Abstract
To establish an animal model for the newly identified Marmota Himalayana hepatovirus, MHHAV, so as to develop a better understanding of the infection of hepatitis A viruses. Five experimental woodchucks (Marmota monax) were inoculated intravenously with the purified MHHAV from wild woodchuck feces. One animal injected with PBS was defined as a control. Feces and blood were routinely collected. After the animals were subjected to necropsy, different tissues were collected. The presence of viral RNA and negative sense viral RNA was analyzed in all the samples and histopathological and in situ hybridization analysis was performed for the tissues. MHHAV infection caused fever but no severe symptoms or death. Virus was shed in feces beginning at 2 dpi, and MHHAV RNA persisted in feces for ~2 months, with a biphasic increase, and in blood for ~30 days. Viral RNA was detected in all the tissues, with high levels in the liver and spleen. Negative-strand viral RNA was detected only in the liver. Furthermore, the animals showed histological signs of hepatitis at 45 dpi. MHHAV can infect M. monax and is associated with hepatic disease. Therefore, this animal can be used as a model of HAV pathogenesis and to evaluate antiviral and anticancer therapeutics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatitis A virus (HAV) is a nonenveloped, positive-sense, single-stranded RNA virus taxonomically classifiable within the genus Hepatovirus, family Picornaviridae. HAV causes acute infectious viral hepatitis worldwide and is related to sudden, explosive outbreaks of hepatitis A [20]. HAV is the only member of the genus Hepatovirus and only one HAV serotype is known [27]. However, viruses similar to HAV have been discovered in animals and small mammals [2, 8, 31], including MHHAV which our group discovered.
Under natural conditions, HAV infects only humans and certain nonhuman primate species [4], and it has been found that quasi-enveloped viral particles exist in the blood of humans and chimpanzees [10]. Understanding of the pathogenesis of HAV infection has been enhanced by studies in nonhuman primates, such as chimpanzees (Pan troglodytes), owl monkeys (Aotus trivirgatus), and marmosets (Saguinus mystax) [3, 7, 22]. HAV variants can replicate in the liver of guinea pigs, but the clinical and laboratory parameters remain normal [14]. Recently, a small animal model has been reported using HAV to infect mice with mutations in key innate immune pathways [13].
The mechanisms underlying the variability in clinical outcomes of HAV infection are unclear. The tropism of HAV for the liver also remains unresolved. A natural animal model would thus enhance our understanding of the pathogenesis of HAV infection in humans and facilitate development of improved therapies. The discovery of MHHAV in wild woodchuck will thus provide useful information on the virology and immunology of HAV infection. In this study, five seronegative, colony-bred M. monax were inoculated intravenously with MHHAV from wild woodchuck feces. The hematological and biochemical parameters and histopathological changes in the infected animals were assessed, and the presence of MHHAV in blood, feces, and tissues was assessed. Natural infection of M. monax with MHHAV caused liver disease similar to that induced by HAV infection in humans. Therefore, the woodchuck represents a useful animal model of HAV infection.
Materials and methods
Animals
Six healthy male woodchucks (M. monax) (age, 1–3 years; weight, 1.8–2.4 kg) were purchased from the Institute of Laboratory Animal Sciences, Chinese Academy of Medical Science and Peking Union Medical College. All woodchucks were confirmed to not have been previously infected with MHHAV or HAV. Of the six woodchucks, five (WC1–5) were subjected to MHHAV infection, and 1 (WC1) was a control animal.
Animal infection and sample collection
MHHAV was purified from stools of wild woodchucks. Stool samples were diluted to 20% suspensions in phosphate-buffered saline (PBS). Beads and chloroform were added to the suspensions, followed by centrifugation for 20 min at 1500 × g. Supernatants were subjected to a single ultracentrifugation step through discontinuous sucrose/glycerol density gradients as described previously for HAV purification [5].
