Abstract
Background
Epstein–Barr virus (EBV)-associated gastric cancer (GC) and microsatellite-instability-high GC are associated with a low prevalence of regional lymph node metastasis (LNM). To evaluate the feasibility of endoscopic treatment of EBV-associated and/or microsatellite-instability-high early GC (EGC), we analyzed the risk factors for LNM using a large series (n = 756) of submucosa-invasive (SM) EGC.
Methods
EBV-encoded RNA in situ hybridization (EBER ISH) and immunohistochemistry for four mismatch repair (MMR) proteins (MLH1, PMS2, MSH2, and MSH6) were performed. The clinicopathologic features and results of EBER ISH and immunohistochemistry were compared according to the LNM status.
Results
Among the cases, 146 EGCs (19.3 %) showed LNM. EBV negativity, larger tumor size (greater than 2 cm), deeper level of submucosal invasion, submucosal invasion depth greater than 500 µm, presence of ulceration, and presence of lymphovascular invasion (LVI) were associated with LNM. However, the MMR deficiency was not correlated with LNM. On multivariate regression analysis, larger tumor size (greater than 2 cm; odds ratio 1.6, p = 0.030), deeper level of submucosal invasion (odds ratio 2.9, p = 0.001), LVI (odds ratio 7.4, p < 0.001), and EBV negativity (p = 0.020) were independent risk factors for LNM in SM EGCs.
Conclusions
EBV positivity was a favorable risk factor for LNM in SM EGC. However, MMR deficiency was not associated with the status of LNM. Thus, we suggest that examination with EBER ISH could be considered for endoscopic resected specimens, especially in cases of SM EGC showing no LVI and clear resection margins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With advances in endoscopic technologies, such as endoscopic mucosal resection and endoscopic submucosal dissection (ESD), the number of patients with early gastric cancer (EGC) treated with endoscopic resection has rapidly increased [1]. The classic indication for endoscopic mucosal resection is a small (2.0 cm or less) differentiated intramucosal adenocarcinoma without ulceration [2]. ESD technology, which overcomes the limitation of the tumor size and achieves a successful en bloc resection, has extended the indications. The currently accepted extended indications of ESD for EGC, based on a proposal by Gotoda et al. [3] are (1) an intramucosal, nonulcerative differentiated adenocarcinoma, regardless of size, (2) an ulcerative intramucosal differentiated adenocarcinoma of 3.0 cm or less, and (3) a differentiated adenocarcinoma of 3.0 cm or less with minute submucosal invasion (500 μm or less). A small (2.0 cm or less) intramucosal undifferentiated adenocarcinoma without ulceration is a marginal indication for ESD. Cases which exceed the above-mentioned criteria and have lymphovascular invasion (LVI) need further gastrectomy with lymph node dissection because of the risk of lymph node metastasis (LNM) [4]. Because of the rapidly increasing number of EGC patients treated by ESD and the impact of the pathology findings for the endoscopically resected specimen on the decision for further surgical treatment, a precise risk prediction of LNM in EGC is more important than ever.
Since Burke et al. [5] reported that Epstein–Barr virus (EBV), a ubiquitous herpes virus found in more than 90 % of adults, was detected in lymphoepithelioma-like gastric cancer (GC) [6], it is now well known that EBV is found in more than 80 % of cases of lymphoepithelioma-like GC, which has the characteristic of dense lymphoid cell infiltration in the stroma [7]. The incidence and clinicopathologic characteristics of EBV-associated GC have also been widely investigated. EBV-associated GC accounts for approximately 8 % of GC worldwide [8–11] and is associated with proximal location, male sex, high incidence in remnant stomach, dense lymphocytic infiltration, and lower prevalence of LNM [8, 9, 12–17]. Intramucosal EBV-associated EGC frequently displays a “lace” pattern, which is formed by fusions of cancer cells, rather than the typical lymphoepithelioma-like features [18]. The lack of tubule formation of lymphoepithelioma-like EGC or EGC with a lace pattern tends to be classified as undifferentiated type. Because only a small portion of undifferentiated EGCs fulfill the current extended criteria, patients with EBV-associated EGC face the possibility of losing the chance to be treated by endoscopic resection, although they may have no LNM. Therefore, the risk evaluation of LNM in EBV-associated EGC is an urgent and mandatory issue that needs to be addressed.
