Abstract
Virotherapy is emerging as an alternative treatment of cancer. Among the candidate oncolytic viruses (OVs), Newcastle disease virus (NDV) has emerged as a promising non-engineered OV. In the present communication, we explored the oncolytic potential of R2B Mukteshwar strain of NDV using SW-620 colon cancer cells. SW-620 cells were xenografted in nude mice and after evaluation of the safety profile, 1 x 107 plaque forming units (PFU) of NDV were inoculated as virotherapeutic agent via the intratumoral (I/T) and intravenous (I/V) route. Tumor growth inhibition was compared with their respective control groups by gross volume and histopathological evaluation. Antibody titer and virus survival were measured by hemagglutination inhibition (HI)/serum neutralization test (SNT) and real-time PCR, respectively. During the safety trial, the test strain did not produce any abnormal symptoms nor weight loss in BALB/c mice. Significant tumor lytic activity was evident when viruses were injected via the I/T route. There was a 43 and 57% tumor growth inhibition on absolute and relative tumor volume basis, respectively, compared with mock control. On the same basis, the I/V route treatment resulted in 40 and 16% of inhibition, respectively. Histopathological examination revealed that the virus caused apoptosis, followed by necrosis, but immune cell infiltration was not remarkable. The virus survived in 2/2 mice until day 10 and in 3/6 mice by day 19, with both routes of administration. Anti-NDV antibodies were generated at moderate level and the titer reached a maximum of 1:32 and 1:64 via the I/T and I/V routes, respectively. In conclusion, the test NDV strain was found to be safe and showed oncolytic activity against the SW-620 cell line in mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is one of the leading causes of death in humans and animals worldwide. At present, cancer treatment relies upon surgery, chemotherapy and radiotherapy, however, there is an urgent need for new approaches which can effectively complement these three mainstays of cancer treatment. Despite a continued improvement in patient care, survival rates of patients have not changed appreciably in the past 20 years [7].
Among the possible alternative approaches, viruses hold the great promise in the treatment of cancers. During transformation, tumor cells acquire a variety of defects that make them selectively permissive to viral replication [20]. This property has been harnessed as a novel form of cancer treatment known as oncolytic virotherapy and the potential candidate viruses are known as oncolytic viruses (OVs) [16]. In the last 20 years, several reports have confirmed that intratumorally- or systematically-delivered viruses can display such antitumor activities [33].
Among the candidates, Newcastle disease virus (NDV), a variant of avian paramyxovirus 1 (APMV-1; species Avian avulavirus 1), has received significant attention as an effective oncolytic agent for virotherapy [39]. Taxonomically, the species Avian avulavirus 1 is classified under the genus Avulavirus of the family Paramyxoviridae. NDV is known among veterinarians for causing Newcastle or Ranikhet disease in poultry [13]. Since the initial reports on the use of NDV as an oncolytic agent, the virus has become one of the highly preferred non-engineered OVs [41]. Many of its strains, notably 73-T, MTH-68, PV701 and NDV-HUJ, have been investigated in different phases of clinical trials [26, 49]. Despite the huge progress in this area at a global level, virotherapy is virtually an unexplored area of research in India. Only recently, work on NDV [24, 25] and chicken anemia virus [38] has been initiated and evidence of the oncolytic potential of these viruses has been limited to cell culture assays. Our study evaluated the oncolytic potential of the R2B Mukteshwar strain of NDV in SW-620 colon cancer cells in nude mice. Growth inhibition or regression of tumor xenografts was used as an indication of the oncolytic efficiency of the virus. The characteristics of this commonly available mesogenic vaccine have been described in detail by Dey et al. [8]. Along with the oncolytic potential of the test strain, virus survival during the virotherapeutic process is important for potential clinical application because the host immune system lacks the ability to distinguish between disease-causing pathogens and therapeutic viruses. Therefore, we aimed to evaluate both virus survival and host immune responses against NDV, to understand the obstacles posed by the immune system during virotherapy.