The woodchucks were anesthetized with ketamine hydrochloride (25 mg/kg) and then received an intravenous injection of 1 × 107 copies/mL MHHAV in 200 µL of PBS. An identical volume of PBS was injected into the control animal. The animals were monitored daily for abnormal appearance, behavior, and body temperature. Feces were collected every other day beginning at 2 dpi. On days 7, 15, 30, 45, 60, and 90 dpi, the live animals were anesthetized for collection of blood samples. At 15, 30, 45, 45, and 90 dpi, WC1–5 were subjected to necropsy after being anesthetized and exsanguinated, and the control animal was subjected to necropsy at 45 dpi. Blood, liver, spleen, lung, kidney, and heart specimens were collected. The control animal and the live infected animals were anesthetized for weight measurement and blood collection at 0, 7, 15, 30, 45, 60, and 90 dpi.
Quantitative polymerase chain reaction
MHHAV RNA was quantified by real-time PCR. A forward primer (MHHAVF: 5′-GTCCTCTTTAAGGCACTCAT-3′), a reverse primer (MHHAVR: 5′-TGGGTCAGTCCATCTGGCAAG-3′), and a probe [MHHAVProbe: 5′-fluorescein amidite (FAM)-CATCTTCATTTCCCTGGCTCTCACC-minor groove binder (MGB)-3′] were designed against the 5′-untranslated region (UTR) sequence of the virus. The reaction conditions were as follows: 50 °C for 30 min, 95 °C for 10 min, and 40 cycles of 95 °C for 15 s and 58 °C for 30 s.
Negative-strand MHHAV RNA testing
To evaluate MHHAV replication in tissue, a tagged primer system was used to detect virus negative-strand RNA. Negative-strand cDNA was generated using a primer (5′-CCTCCGCTGCCATCTGATTGCGTCCTCTTTAAGGCACTCAT-3’) specific for the 5′-UTR of MHHAV at 65 °C for 5 min, 25 °C for 10 min, 50 °C for 50 min and 72 °C for 15 min. Quantitative PCR was performed to quantify negative-strand RNA using a forward primer (TagF: 5′-CCTCCCGATCATCTGGTTGC-3′), reverse primer (UTRR: 5′-TGGGTCAGTCCATCTGGCAAG-3′) and probe (NProbe: 5′-FAM-CATCTTCATTTCCCTGGCTCTCACC-MGB-3′). The reaction conditions were as follows: 50 °C for 2 min, 95 °C for 10 min, and 40 cycles of 95 °C for 15 s and 58 °C for 30 s.
In situ hybridization
Three FAM-labeled probes were designed based on the MHHAV 5’-UTR sequence (probe 1, TATTAATTCCAGCAGGTTCTGGTTTCTGAATTTGTCTCTTTAAGGCACTC; probe 2, GATCTTTGGTATGGGTAGGCTACGGGTGAAACCCCATAGGTTAATACTA; and probe 3, TGGCCTTGCTTCTGTTGAACAGACACTGGGGCTTATGGTATTCACCCTG). Liver sections were fixed with 4% formaldehyde and acetic anhydride, and dehydrated, permeabilized, and stained with Fast Red substrate (QG View RNA Chromogenic Signal Amplification Kit, Panomics/Affymetrix) for light and fluorescence microscopy.
Histopathological analysis and immuno-electron microscopy
Tissues were fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). Four hundred and fifty microliters of chloroform-purified MHHAV (from day-30 feces) were incubated with 50 µL of WC3 woodchuck serum (1:10 dilution) at 37 °C for 1 h, centrifuged at 23,000 rpm for 1 h, and the pellet was resuspended in 50 µL of PBS. The resulting viral suspension was subjected to negative staining. Grids were visualized by transmission electron microscopy (TECNAI 12, FEI, Blackwood, NJ).