Microsatellite instability (MSI) is a form of genetic instability characterized by varying sizes of repetitive sequences. MSI is caused by a failure of the DNA mismatch repair (MMR) system [19]. A standard panel of microsatellite markers, including BAT26, BAT25, D2S123, D5S346, and D17S250, has been recommended for MSI testing [20]. With the use of the reference panel, tumors showing MSI at two or more markers were defined as MSI high (MSI-H) type [20]. MSI-H has been found in approximately 8.2–34.4 % of GC, depending on the definition system used [21, 22]. We previously reported a similar prevalence (9.0 %) of MSI-H-type GC [23]. The MSI-H phenotype is associated with intestinal type according to the Lauren classification, prominent lymphoid infiltration, older age, antral location, lower prevalence of LNM, lower pTNM stage, and better prognosis [22–27]. Several studies reported immunohistochemistry (IHC) using antibodies for DNA MMR proteins, including MLH1, MSH2, MSH6, and PMS2, as a useful alternative method to detect MSI-H [28, 29].
As EBV-associated and MSI-H types of GC are associated with a lower prevalence of LNM, we assumed that EBV-associated and/or MSI-H types of EGC could be candidate groups for endoscopic resection. To evaluate the feasibility of our hypothesis, we analyzed the risk factors for LNM using a large series of submucosa-invasive (SM) EGC, focusing on EBV positivity and MSI-H type.
Materials and methods
Patients and tissue collection
Information on 756 SM EGC patients (485 males and 271 females) who underwent gastrectomy with D2 lymph node dissection at Yonsei University College of Medicine between January 2010 and December 2012 was retrieved. The TNM stage was reviewed according to the seventh edition of the American Joint Committee on Cancer Cancer Staging Manual [30]. The patients’ clinical information was obtained from the medical records. The mean age was 63 years (range 27–86 years). The study was approved by the Institutional Review Board of Yonsei University College of Medicine (approval number 4-2014-0665).
Pathology analysis
A series of pathologic factors, including tumor size, histologic classification, submucosa invasion level, depth of submucosal invasion, presence of ulceration, stromal lymphoid reaction, and presence of LVI were reviewed. For histologic classification, the gastric carcinomas were classified according to the WHO classification system (well, moderately, and poorly differentiated tubular adenocarcinomas and signet ring cell carcinomas) [31] and the Lauren classification system (intestinal and diffuse type) [32]. Well and moderately differentiated tubular adenocarcinomas and papillary adenocarcinomas were classified as differentiated, whereas poorly differentiated tubular adenocarcinomas, poorly cohesive carcinomas (including signet ring cell carcinomas), and others were classified as undifferentiated [33]. Ulceration was defined histologically as a disruption of the muscularis mucosae with or without granulation tissue formation or submucosal fibrosis. The depth of submucosal invasion was defined as the distance from the lowest level of the muscularis mucosae (or surface of the ulceration) to the end of the deepest tumor invasion. Stromal lymphoid reaction was classified into three groups: none or mild (0–10 % lymphocytic infiltration in the stroma), moderate (10–50 %), and severe (more than 50 %).
Tissue microarray construction
Two cores of tumor tissue (3-mm diameter) were punched out from individual formalin-fixed and paraffin-embedded tumor blocks and arrayed in a new tissue microarray block. A core of adjacent nonneoplastic mucosa was arrayed in each tissue microarray block as a landmark and internal control. Sections (4-μm thick) from each tissue microarray block were prepared for immunohistochemical staining and EBV-encoded RNA in situ hybridization (EBER ISH). Hematoxylin and eosin and cytokeratin immunohistochemical staining were performed to confirm the presence of tumor cells.
