LELC), Crohn’s-like lymphocytic reaction (CLR), and conventional adenocarcinoma (CA), based on the pattern of immune cell infiltration and the extent of desmoplasia [23]. As shown in Fig. 1, LELC was characterized by poorly formed glandular structures with well-defined tumor margins, dense tumor-infiltrating lymphocytes, and local formation of secondary lymphoid follicles, typically without desmoplasia (Fig. 1A1). CLR was defined by frequent tubule or gland formation with patchy lymphocytic infiltration, often accompanied by minimal or no desmoplasia (Fig. 1B1). CA was classified as having scattered lymphocyte infiltration with prominent desmoplasia (Fig. 1C1). Among the EBVaGC patients, those with the LELC subtype had the best prognosis, followed by CLR, and then CA. The LELC subtype accounted for 60% (21/35) of EBVaGC cases, while CLR and CA accounted for 22.8% (8/35) and 17.1% (6/35), respectively.
HE staining, EBER-ISH, and immunohistochemistry (×200) of EBVaGC of different histological forms and EBVnGC (red arrow, carcinoma cells; green arrow, fibrous proliferation; yellow arrow, lymphocytic infiltration; scale, 100 µm). Column A: EBVaGC (LELC) was poorly differentiated, with prominent lymphocytic infiltration, and the scattered individual carcinoma cells were difficult to identify without immunohistochemical analysis. A1, HE staining; A2, EBER-ISH positive; A3, HER2 score of 2+; A4, PD-L1 (CPS) score of 60; A5, Ki67 value 90%. Column B: EBVaGC (CLR) had tubular gland formation, patchy lymphocytic infiltration in the mesenchyme, and little fibrous proliferation. B1, HE staining; B2, EBER-ISH positive; B3, HER2 score of 2+; B4, PD-L1 (CPS) score of 5; B5, Ki67 value 70%. Column C: EBVaGC (CA) exhibiting moderately differentiated glands with no or very little interstitial lymphocytic infiltration and localized with marked fibrous tissue hyperplasia. C1, HE staining; C2, EBER-ISH positive; C3, HER2 score of 1+; C4, PD-L1 (CPS) score of 2; C5, Ki67 value 40%. Column D: EBVnGC signet-ring cells seen in the mucosa. D1, HE staining; D2, EBER-ISH negative; D3, HER2 score of 3+; D4, PD-L1 (CPS) score of 0; D5, Ki67 value 10%. LELC, lymphoepithelioma-like carcinoma; CLR, Crohn’s-like lymphoid reaction; CA, conventional adenocarcinoma; EBVaGC, Epstein-Barr virus-associated gastric cancer; EBVnGC, Epstein-Barr virus-negative gastric cancer; CPS, combined positive score; EBER-ISH, Epstein-Barr virus-encoded RNA in situ hybridization; HER2, human epidermal growth factor receptor 2; PD-L1, programmed death ligand 1
Regarding the Lauren classification, 74.3% (26/35) were categorized as diffuse type (DT), 14.3% (5/35) as mixed type (MT), and 11.4% (4/35) as intestinal type (IT). Concerning the degree of differentiation, 71.4% (25/35) exhibited low differentiation, 22.9% (8/35) had moderate-to-low differentiation, and 5.7% (2/35) displayed moderate differentiation (Table 1). Signet-ring cells in EBVnGC mucosa are shown in Fig. 1D1.
Comparison of HER2, PD-L1 expression, and Ki67 values in EBVaGC and EBVnGC
Details of the prevalence of HER2, PD-L1, and Ki67 expression are shown in Fig. 2. PD-L1 expression was found to be significantly higher in the EBVaGC group, with a CPS of 38.66 ± 28.85, than that in the EBVnGC group (Table 2). Specifically, six patients had a CPS ranging from 0 to 5, 14 had a CPS of 6–49, and 15 had a CPS ≥ 50. PD-L1 expression in EBVaGC was associated with the degree of tumor differentiation, TNM stage, and clinical stage, especially when the PD-L1 CPS was ≥ 50. In these cases, the tumors were mostly classified as low-differentiated, T3/T4 stage, and clinical stage II/III (Table 3).