Neutralization test
Serial dilutions of serum samples from WC1–6 were mixed with 500 µL of 100 CCID 50/mL HAV and incubated at 37 °C for 1.5 h. The mixture was then added in quadruplicate to a six-well plate containing 2BS cells. The negative control was dilution medium and the positive control was a monoclonal antibody to HAV. The plates were incubated at 33 °C in a 5% CO2 atmosphere for 21 d. Viral infection in individual wells of the plates was detected using a specific enzyme-linked immunosorbent assay (ELISA) in serum samples from patients with laboratory-confirmed infection. The end-point titer was expressed as the reciprocal of the highest dilution of serum that prevented infection.
Serum aminotransferase levels
Serum ALT and AST levels were assessed using an Alanine Aminotransferase (ALT or SGPT) Activity Colorimetric/Fluorometric Assay Kit and an Aspartate Aminotransferase (AST or SGOT) Activity Colorimetric Assay Kit (BioVision, USA), respectively. Baseline ALT and AST levels were obtained from pre-inoculation and control blood samples.
Results
Experimental infection of M. monax with MHHAV
At 15, 30, 45, 60 and 90 days post-inoculation (dpi) with MHHAV intravenously, five woodchucks (WC1–5) were subjected to necropsy after exsanguination, while the control animal (WC6) was subjected to necropsy at 45 dpi. Daily observations indicated no abnormal appearance or behavior in the infected woodchucks, such as weariness, anorexia, ruffled hair, or diarrhea. Also, there was no obvious weight change in the animals during the infection. However, all five of the infected animals developed mild fever; the highest temperature was 35 °C in WC3 at 24 dpi (Fig. 1), while the temperature of the uninfected animal was very stable, with temperatures between 30.2 and 30.9 °C.
All of the infected woodchucks showed similar viral replication patterns. Virus shedding in feces began at 3 dpi and exhibited a biphasic increase, peaking at 106 to 109 RNA copies/g at 8–9 dpi in all animals, with the exception of WC4, followed by a slow decrease at 23 dpi and a transient rebound at 23 dpi. Virus shedding in WC4 peaked (106 copies/g) at 7 dpi, with a transient rebound at 16 dpi to a level 10-fold that of the first peak (Fig. 2A). In the early stage of infection, peak viremia ranged from 104 to 106 RNA copies/mL at 7 dpi in all woodchucks, with the exception of WC4. WC4 became viremic at 7 dpi, reached peak viremia at 15 dpi, and exhibited a higher viral load than the other animals (Fig. 2B). MHHAV was cleared from the blood beginning at ~45 dpi; in contrast, MHHAV RNA persisted in feces until 67 dpi in WC5. Peak MHHAV Shedding in feces occurred simultaneously with peak viremia.
Persistence of MHHAV RNA in woodchucks. (A) MHHAV RNA in feces. Virus shedding in feces began at 3 dpi and exhibited a biphasic increase. (B) MHHAV RNA levels in blood. Peak viremia ranged from 104 to 106 RNA copies/mL by 7 dpi in all woodchucks, with the exception of WC4. WC4 became viremic at 7 dpi, reached peak viremia at 15 dpi, and exhibited a higher viral load than the other animals
Immuno-electron microscopy showed that the virus particles in feces were identical to those in wild woodchucks; both virus particles and empty capsids were present (Supplemental Fig. 1).
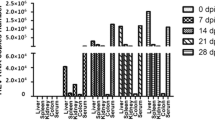
Distribution and replication of MHHAV
To assess the distribution of MHHAV, we measured viral copy numbers in the liver, spleen, lung, kidney, heart, salivary gland, duodenum, and small intestine by real-time PCR. The duodenum and small intestine of WC1 were not collected. MHHAV RNA was detected in all tissues of WC1–5 (Fig. 3).
To assess MHHAV replication, we used real-time RT-PCR to quantify MHHAV negative-strand RNA in the blood, liver, spleen, kidney, lung, heart, salivary gland, duodenum, and small intestine of the woodchucks. High levels of MHHAV negative-strand RNA were detected only in the liver (Fig. 4). The blood, spleen, kidney, lung, heart, salivary gland, duodenum, and small intestine, which were positive for viral RNA, were negative for viral negative-strand RNA. MHHAV was detected in the liver of the infected animals by in situ hybridization (Supplemental Fig. 2).