EBER ISH
EBER ISH was performed with a Ventana BenchMark in situ hybridization system (ISH iView kit, Ventana, Tucson, AZ, USA). Paraffin-embedded tissue sections were deparaffinzed with EZ Prep buffer (Ventana), and then digested with protease I for 4 min. Probes were applied and then denaturation was performed at 85 °C (10 min), followed by hybridization at 37 °C (1 h). The probes labeled with fluorescein contained a cocktail of oligonucleotides dissolved in a formamide-based diluent. After hybridization, tissues were washed 3 times with 2× saline sodium citrate buffer at 57 °C. Incubation with antifluorescein monoclonal antibody was performed for 20 min and then an Alkaline Blue detection kit (Ventana) was used according to the manufacturer’s protocol. The slides were counterstained with Nuclear Fast Red for 10 min.
Immunohistochemistry
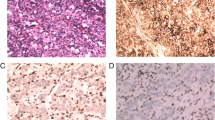
IHC was performed with a Ventana XT automated stainer with antibodies for cytokeratin (1:300, AE1/AE3, DAKO, Carpinteria, CA, USA), MLH1 (ready to use, clone M1, Roche, Indianapolis, IN, USA), MSH2 (ready to use, clone G219-1129, Roche), MSH6 (1:100, clone 44, Cell Marque, Rocklin, CA, USA), and PMS2 (1:40, clone MRQ28, Cell Marque). Sections were deparaffinized with EZ Prep solution (Ventana). CC1 standard [pH 8.4 buffer containing tris(hydroxymethyl)aminomethane–borate–EDTA] was used for antigen retrieval and blocked with 3 % H2O2 for 4 min at 37 °C. Slides were incubated with primary antibody for 40 min at 37 °C followed by a universal secondary antibody for 20 min at 37 °C. Slides were incubated in streptavidin–horseradish peroxidase for 16 min at 37 °C and then the substrate, 3,3′-diaminobenzidine tetrahydrochloride in H2O2, was added for 8 min followed by hematoxylin and bluing reagent counterstaining at 37 °C. A loss of MMR protein expression (MMR deficiency) was defined as when none of the neoplastic epithelial cells showed nuclear staining, whereas normal expression was defined as the presence of nuclear staining of tumor cells, irrespective of the proportion or intensity (Fig. 1). Infiltrating lymphocytes, stromal cells, and adjacent nonneoplastic epithelium served as internal positive controls. An MMR-deficient tumor was defined as a tumor showing loss of expression of any of the four MMR proteins.
Epstein–Barr virus (EBV)-encoded RNA in situ hybridization (EBER ISH) (a) and immunohistochemistry for mismatch repair (MMR) proteins (b). a An EBV-positive case shows tumor nests with dense lymphocytic infiltration in the stroma. On EBER ISH for this case, a strong nuclear positivity is evident. b An MMR-deficient case shows the loss of MLH1 and PMS2 expression and the nuclear expression of MSH2 and MSH6. However, all four MMR proteins are present in the nuclei of the tumor cells in an MMR-competent case. Original magnification ×100. H&E hematoxylin and eosin
Statistical analysis
The clinical and pathologic data were analyzed with IBM SPSS version 20.0 (IBM, Armonk, NY, USA). Pearson’s chi-square test, Fisher’s exact test, and logistic regression analysis were applied for the statistical analysis of the correlation between clinicopathologic variables and the status of LNM, EBV positivity, and MMR deficiency. Statistical significance was defined as p < 0.05.
Results
Clinicopathologic characteristics of SM EGCs according to LNM status
Among 756 SM EGCs, 146 EGCs (19.3 %) showed LNM. The clinicopathologic features of SM EGCs according to LNM status are summarized in Table 1. Univariate analysis revealed that smaller tumor size (2 cm or less) (p = 0.002), lower level of submucosa invasion (p = 0.001), submucosal invasion depth less than 500 μm (p = 0.004), absence of ulceration (p = 0.020), absence of LVI (p < 0.001), and EBV positivity (p = 0.002) were associated with EGCs without LNM. MSI status and stromal lymphoid reaction showed no correlation with LNM. Only three EBV-positive cases (4.7 %) showed LNM, and of these, two cases showed LVI, which is the most important risk factor for LNM. The remaining cases had a submucosal invasion depth of 4.0 mm.