Expression of Ki67, PD-L1, and HER2 in 35 EBVaGC cases and 35 matched EBVnGC cases (A) Expression of Ki67. (B) Expression of PD-L1. (C) Expression of HER2 (P < 0.05, EBVnGC vs. EBVaGC). EBVaGC, Epstein-Barr virus-associated gastric cancer; EBVnGC, EBV-negative gastric cancer; CPS, combined positive score; PD-L1, programmed death ligand 1; HER2, human epidermal growth factor receptor 2
The Ki67 values in EBVaGC were notably elevated, with a mean of 59.71 ± 14.24 (Table 2), and in 15 cases, the values were ≥ 70%. Subsequent analysis using Fisher’s exact test revealed a negative correlation between Ki67 expression in the 35 EBVaGC cases and the age at onset (P = 0.041) (Table 3), suggesting that a younger onset age was associated with a higher Ki67 expression level. The results of the chi-square test indicated that both Ki67 and PD-L1 expression levels in EBVaGC tissues were higher than those in the EBVnGC group (Table 2 and Fig. 2A and B).
Among the 35 EBVaGC cases, one had an HER2 score of 3+, six had a score of 2+, 20 had a score of 1+, and eight had a score of 0. HER2 expression was higher in the EBVaGC group than in the EBVnGC group, but there was no statistical difference (Table 2 and Fig. 2C).
Prognostic significance between EBVaGC and EBVnGC
The follow-up period ranged from 3 to 59 months (last date of follow-up, January 31, 2023), with a median duration of 26 months. Of the 35 EBVaGC patients, seven experienced disease progression and ultimately succumbed, with a mean survival time of 23 months. In comparison, among the 35 EBVnGC patients, 11 had disease progression, and seven of them passed away, with a mean survival time of 18.9 months. The three-year OS rate for the 35 EBVaGC cases was 64.2%, while for 35 EBVnGC cases, it was 63.9%, with no statistically significant difference observed.
Statistical analysis was conducted with regard to different clinical characteristics of EBVaGC patients. The logrank test was used for univariate survival analysis, considering related clinicopathological features as well as the expression of PD-L1, Ki67, and HER2. The results indicated that Ki67 (P = 0.021), PD-L1 (P = 0.016), and clinical stage (P = 0.018) were significant factors affecting patient OS (Table 4 and Fig. 3). However, HER2 did not emerge as a significant factor for OS in EBVaGC patients (Table 4 and Fig. 3). Multivariate survival analysis including these statistically significant factors identified clinical stage (P = 0.028) and Ki67 expression (P = 0.040) as independent risk factors influencing the prognosis of EBVaGC patients (Table 4).
Survival curves of the study groups (n = 35). (A) Survival curves of EBVaGC with different Ki67 expression levels (P < 0.05). (B) Survival curves of EBVaGC with different PD-L1 expression levels (P < 0.05). (C) Survival curves of EBVaGC with different HER2 expression levels (P > 0.05). (D) Survival curves of EBVaGC with different clinical stages (P < 0.05). (E) Survival curves of EBVaGC and matched EBVnGC (P > 0.05). CPS, combined positive score; EBVaGC, Epstein-Barr virus-associated gastric cancer; EBVnGC, Epstein-Barr virus-negative gastric cancer; CPS, combined positive score; PD-L1, programmed death ligand 1; HER2, human epidermal growth factor receptor 2
Therapeutic strategies of EBVaGC
Of the 35 EBVaGC patients, five underwent immunotherapy (Table 5). Notably, two patients with stage IIIB/C disease among these five cases achieved long-term survival and did not experience disease progression following postoperative immune adjuvant therapy. To elaborate, one patient received postoperative adjuvant therapy involving irinotecan and pabrolizumab, with a follow-up period of 40 months. The other patients were treated with a combination of S-1 and oxaliplatin along with camrelizumab, and the treatment was ongoing. The remaining two out of the five patients who underwent immunotherapy also had favorable survival outcomes, even though they experienced disease progression, specifically abdominal metastasis. One of them, with a HER2 score of 3 + and cancer stage pT3N2M0, had a disease-free survival (DFS) of 13 months following postoperative adjuvant chemotherapy with SOX (oxaliplatin and capecitabine). Unfortunately, the disease progressed, leading to the administration of a combination treatment consisting of anti-HER2 (trastuzumab) and PD-1 monoclonal antibody (camrelizumab), followed by antivascular targeting and RC48 (disitamab vedotin) treatment, after which the patient survived for a total of 33 months. The other patient had a DFS of seven months and received a combination of anti-HER2 (trastuzumab) and PD-1 monoclonal antibody (camrelizumab) after disease progression, resulting in an OS of 25 months. Comparatively, among the 35 EBVnGC patients, two experienced recurrent metastases after postoperative adjuvant therapy. Sindilizumab immunotherapy combined with chemotherapy increased the survival of one patient, demonstrating a DFS of five months, while the other patient (with a DFS of seven months) survived for only 10 months. In our cohort, one EBVaGC patient with stage IIIB disease achieved a DFS of 40 months, demonstrating a significant response to combined therapy, including immune checkpoint blockade. Table 5 provides preliminary evidence of the promising clinical efficacy of early immunotherapy in EBVaGC patients.