Histopathological changes associated with MHHAV infection
To assess the histopathology of MHHAV infection, we examined the livers of infected woodchucks after necropsy. In the livers of WC-3 and WC-4, which were euthanized at 45 dpi and 60 dpi, typical granular degeneration of hepatic cells was observed, with hepatocyte swelling and rounding (Fig. 5C). In the spleen of WC2, which was euthanized at 30 dpi, splenic white pulp atrophy and decreased numbers of lymphocytes were observed (Fig.5E). However, serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels remained unchanged.
Histopathological analysis of the liver and spleen. (A) the normal control animal liver, (B) WC-3 liver, (C) WC-4 liver, (D) the normal control animal spleen and (E) WC2 spleen. Typical granular degeneration of hepatic cells was observed in WC-3 and WC-4 livers, while splenic white pulp atrophy and decreased numbers of lymphocytes were observed in the WC2 spleen
Neutralizing activity assay
Sequence analysis suggests that MHHAV may be a new serotype of HAV [31]. In order to confirm whether there is any antibody cross protection between the MHHAV and HAV, the serum from the six woodchucks in this study, that contained MHHAV antibody, was used to neutralize HAV cultured in 2BS cells. The HAV neutralizing antibody titers of WC1–WC6 were 4, 12, < 4, 4, 24, and 8, respectively, while that of the positive control was 1,024. Therefore, there was no cross-reactivity between antibodies for MHHAV and those for HAV. Since growth of MHHAV in cell culture was unsuccessful in this study, the ability of anti-HAV antibodies to neutralize MHHAV was not assessed.
Discussion
Since HAV targets the liver and results in considerable morbidity and mortality despite the availability of an effective vaccine [6], research on MHHAV infection in M. monax is of great importance. MHHAV RNA was detected in the feces, blood and different tissues, but its negative-strand RNA was detected only in the liver, which suggests MHHAV can infect M. monax and is distributed in various organs, but replicates to a detectable level only in the liver, similarly to HAV infection. Viral particles were shed in the feces of the infected animals, and were spherical, non-enveloped, and of an average diameter of ~30 nm, which is similar to other members of the Picornaviridae.
The incubation period of HAV infection is ~28 days (range, 15–50 days) [12]. Typical symptoms include gastrointestinal disturbance, weakness, anorexia, vomiting, and fever, which can develop into clinical hepatitis with jaundice and elevated serum liver enzyme levels. The woodchucks infected with MHHAV in this study developed only fever. MHHAV virions and viral RNA were detected in feces as early as 3 dpi. Viral RNA persisted in feces longer than in blood. In WC5, MHHAV RNA was cleared from blood beginning at 14 dpi and was no longer detectable at 45 dpi. In children, virus is shed in feces during the incubation period and can be detected until day 90 after symptom onset, while the duration of viremia is shorter than that of virus shedding in feces [23]. In owl monkeys, HAV viremia develops at 7–11 dpi, in parallel with fecal shedding, and persists for a mean of 20.5 days; although virus was not recovered from the liver at 96 dpi [18]. In this study, the duration of virus shedding was identical to that of HAV in human and owl monkeys; however, because the last experimental animal was euthanized at 90 dpi, when MHHAV RNA remained detectable in the liver and spleen, the time to viral clearance is unclear.
Liver is the primary site of HAV replication. HAV antigens have been detected in hepatocytes of infected animals within hours after infection [16, 21, 24]; HAV is released into the bile and passes into the intestine [25]. HAVcr1, the putative cellular receptor for HAV [9], is distributed in various tissues, but HAV replicates only in the liver. The presence of the replication intermediate is a marker of viral replication [1, 15]. In our study, although viral RNA was detected in various tissues, high levels of MHHAV negative-strand RNA were detected only in the liver, suggesting that the distribution pattern and replication of MHHAV are similar to those of HAV.