Multivariate regression analysis revealed that EBV negativity (odds ratio 4.2, p = 0.020), larger tumor size (greater than 2 cm) (odds ratio 1.6, p = 0.030), the level of submucosal invasion (odds ratio 2.9, p = 0.001), and LVI (odds ratio 7.4, p < 0.001) were independent risk factors for LNM in SM EGCs (Table 2).
Clinicopathologic characteristics of SM EGC according to EBV status
Among 756 SM EGCs, 64 EGCs (8.5 %) were positive by EBER ISH (Fig. 1). The clinicopathologic features of SM EGCs according to EBV status are summarized in Table 3. Univariate analysis showed that younger age (60 years or younger) (p = 0.012), male sex (p < 0.001), proximal location (p < 0.001), elevated gross type (p = 0.015), undifferentiated histologic type (p = 0.007), intestinal type according to the Lauren classification (p < 0.001), absence of LVI (p = 0.005), moderate to severe lymphoid stroma (p < 0.001), and absence of LNM (p = 0.002) were associated with EBV-positive SM EGCs.
Clinicopathologic characteristics of SM EGCs according to MMR deficiency
Among 744 cases, loss of MLH1 and PMS2 expression occurred in 68 cases (9.1 %) and 69 cases (9.3 %), respectively (Fig. 1). Sixty-eight cases showed simultaneous loss of expression of MLH and PMS2 and only one case showed loss of expression of PMS2 only. No cases showed loss of MSH2 or MSH6 expression. MMR deficiency was defined as loss of expression of either MLH1 or PMS2.The clinicopathologic features of SM EGCs according to MMR deficiency status are summarized in Table 4. Older age (p < 0.001), distal location (p = 0.011), differentiated histologic type (p < 0.001), intestinal type according to the Lauren classification (p < 0.001), deeper level of submucosal invasion (p = 0.020), presence of LVI (p = 0.003), and moderate to severe lymphoid stroma (p = 0.003) were related to MMR deficiency. However, MMR deficiency was not correlated with LNM (p = 1.000). The MMR-deficient EGCs and EBV-positive EGCs were mutually exclusive, except in one case. Among the 745 cases, MSI analysis using five microsatellite markers (BAT26, BAT25, D2S123, D5S346, and D17S250DNA) was performed in 144 cases as previously reported [23]. Twenty EGCs (13.9 %) showed an MSI-H phenotype. The clinicopathologic characteristics of MSI-H EGCs based on DNA analysis were older age (p = 0.001), intestinal type according to the Lauren classification (p = 0.004), and moderate to severe lymphoid stroma (p < 0.001) (Table S1). Among the 144 cases, 12 EGCs (8.3 %) were positive by EBER ISH. The EBV positivity and the MSI-H type based on the DNA analysis were also mutually exclusive. The correlation between the results of IHC using antibodies for four MMR proteins and the results of the DNA analysis had a high concordance rate (k = 0.838, p < 0.001) and high sensitivity (90 %) and specificity (100 %) (Table S2).