Discussion
Previous studies by Li et al. found no detectable differences in overall survival and disease-free survival between EBVaGC and EBVnGC patients [24]. In the present study, 3.4% of the patients were diagnosed with EBVaGC. Notably, EBVaGC patients showed significantly higher Ki67 and PD-L1 expression levels and a lower prevalence of pericancerous nerve invasion when compared to EBVnGC patients. Ki67, PD-L1 expression, and clinical stage emerged as significant factors influencing OS, with Ki67 and clinical stage being independent risk factors. The clinical implications of this study on EBVaGC might be significant. The median age of 59 years and notable male predominance (91.4%) of the EBVaGC patients suggests a need for targeted screening in this demographic. The distinct tumor characteristics, such as preferential localization proximal to the stomach and the diffuse Lauren classification, and the observed trend towards low differentiation, highlight the unique characteristics of EBVaGC, which could guide personalized treatment approaches. The observation that most of the cases were in an advanced stage also underscores the urgent need for early detection strategies in individuals at high risk of developing GC. Additionally, the history of partial gastrectomy in some patients suggests a potential risk factor, indicating a need for vigilant postoperative monitoring. These insights offer a deeper understanding of EBVaGC’s clinical behavior, which will contribute to the development of more-effective personalized management strategies for this cancer subtype.
The differences in EBVaGC prevalence across different studies, including our present study, in which it accounted for 3.4% of all GC cases, can be attributed to several factors [25, 26]. First, geographical variations may play a significant role. EBVaGC prevalence has been observed to differ significantly across regions, possibly due to differences in genetic susceptibility, environmental factors, and dietary habits [27]. Second, the methodologies used for detecting EBV in tumor cells, such as in situ hybridization or PCR, may vary in sensitivity and specificity, leading to differing rates of EBVaGC detection [8]. Additionally, variations in study design, including differences in patient selection criteria and sample sizes, could also contribute to these disparities [28, 29]. Hence, understanding these variations is crucial for accurately assessing the global burden of EBVaGC and tailoring region-specific strategies for its diagnosis and treatment.
Our results showed that HER2 expression did not significantly impact OS in EBVaGC patients. HER2 status has been reported to vary significantly across different histological types of GC, with its positivity rate reported to be notably higher in intestinal-type GCs, ranging from 21.5–32.7%, compared to diffuse-type GCs, where the positivity rate ranges from 2.9–11.7% [30]. Moreover, association of HER2 overexpression with elevated phosphorylated protein kinase (pAkt) expression is significantly linked to poor prognosis in cancer patients, highlighting the potential importance of the HER2-Akt axis in GC. Interestingly, EBV infection has been shown to lead to the loss of phosphatase and tensin homolog (PTEN) expression through CpG island methylation of its promoter, consequently activating the PI3K-Akt signaling pathway in EBVaGC [31, 32]. Therefore, EBVaGC may exhibit distinct changes in the PI3K-Akt signaling pathway compared to EBVnGC, which could contribute to differences in prognosis and HER2 expression between the two subtypes [33]. For example, previous studies have reported fewer cases of HER2 overexpression in EBVaGC compared to EBVnGC, with percentages ranging from 1.6–5.1% in EBVaGC and 13.5–23.7% in EBVnGC [34, 35]. In contrast to these earlier findings, our study showed that the overall HER2 expression level was higher in EBVaGC than in EBVnGC. Our observation that HER2 expression did not significantly influence OS in EBVaGC patients in our study may be attributed to several factors. First, EBVaGC patients exhibited lower HER2 expression levels when compared to EBVnGC counterparts, possibly falling below the threshold required for HER2 to exert a substantial impact on OS. Second, EBVaGC and EBVnGC are associated with distinct tumorigenic mechanisms and genetic alterations, suggesting that HER2 may play a less prominent role in EBVaGC prognosis due to unique underlying molecular pathways. Third, significant clinicopathological factors such as Ki67 expression and clinical stage were identified as strong determinants of OS in EBVaGC patients, potentially overshadowing the influence of HER2 expression.