Interestingly, when animal WC5 was sacrificed at 90 dpi, no viral RNA was detected in feces or blood, while it was detected in other tissues, and MHHAV negative-strand RNA was detected in the liver, albeit at a low level. Although HAV infection is usually acute, Lanford et al. reported that HAV RNA persists in the liver for several months, remaining present long after clearance from serum and feces [17]. Our results suggest that MHHAV also persists in infected animals. In addition, Glikson reported that up to 20% of patients with HAV infection relapse after normalization of liver transaminase levels and control of virus replication [11]. Whether MHHAV infection has similar characteristics should be investigated.
HAV infection usually causes mild liver damage in humans and nonhuman primates. In this study, liver biopsies showed histological changes characteristic of viral hepatitis, including ballooning degeneration of hepatocytes or swollen hepatocytes with granular cytoplasm, which are typical lesions of HAV infection [3, 24, 26]. Also, we found white pulp atrophy and decreased numbers of lymphocytes in the spleen. The liver tissues of WC1, WC2 and WC5, which were euthanized at 15, 30 and 90 dpi, were normal, as was that of the control animal. These results suggest that the pathological lesions might develop in the middle phase of MHHAV infection and resolve in the late phase.
Elevated serum AST and ALT levels suggest hepatocyte death, possibly due to killing of infected or bystander liver cells by the host immune response [19]. However, HCV kinetics in experimentally infected chimpanzees, as reported by Thimme et al., did not show a consistent pattern, since some of the animals did not exhibit an elevated serum ALT level [29]. No significant increase in serum ALT and AST levels was observed in the infected woodchucks, possibly because of the mild and/or transient hepatic injury. Alternatively, a mechanism other than destruction of infected cells might have been responsible for viral clearance [29].
The woodchuck is used as a model for the study of the virological, pathological, and immunological features of woodchuck hepatitis virus (WHV) and HBV [28, 30]. Although nonhuman primates are good animal models for investigation of HAV, they have disadvantages; e.g., their considerable size and difficulty of manipulation. We found that the woodchuck is susceptible to MHHAV, suggesting that MHHAV could be used as a surrogate for studies of HAV. In this study, MHHAV was administered intravenously and M. monax was used; therefore, future work should address natural MHHAV infection of wild woodchuck (M. himalayana) by the oral route.
References
Amado LA, Marchevsky RS, de Paula VS, Hooper C, Freire Mda S, Gaspar AM, Pinto MA (2010) Experimental hepatitis A virus (HAV) infection in cynomolgus monkeys (Macaca fascicularis): evidence of active extrahepatic site of HAV replication. Int J Exp Pathol 91:87–97
Anthony SJ, St Leger JA, Liang E, Hicks AL, Sanchez-Leon MD, Jain K, Lefkowitch JH, Navarrete-Macias I, Knowles N, Goldstein T, Pugliares K, Ip HS, Rowles T, Lipkin WI (2015) Discovery of a novel hepatovirus (Phopivirus of Seals) related to human hepatitis A virus. MBio 6:e01180
Asher LV, Binn LN, Mensing TL, Marchwicki RH, Vassell RA, Young GD (1995) Pathogenesis of hepatitis A in orally inoculated owl monkeys (Aotus trivirgatus). J Med Virol 47:260–268
Balayan MS (1992) Natural hosts of hepatitis A virus. Vaccine 10(Suppl 1):S27–S31