Discussion
The rates of LNM in mucosa-confined and SM EGCs are 2.2–4.6 % and 14.0–23.6 %, respectively [3, 34–38]. In this study, the overall LNM rate of SM EGCs was 19.3 % (146/759). However, the LNM rate in EBV-positive SM EGCs was only 4.7 % (3/64), similar to that of mucosa-confined EGCs and dramatically lower than that of EBV-negative SM EGCs (20.1 %; 143/692). Similarly to our finding, Tokunaga et al. [16] reported a negative association between EBV positivity and LNM in a large series (n = 1760) of GCs. Among these, 323 cases were submucosa-confined (pT1b) cancers. Of the 323 cases, 43 (14.9 %) showed LNM and 45 (13.9 %) were EBV-positive cancers. In the EBV-positive group, none of the cases showed LNM. Therefore, on the basis of this finding, the Tokunaga et al. suggest that routine assay of a biopsy specimen for EBV may be important, especially in the case of EGC. However, because the study was performed in the pre-ESD era, there were no details regarding other important risk factors, including tumor size, tumor histologic type, ulceration, submucosal invasion depth, or LVI. Van Beek et al. [17] also reported a low frequency of LNM in EBV-positive GC in cohorts of the Dutch D1D2 trial (n = 566). In tar study, 41 cases (7.2 %) were EBV positive, and a significantly lower N category was found in the EBV-positive group. Amomg the cohorts, 150 cases were EGC (pT1), and among these, 13 (8.7 %) were EBV positive. However, there was no further information regarding the EGC subgroup. In our study, we observed a similar prevalence (8.5 %) of EBV positivity. In accordance with the observations of Tokunaga et al., we also found a significant difference in the frequency of LNM between the EBV-negative group (20.7 %) and the EBV-positive group (4.7 %). This suggests that if we use not only the current ESD criteria but also EBV positivity as an additional factor for prediction of LNM in an ESD specimen, we could clearly select the cases with a very low risk of LNM, even among SM EGC patients. As a true instance of this possibility, a recent case series study reported four cases of EBV-associated early lymphoepithelioma-like GC that were treated by ESD [39]. All the cases were resected en bloc with free resection margins and no LVI was found. However, all showed submucosal invasion of more than 500 µm (1.8–2.5 mm). In spite of this, one patient who underwent additional radical gastrectomy was found to have no LNM, and the other patients refused additional surgical treatment and none of them reported recurrence or metastasis for more than 27 months after ESD [39].
Among the 64 EBV-positive cases, 72 % of cases (46) were of an undifferentiated type. Because an SM undifferentiated carcinoma case needs further gastrectomy to dissect regional lymph nodes, on the basis of the proposal of Gotoda et al. [3] and Japanese guidelines [33], our results imply that, according to the current guidelines, most patients with EBV-positive SM EGCs could lose the chance to be treated by endoscopic resection, the organ-preserving treatment. Therefore, if a case of undifferentiated SM EGCs shows only minute submucosal invasion with neither LVI nor resection margin involvement, EBER ISH should be performed to avoid unnecessary surgical treatment. Particularly in cases with a mucosal lace pattern or moderate to severe stromal lymphoid reaction, EBER ISH should be mandatory.
Lymphoid stromal reaction is a well-known feature of EBV-associated GC [14, 18, 40–43]. Song et al. [43] divided EBV-associated GC into three groups depending on the host inflammatory reaction; lymphoepithelioma-like carcinoma, GC with Crohn’s diease-like lymphocytic reaction, and conventional adenocarcinoma. They then demonstrated that the prognosis of EBV-associated GCs depended on the stromal inflammatory reaction. In their study, a higher proportion of pN0 tumors was found in the lymphoepithelioma-like carcinoma group. However, from our results, although a severe or moderate degree of lymphoid stromal reaction was more frequently found in the EBV-positive group, the degree of lymphoid reaction was not associated with LNM in the case of SM EGCs.
In terms of tumor location, the tendency toward a proximal location is also a well-known feature of EBV-associated GC. In our series, 84.4 % of EBV-positive cases (n = 54) were located in the upper or middle third, and half (n = 27) were in the upper third. Because of the high proportion of proximally located EBV-positive tumors, a routine EBER ISH on ESD specimens of proximally located EGC may save more patients from having a total gastrectomy procedure, which has a more serious effect on quality of life than a subtotal or distal gastrectomy.