The lymphocyte response plays a crucial role in preventing lymph node metastasis (LNM) and the invasion of EBV-positive cancer cells into the deeper layers of the stomach wall. A study by Park et al. involving 756 patients with pathologic stage of tumor 1b (pT1b) GC showed that, of 64 patients with EBVaGC, only three had LNM [36]. Similarly, among 592 pT1b GC patients without lymphatic invasion (LVI), 59 were identified as EBVaGC, and only one patient (1.7%) developed LNM. These findings suggest that the absence of EBV infection is an independent risk factor for submucosal LNM in early gastric carcinoma. Patients with early GC who underwent curative resection could achieve a high 5-year survival rate, which may often exceed 90%. In our study, all 35 EBVaGC patients underwent radical gastrectomy, with the majority being stage II/III patients (82.9%). Their three-year OS rate was 64.2%. Among the TCGA GC classification subtypes, EBVaGC is associated with the most favorable prognosis. Consequently, it has been proposed to broaden the indications for endoscopic submucosal dissection (ESD) in stage pT1 (especially pT1b) EBVaGC cases that are amenable to complete resection and have a very low risk of LNM [37].
Although GC cells can employ various mechanisms to evade immune regulation, the current use of PD-L1/PD-1 inhibitors to treat advanced GC patients has shown the potential to significantly improve patients’ treatment outcomes and quality of life [38]. A study conducted by Kim et al. demonstrated that six EBVaGC patients achieved partial responses following pembrolizumab treatment, with a median remission duration of 8.5 months [39]. Remarkably, one patient with multiple liver metastases achieved a pathologic stage of the tumor 0 node 0 (pT0N0) EBVaGC status after radical surgery and eight cycles of pembrolizumab treatment. Furthermore, Mishima et al. reported that GC patients with high PD-L1 expression tended to exhibit more favorable responses to nivolumab treatment [39]. Our study indicated the successful use of immune adjuvant therapy in two patients with advanced stage IIIB/C EBVaGC, achieving long-term survival without disease progression, thereby demonstrating the potential of immunotherapy in improving outcomes for this specific cancer subtype. The use of various treatment regimens, including irinotecan, pabrolizumab, S-1, oxaliplatin, and camrelizumab, highlights the effectiveness of personalized therapy based on individual patient profiles. Notably, even in cases where the disease progressed, such as in patients receiving a combination of anti-HER2 and PD-1 monoclonal antibody therapy, there was still a notable extension of survival [40]. This contrasts with the outcomes observed in EBVnGC patients, in which the response to similar therapies was less pronounced. Taken together, these observations suggest that EBVaGC might be more responsive to immunotherapy and could guide future clinical approaches in treating this distinct GC subtype, emphasizing the importance of early and tailored immunotherapy strategies.
There are several limitations worth mentioning in this research. First, the retrospective analysis of patient data inherently restricted the ability to establish more-detailed correlations of clinicopathological features with EBVaGC diagnosis and subgroup analyses. Secondly, as a single-center study, our findings may not be entirely generalizable to other populations. The prevalence and characteristics of EBVaGC can vary based on geographic and ethnic differences, which may not be fully represented in this study. Additionally, the relatively small sample size might be associated with reduced statistical power, which might not be adequate to detect significant differences between EBVaGC and EBVnGC cases, such as a possible HER2-OS relationship. Furthermore, data regarding other possibly relevant factors, such as tumor markers, previous treatments, etc., were not available for all patients. Hence, further research involving diverse approaches and populations is essential to better elucidate the association between EBV and GC.