Bishop NE, Hugo DL, Borovec SV, Anderson DA (1994) Rapid and efficient purification of hepatitis A virus from cell culture. J Virol Methods 47:203–216
Deinhardt F (1992) Prevention of viral hepatitis A: past, present and future. Vaccine 10(Suppl 1):S10–S14
Dienstag JL, Feinstone SM, Purcell RH, Hoofnagle JH, Barker LF, London WT, Popper H, Peterson JM, Kapikian AZ (1975) Experimental infection of chimpanzees with hepatitis A virus. J Infect Dis 132:532–545
Drexler JF, Corman VM, Lukashev AN, van den Brand JM, Gmyl AP, Brunink S, Rasche A, Seggewibeta N, Feng H, Leijten LM, Vallo P, Kuiken T, Dotzauer A, Ulrich RG, Lemon SM, Drosten C (2015) Evolutionary origins of hepatitis A virus in small mammals. Proc Natl Acad Sci USA 112:15190–15195
Feigelstock D, Thompson P, Mattoo P, Zhang Y, Kaplan GG (1998) The human homolog of HAVcr-1 codes for a hepatitis A virus cellular receptor. J Virol 72:6621–6628
Feng Z, Hensley L, McKnight KL, Hu F, Madden V, Ping L, Jeong SH, Walker C, Lanford RE, Lemon SM (2013) A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature 496:367–371
Glikson MGE, Oren R, Tur-Kaspa R, Shouval D (1992) Relapsing hepatitis A. Review of 14 cases and literature survey. Medicine (Baltimore) 71:14–23
Gluud LL, Gluud C (2009) Meta-analyses on viral hepatitis. Infect Dis Clin N Am 23:315–330
Hirai-Yuki A, Hensley L, McGivern DR, Gonzalez-Lopez O, Das A, Feng H, Sun L, Wilson JE, Hu F, Feng Z, Lovell W, Misumi I, Ting JP, Montgomery S, Cullen J, Whitmire JK, Lemon SM (2016) MAVS-dependent host species range and pathogenicity of human hepatitis A virus. Science 353:1541–1545
Hornei B, Kammerer R, Moubayed P, Frings W, Gauss-Muller V, Dotzauer A (2001) Experimental hepatitis A virus infection in guinea pigs. J Med Virol 64:402–409
Jiang YJ, Liao GY, Zhao W, Sun MB, Qian Y, Bian CX, Jiang SD (2004) Detection of infectious hepatitis A virus by integrated cell culture/strand-specific reverse transcriptase-polymerase chain reaction. J Appl Microbiol 97:1105–1112
Krawczynski KK, Bradley DW, Murphy BL, Ebert JW, Anderson TE, Doto IL, Nowoslawski A, Duermeyer W, Maynard JE (1981) Pathogenetic aspects of hepatitis A virus infection in enterally inoculated marmosets. Am J Clin Pathol 76:698–706
Lanford RE, Feng Z, Chavez D, Guerra B, Brasky KM, Zhou Y, Yamane D, Perelson AS, Walker CM, Lemon SM (2011) Acute hepatitis A virus infection is associated with a limited type I interferon response and persistence of intrahepatic viral RNA. Proc Natl Acad Sci USA 108:11223–11228
Lemon SM, Binn LN, Marchwicki R, Murphy PC, Ping LH, Jansen RW, Asher LV, Stapleton JT, Taylor DG, LeDuc JW (1990) In vivo replication and reversion to wild type of a neutralization-resistant antigenic variant of hepatitis A virus. J Infect Dis 161:7–13
Major MEDH, Mihalik K, Puig M, Rice CM, Neumann AU, Feinstone SM (2004) Hepatitis C virus kinetics and host responses associated with disease and outcome of infection in chimpanzees. Hepatology 39:1709–1720
Martin A, Lemon SM (2006) Hepatitis A virus: from discovery to vaccines. Hepatology 43:S164–S172
Mathiesen LR, Drucker J, Lorenz D, Wagner JA, Gerety RJ, Purcell RH (1978) Localization of hepatitis A antigen in marmoset organs during acute infection with hepatitis A virus. J Infect Dis 138:369–377
Mathiesen LR, Moller AM, Purcell RH, London WT, Feinstone SM (1980) Hepatitis A virus in the liver and intestine of marmosets after oral inoculation. Infect Immun 28:45–48
Munne MS, Canero Velasco MC, Moreiro R, Vladimirsky S, Otegui L, Castro R, Brajterman L, Soto S, Mutti J, Nucifora S, Lara E, Sosa A, Godoy P, Ciocca M, Cuarterolo M, Quarleri JF, Gonzalez JE (2006) Duration of viremia and fecal shedding of the virus in hepatitis A infected children. Acta Gastroenterol Latinoam 36:182–189