In many studies, the presence of LVI has been proven to be the most important and reliable risk factor for LNM [44–46]. In our previous study using a series (n = 79) of endoscopically resected EGCs sequentially treated by gastrectomy and lymph node dissection, the odds ratio of LVI for LNM was 21.41 (95 % confidence interval 2.11–217.28, p = 0.010) [46]. In our current study, the presence of LVI was also found to be the most important risk factor (odd ratio 7.45). Among the 64 EBV-positive cases, only five cases (7.8 %) were observed to have LVI. Of the five cases, two cases showed LNM—that is, 40 % of EBV-positive cases with LVI had LNM (2/5). Thus, even in the case of EBV-positive EGC, if LVI is found in the ESD specimen, a further surgical intervention to dissect lymph nodes is inevitable.
At the beginning of this study, we expected that the MSI-H phenotype would be a favorable predictive factor for LNM, as well as EBV positivity, because several studies have reported an association between the MSI-H phenotype and a low prevalence of LNM in GC [23, 24, 26, 27]. Good concordance between the IHC method and the PCR-based assay has been reported to identify MSI-H type [22, 25, 29]; therefore, we evaluated the expression of MMR proteins as a marker of MSI-H phenotype using IHC. To confirm the reliability of the IHC method, we compared the results obtained by IHC and a PCR-based assay in a subgroup (n = 144) in which the PCR-based MSI test had already been performed. The high concordance rate between the IHC method and the PCR-based assay (k = 0.838) and the satisfactory sensitivity (90 %) and specificity (100 %) of the IHC method confirmed that IHC was a competent method for detection of the MSI-H phenotype in GC. According to the results obtained by IHC, the MMR-deficient EGCs and EBV-positive EGCs were mutually exclusive, except in one case. However, MMR deficiency was not correlated with LNM status. LVI, which is the strongest risk factor for LNM, was more frequently observed in the MMR-deficient group. However, differentiated histologic type and intestinal type, which are generally believed to be favorable factors for LNM, and EBV negativity, which was the second most important risk factor in this study, were more frequent in the MMR-competent (microsatellite stable) group. Thus, the lack of a relationship between MMR deficiency and LNM may be a cumulative result of those contradictory effects. In addition, we found no relationship between MSI-H type and LNM in the PCR-based test subgroup.
In conclusion, in this study, EBV positivity was a favorable factor for LNM in SM EGC; however; MMR-deficiency was not associated with LNM status. Therefore, EBV positivity might be considered as an additive criterion for endoscopic resected specimens especially in cases of SM EGC without LVI and tumor involvement of resection margins.
References
Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228–35.
Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23:4490–8.
Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–25.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113–23.
Burke AP, Yen TS, Shekitka KM, Sobin LH. Lymphoepithelial carcinoma of the stomach with Epstein–Barr virus demonstrated by polymerase chain reaction. Mod Pathol. 1990;3:377–80.
Thompson MP, Kurzrock R. Epstein–Barr virus and cancer. Clin Cancer Res. 2004;10:803–21.
Watanabe H, Enjoji M, Imai T. Gastric carcinoma with lymphoid stroma. Its morphologic characteristics and prognostic correlations. Cancer. 1976;38:232–43.
Tokunaga M, Land CE, Uemura Y, Tokudome T, Tanaka S, Sato E. Epstein–Barr virus in gastric carcinoma. Am J Pathol. 1993;143:1250–4.
Tokunaga M, Uemura Y, Tokudome T, Ishidate T, Masuda H, Okazaki E, et al. Epstein–Barr virus related gastric cancer in Japan: a molecular patho-epidemiological study. Acta Pathol Jpn. 1993;43:574–81.
Lee JH, Kim SH, Han SH, An JS, Lee ES, Kim YS. Clinicopathological and molecular characteristics of Epstein–Barr virus-associated gastric carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2009;24:354–65.
Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein–Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009;137:824–33.
Kijima Y, Ishigami S, Hokita S, Koriyama C, Akiba S, Eizuru Y, et al. The comparison of the prognosis between Epstein–Barr virus (EBV)-positive gastric carcinomas and EBV-negative ones. Cancer Lett. 2003;200:33–40.