Conclusion
In summary, our study provides new insights into the clinicopathological characteristics and prognostic factors of EBVaGC, highlighting its distinct features, such as higher Ki67 and PD-L1 expression. These findings suggest potential for targeted therapies, particularly with immune checkpoint inhibitors. However, the limitations of a single-center study and a small EBVaGC sample size call for cautious interpretation. Consequently, future research should focus on multicenter studies with diverse cohorts to validate our findings and explore long-term outcomes, thereby advancing the clinical management of EBVaGC.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
World Health Organization (WHO). Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019. WHO (2020) Accessed December 11, 2020. whowww.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death
Costache S, Sajin M, Wedden S et al A consolidated working classification of gastric cancer for histopathologists (Review). Biomed Rep. 2023 July 19;19(3):58
Jia X, Guo T, Li Z et al (2021) Clinicopathological and Immunomicroenvironment Characteristics of Epstein-Barr Virus-Associated Gastric Cancer in a Chinese Population. Front Oncol 10:586752
Lieberman PM (2016) Epigenetics and Genetics of Viral Latency. Cell Host Microbe 19(5):619–628
Lu J, Murakami M, Verma SC et al (2011) Epstein-Barr Virus nuclear antigen 1 (EBNA1) confers resistance to apoptosis in EBV-positive B-lymphoma cells through up-regulation of survivin. Virology 410(1):64–75
Frappier L (2012) Contributions of Epstein-Barr nuclear antigen 1 (EBNA1) to cell immortalization and survival. Viruses 4(9):1537–1547
Münz C (2019) Latency and lytic replication in Epstein-Barr virus-associated oncogenesis. Nat Rev Microbiol 17(11):691–700
Shinozaki-Ushiku A, Kunita A, Fukayama M (2015) Update on Epstein-Barr virus and gastric cancer (review). Int J Oncol 46(4):1421–1434. https://doi.org/10.3892/ijo.2015.2856
Farrell PJ Epstein-Barr Virus and Cancer. Annu Rev Pathol 2019 January 24;14:29–53
Burke AP, Yen TS, Shekitka KM, Sobin LH (1990) Lymphoepithelial carcinoma of the stomach with Epstein-Barr virus demonstrated by polymerase chain reaction. Mod Pathol 3(3):377–380
Roy S (2021) Physicians’ Dilemma of False-Positive RT-PCR for COVID-19: a Case Report. SN Compr Clin Med 3(1):255–258
AbuSalah MAH, Gan SH, Al-Hatamleh MAI et al (2020) Recent Advances in Diagnostic Approaches for Epstein-Barr Virus. Pathogens 9(3):226
Owliaee I, Khaledian M, Boroujeni AK et al (2023) Engineered small extracellular vesicles as a novel platform to suppress human oncovirus-associated cancers. Infect Agents Cancer 69(18):1750–9378
Tokunaga M, Uemura Y, Tokudome T et al (1993 Oct) Epstein-Barr virus virus-related gastric cancer in Japan: a molecular patho-epidemiological study. Acta Pathol Jpn 43(10):574–581
Chen JN, Ding YG, Feng ZY et al (2010) Association of distinctive Epstein-Barr virus variants with gastric carcinoma in Guangzhou, southern China. J Med Virol 82(4):658–667
Qiu MZ, He CY, Lu SX et al (2020) Prospective observation: Clinical utility of plasma Epstein-Barr virus DNA load in EBV-associated gastric carcinoma patients. Int J Cancer 146(1):272–280
Lee JH, Kim SH, Han SH et al (2009) Clinicopathological and molecular characteristics of Epstein-Barr virus-associated gastric carcinoma: a meta-analysis. J Gastroenterol Hepatol 24(3):354–365
Camargo MC, Kim WH, Chiaravalli AM et al (2014) Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: an international pooled analysis. Gut 63(2):236–243
Cancer Genome Atlas Research Network (2014) Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513(7517):202–209
Au WY, Pang A, Chan EC et al (2005) Epstein-barr virus-related gastric adenocarcinoma: an early secondary cancer post hemopoietic stem cell transplantation. Gastroenterology 129(6):2058–2063
Hofmann M, Stoss O, Shi D et al (2008) Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 52(7):797–805
Kulangara K, Zhang N, Corigliano E et al (2019) Clinical Utility of the Combined Positive Score for Programmed Death Ligand-1 Expression and the Approval of Pembrolizumab for Treatment of Gastric Cancer. Arch Pathol Lab Med 143(3):330–337