Pinto MA, Marchevsky RS, Baptista ML, de Lima MA, Pelajo-Machado M, Vitral CL, Kubelka CF, Pissurno JW, Franca MS, Schatzmayr HG, Gaspar AM (2002) Experimental hepatitis A virus (HAV) infection in Callithrix jacchus: early detection of HAV antigen and viral fate. Exp Toxicol Pathol 53:413–420
Schulman AN, Dienstag JL, Jackson DR, Hoofnagle JH, Gerety RJ, Purcell RH, Barker LF (1976) Hepatitis A antigen particles in liver, bile, and stool of chimpanzees. J Infect Dis 134:80–84
Shevtsova ZV, Lapin BA, Doroshenko NV, Krilova RI, Korzaja LI, Lomovskaya IB, Dzhelieva ZN, Zairov GK, Stakhanova VM, Belova EG et al (1988) Spontaneous and experimental hepatitis A in Old World monkeys. J Med Primatol 17:177–194
Stapleton JT, Jansen R, Lemon SM (1985) Neutralizing antibody to hepatitis A virus in immune serum globulin and in the sera of human recipients of immune serum globulin. Gastroenterology 89:637–642
Summers J, Smolec JM, Snyder R (1978) A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc Natl Acad Sci USA 75:4533–4537
Thimme RBJ, Spangenberg HC, Wieland S, Pemberton J, Steiger C, Govindarajan S, Purcell RH, Chisari FV (2002) Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci 99:15661–15668
Wong DC, Shih JW, Purcell RH, Gerin JL, London WT (1982) Natural and experimental infection of woodchucks with woodchuck hepatitis virus, as measured by new, specific assays for woodchuck surface antigen and antibody. J Clin Microbiol 15:484–490
Yu JM, Li LL, Zhang CY, Lu S, Ao YY, Gao HC, Xie ZP, Xie GC, Sun XM, Pang LL, Xu JG, Lipkin WI, Duan ZJ (2016) A novel hepatovirus identified in wild woodchuck Marmota himalayana. Sci Rep 6:22361
Funding
The study was supported by the National Natural Science Foundation of China (Grant No. 81702007 and 81290345).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Six healthy male woodchucks (M. monax) (age, 1–3 years; weight, 1.8–2.4 kg) were purchased from the Institute of Laboratory Animal Sciences, Chinese Academy of Medical Science and Peking Union Medical College.
All experiments involving MHHAV-infected animals were performed in biosafety level 2 containment at the Experimental Animal Center of the China CDC, in accordance with institutional guidelines. The animal study protocol was approved by the China CDC Animal Welfare Committee.
Additional information
Handling Editor: Michael Carpenter.
Electronic supplementary material
Below is the link to the electronic supplementary material.
705_2018_3715_MOESM1_ESM.tif
Supplemental Fig. 1. Electron micrographs of immune-complexed MHHAV. The virus particles shed in feces of the infected animals were identical to those shed by wild woodchucks, and both virus particles and empty capsids were present in feces (TIFF 825 kb)
705_2018_3715_MOESM2_ESM.tif
Supplemental Fig. 2. In situ hybridization of MHHAV RNA in woodchuck liver. (A) Liver of the control animal. (B) Liver of an MHHAV-infected animal. Left, middle, and right; fluorescence, bright-field, and superimposed images, respectively. Bright green dots indicate probe bound to MHHAV genomic RNA (TIFF 678 kb)
Rights and permissions
About this article
Cite this article
Yu, Jm., Li, Ll., Xie, Gc. et al. Experimental infection of Marmota monax with a novel hepatitis A virus. Arch Virol 163, 1187–1193 (2018). https://doi.org/10.1007/s00705-018-3715-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-018-3715-z