Koriyama C, Akiba S, Corvalan A, Carrascal E, Itoh T, Herrera-Goepfert R, et al. Histology-specific gender, age and tumor-location distributions of Epstein–Barr virus-associated gastric carcinoma in Japan. Oncol Rep. 2004;12:543–7.
Lee HS, Chang MS, Yang HK, Lee BL, Kim WH. Epstein–Barr virus-positive gastric carcinoma has a distinct protein expression profile in comparison with Epstein–Barr virus-negative carcinoma. Clin Cancer Res. 2004;10:1698–705.
van Beek J, zur Hausen A, Klein Kranenbarg E, van de Velde CJ, Middeldorp JM, van den Brule AJ, et al. EBV-positive gastric adenocarcinomas: a distinct clinicopathologic entity with a low frequency of lymph node involvement. J Clin Oncol. 2004;22(4):664–70.
Tokunaga M, Land CE. Epstein–Barr virus involvement in gastric cancer: biomarker for lymph node metastasis. Cancer Epidemiol Biomarkers Prev. 1998;7:449–50.
van Beek J, zur Hausen A, Kranenbarg EK, van de Velde CJ, Middeldorp JM, van den Brule AJ, et al. EBV-positive gastric adenocarcinomas: a distinct clinicopathologic entity with a low frequency of lymph node involvement. J Clin Oncol. 2004;22:664–70.
Uemura Y, Tokunaga M, Arikawa J, Yamamoto N, Hamasaki Y, Tanaka S, et al. A unique morphology of Epstein–Barr virus-related early gastric carcinoma. Cancer Epidemiol Biomarkers Prev. 1994;3:607–11.
Duval A, Hamelin R. Genetic instability in human mismatch repair deficient cancers. Ann Genet. 2002;45:71–5.
Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–57.
dos Santos NR, Seruca R, Constancia M, Seixas M, Sobrinho-Simoes M. Microsatellite instability at multiple loci in gastric carcinoma: clinicopathologic implications and prognosis. Gastroenterology. 1996;110:38–44.
Seo HM, Chang YS, Joo SH, Kim YW, Park YK, Hong SW, et al. Clinicopathologic characteristics and outcomes of gastric cancers with the MSI-H phenotype. J Surg Oncol. 2009;99:143–7.
Kim H, An JY, Noh SH, Shin SK, Lee YC, Kim H. High microsatellite instability predicts good prognosis in intestinal-type gastric cancers. J Gastroenterol Hepatol. 2011;26:585–92.
Lee HS, Choi SI, Lee HK, Kim HS, Yang HK, Kang GH, et al. Distinct clinical features and outcomes of gastric cancers with microsatellite instability. Mod Pathol. 2002;15:632–40.
Laghi L, Bianchi P, Malesci A. Differences and evolution of the methods for the assessment of microsatellite instability. Oncogene. 2008;27:6313–21.
Beghelli S, de Manzoni G, Barbi S, Tomezzoli A, Roviello F, Di Gregorio C, et al. Microsatellite instability in gastric cancer is associated with better prognosis in only stage II cancers. Surgery. 2006;139:347–56.
Falchetti M, Saieva C, Lupi R, Masala G, Rizzolo P, Zanna I, et al. Gastric cancer with high-level microsatellite instability: target gene mutations, clinicopathologic features, and long-term survival. Hum Pathol. 2008;39:925–32.
Chaves P, Cruz C, Lage P, Claro I, Cravo M, Leitao CN, et al. Immunohistochemical detection of mismatch repair gene proteins as a useful tool for the identification of colorectal carcinoma with the mutator phenotype. J Pathol. 2000;191:355–60.
Lindor NM, Burgart LJ, Leontovich O, Goldberg RM, Cunningham JM, Sargent DJ, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol. 2002;20:1043–8.
Stephen BE, David RB, Carolyn CC, April GF, Frederick LG, Andy T III. American Joint Committee on Cancer cancer staging manual; 2010. p. 117–26.