Song HJ, Srivastava A, Lee J et al (2010) Host inflammatory response predicts survival of patients with Epstein-Barr virus-associated gastric carcinoma. Gastroenterology 139(1):84–92e2
Li GH, Zhou ZH, Wang ZX et al (2023) Assessing Epstein-Barr virus in gastric cancer: clinicopathological features and prognostic implications. Infect Agent Cancer 18:11
Atri-Schuller A, Abushukair H, Cavalcante L et al (2022) Tumor Molecular and Microenvironment Characteristics in EBV-Associated Malignancies as Potential Therapeutic Targets: Focus on Gastric Cancer. Curr Issues Mol Biol 44(11):5756–5767
Salnikov MY, Fonseca GJ, Mymryk JS (2023) Differences in the Tumor Microenvironment of EBV-Associated Gastric Cancers Revealed Using Single-Cell Transcriptome Analysis. Cancers (Basel) 15(12):3178
Wong Y, Meehan MT, Burrows SR et al (2022) Estimating the global burden of Epstein-Barr virus-related cancers. J Cancer Res Clin Oncol 148(1):31–46
Tatematsu D, Akao M, Park H et al (2023 March) Relationship between the inclusion/exclusion criteria and sample size in randomized controlled trials for SARS-CoV-2 entry inhibitors. J Theor Biol 21:561:111403
Chen JN, He D, Tang F et al (2012) Epstein-Barr virus-associated gastric carcinoma: a newly defined entity. J Clin Gastroenterol 46(4):262–271
Van Cutsem E, Bang YJ, Feng-Yi F et al (2015) HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 18(3):476–484
Chakravorty S, Afzali B, Kazemian M (2022 October) EBV-associated diseases: Current therapeutics and emerging technologies. Front Immunol 27:13:1059133
Dugan JP, Coleman CB, Haverkos B (2019 March) Opportunities to Target the Life Cycle of Epstein-Barr Virus (EBV) in EBV-Associated Lymphoproliferative Disorders. Front Oncol 15:9:127
Sukawa Y, Yamamoto H, Nosho K et al (2012 December) Alterations in the human epidermal growth factor receptor 2-phosphatidylinositol 3-kinase-v-Akt pathway in gastric cancer. World J Gastroenterol 7(45):6577–6586
Zhang YW, Zhao XX, Tan C et al (2015) Epstein-Barr virus latent membrane protein 2A suppresses the expression of HER2 via a pathway involving TWIST and YB-1 in Epstein-Barr virus-associated gastric carcinomas. Oncotarget 6(1):207–220
Lee HS, Chang MS, Yang HK et al (2004) Epstein-barr virus-positive gastric carcinoma has a distinct protein expression profile in comparison with epstein-barr virus-negative carcinoma. Clin Cancer Res. March 1;10(5):1698–1705
Park JH, Kim EK, Kim YH et al (2016) Epstein-Barr virus positivity, not mismatch repair- deficiency, is a favorable risk factor for lymph node metastasis in submucosa-invasive early gastric cancer. Gastric Cancer 19(4):1041–1051
Osumi H, Kawachi H, Murai K et al (2019) Risk stratification for lymph node metastasis using Epstein-Barr virus status in submucosal invasive (pT1) gastric cancer without lymphovascular invasion: a multicenter observational study. Gastric Cancer 22(6):1176–1182
Zhang Y, Yang Y, Chen Y et al (2022) PD-L1: Biological mechanism, function, and immunotherapy in gastric cancer. Front Immunol 13:1060497
Mishima S, Kawazoe A, Nakamura Y et al (2019) Clinicopathological and molecular features of responders to nivolumab for patients with advanced gastric cancer. J Immunother Cancer 7(1):24
Tsao LC, Force J, Hartman ZC Mechanisms of Therapeutic Antitumor Monoclonal Antibodies. Cancer Res 2021 September 15;81(18):4641–4651
Funding
This work was supported by Xuzhou Medical Key Talent Training Project (XWRCHT20220068); Xuzhou Medical University Hospital Development Fund Innovation Team Project (XYFC2021001), and the surface project (XYFM2021014).
Author information
Authors and Affiliations
Contributions
L.L, A.Y., M.Z., and H.W. conceptualized and designed the study and drafted the initial manuscript. L.M., M.C., W.L., X.Q., and C.G. collected the data and carried out the initial analyses. Z.H. and H.W. critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This study was approved by the Medical Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (XYFY2021-KL063-01).
Additional information
Communicated by Zhongjie Shi.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Ll., Yu, Ay., Zhu, M. et al. Clinicopathological characteristics and prognosis of Epstein-Barr virus–associated gastric cancer. Arch Virol 169, 114 (2024). https://doi.org/10.1007/s00705-024-06033-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00705-024-06033-3