Lauwers GY, Carneiro F, Graham DY, Curado MP, Franceschi S, Montgomery E, et al. Gastric carcinoma. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the digestive system. Lyon: IARC Press; 2010. p. 48–58.
Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12.
Yamao T, Shirao K, Ono H, Kondo H, Saito D, Yamaguchi H, et al. Risk factors for lymph node metastasis from intramucosal gastric carcinoma. Cancer. 1996;77:602–6.
Seto Y, Shimoyama S, Kitayama J, Mafune K, Kaminishi M, Aikou T, et al. Lymph node metastasis and preoperative diagnosis of depth of invasion in early gastric cancer. Gastric Cancer. 2001;4:34–8.
An JY, Baik YH, Choi MG, Noh JH, Sohn TS, Kim S. Predictive factors for lymph node metastasis in early gastric cancer with submucosal invasion: analysis of a single institutional experience. Ann Surg. 2007;246:749–53.
Holscher AH, Drebber U, Monig SP, Schulte C, Vallbohmer D, Bollschweiler E. Early gastric cancer: lymph node metastasis starts with deep mucosal infiltration. Ann Surg. 2009;250:791–7.
Nam MJ, Oh SJ, Oh CA, Kim DH, Bae YS, Choi MG, et al. Frequency and predictive factors of lymph node metastasis in mucosal cancer. J Gastric Cancer. 2010;10:162–7.
Lee JY, Kim KM, Min BH, Lee JH, Rhee PL, Kim JJ. Epstein–Barr virus-associated lymphoepithelioma-like early gastric carcinomas and endoscopic submucosal dissection: case series. World J Gastroenterol. 2014;20:1365–70.
Takano Y, Kato Y, Sugano H. Epstein–Barr virus-associated medullary carcinomas with lymphoid infiltration of the stomach. J Cancer Res Clin Oncol. 1994;120:303–8.
Matsunou H, Konishi F, Hori H, Ikeda T, Sasaki K, Hirose Y, et al. Characteristics of Epstein–Barr virus-associated gastric carcinoma with lymphoid stroma in Japan. Cancer. 1996;77:1998–2004.
Saiki Y, Ohtani H, Naito Y, Miyazawa M, Nagura H. Immunophenotypic characterization of Epstein–Barr virus-associated gastric carcinoma: massive infiltration by proliferating CD8+ T-lymphocytes. Lab Invest. 1996;75:67–76.
Song HJ, Srivastava A, Lee J, Kim YS, Kim KM, Ki Kang W, et al. Host inflammatory response predicts survival of patients with Epstein–Barr virus-associated gastric carcinoma. Gastroenterology. 2010;139(84–92):e2.
Kwee RM, Kwee TC. Predicting lymph node status in early gastric cancer. Gastric Cancer. 2008;11:134–48.
Kang HJ, Kim DH, Jeon TY, Lee SH, Shin N, Chae SH, et al. Lymph node metastasis from intestinal-type early gastric cancer: experience in a single institution and reassessment of the extended criteria for endoscopic submucosal dissection. Gastrointest Endosc. 2010;72:508–15.
Kim H, Kim JH, Park JC, Lee YC, Noh SH, Kim H. Lymphovascular invasion is an important predictor of lymph node metastasis in endoscopically resected early gastric cancers. Oncol Rep. 2011;25:1589–95.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning (2012R1A1A1004403) and by a faculty research grant from Yonsei University College of Medicine for 2012 (6-2012-0044).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (the Institutional Review Board of Yonsei University College of Medicine, approval number 4-2014-0665) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for their being included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
J. H. Park and E. K. Kim contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Park, J.H., Kim, E.K., Kim, Y.H. et al. Epstein–Barr virus positivity, not mismatch repair-deficiency, is a favorable risk factor for lymph node metastasis in submucosa-invasive early gastric cancer. Gastric Cancer 19, 1041–1051 (2016). https://doi.org/10.1007/s10120-015-0565-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-015-0565-1