Materials and methods
Virus, cells, culture media and animals
R2B Mukteshwar strain of NDV was obtained as a vaccine vial from a commercial source. Each vial was labeled to contain 1 x 108 EID50 of virus in lyophilized form. Vero and SW-620 cell lines were propagated in DMEM and RPMI-1640 culture media, respectively, supplemented with 10 % fetal bovine serum (Invitrogen).The cells were grown under 5% CO2 concentration at 37 °C. To generate a xenograft, 1 x 106 SW-620 cells were used in each mouse. Virus safety was determined in Balb/c mice while tumor regression experiments were conducted on nu/nu nude mice at the animal research facility, Zydus lab, Ahmedabad. All the required permissions were obtained from the ethical committee to carry out both experiments.
Virus counting
The vaccine was reconstituted in the vial in 1 ml of NDV diluent (provided by Biological Product Lab, Gujarat state) and all further dilutions were made from this suspension. Viruses were counted as hemagglutination (HA) units, using serial dilutions of 25 µl of viral suspension and 1% chicken red blood cells (RBC) [42]. 100 µl of virus suspension was used to calculate the tissue culture infective dose-50 (TCID50) [2] and plaque forming units (PFU) [23] in Vero cells. The HA titer was obtained as 1:128, whereas TCID50 and PFU were calculated as 1 x 108.16/ml and 4.2(=4) x 107/ml, respectively. The PFU count was used for the safety trial and virotherapy experiments, whereas the HA units and TCID50 were used during the hemagglutination inhibition (HI) experiment and serum neutralization test (SNT), respectively.
Virus safety determination
The virus was inoculated intravenously at 2 x 107 PFU in immune-competent Balb/c mice. Safety was determined on the basis of absence of any undesirable gastro-intestinal, respiratory and nervous system signs, as well as death in the inoculated group. Weight values of inoculated mice were compared with control mice (Student’s t test).
Tumor xenografts generation and treatments
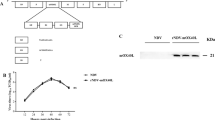
Nude mice were injected with 1 x 106 SW-620 cells using the subcutaneous route along with Matrigel (BD bioscience), a connective tissue equivalent, in a 1:1 ratio. After 10 days, when tumors reached a palpable size of about 200-300 mm3 (calculated using the formula: [length x width2]/2) [15], animals were divided into four groups (Table 1). In the treatment group, NDV was inoculated via the I/T and I/V routes at a dose of 1 x 107 PFU per animal, in 50 (I/T) and 100 µl (I/V) of sterile normal saline. The control groups were inoculated with a similar volume of normal saline solution (NSS), as per the designated route of administration. The tumor volumes were measured on alternate days. On day 10, two mice were sacrificed from each group. A 1800 mm3 tumor volume for the treatment group was set as the endpoint of the experiment. The mean of the absolute tumor volumes of the treatment and control groups were compared using the Student’s t test. As the mean volumes of the two groups, for both treatment routes, were unequal at the beginning of the experiment, the relative tumor volume (RTV) was calculated for the two groups using the formula: RTV = volume on day n/volume at day 1.
Histopathology of tumor mass
Tumor mass was aseptically collected from sacrificed mice and halved. One part of each tumor lump was used for histopathological evaluation using hematoxyllin and eosin staining method, and the other part was collected in RNA protecting agent (RNAlater, Qiagen) and used for virus survival determination.
NDV survival in tumor mass
The survival of therapeutic virus was determined using real-time PCR. Total RNA was isolated from 100 mg of tumor mass using Trizol (Invitrogen). The initial processing of sample was carried out as per manufacturer’s instructions. The tissue was snap-frozen with liquid nitrogen and triturated in RNAase-free pestle and mortar. Subsequently, 100 ng of total RNA were used for each reaction. The one-step Taqman chemistry-based, NDV real-time PCR kit (Vetmax, Invitrogen) was used for viral RNA amplification. NDV RNA (10,000 molecules/µl) supplied with the kit was used as reference control. After preparation of the master mix, one-step real-time PCR was carried out using an Applied Biosystem Real-Time PCR Instrument (ABI, 7500). The annealing temperature was 55 °C and other parameters were set as per the ABI instruction manual. The real-time PCR instrument calculated the cycle threshold (Ct) values, which were used as the final result.
Immunogenicity of NDV in mice
At the time of animal sacrifice, blood was collected aseptically and serum was harvested. Generation of anti-NDV antibodies was determined by SNT and HI test [2, 42]. HI was done in 96-well V-bottom microtiter plates (Laxbro) using 2-fold dilutions of serum samples against 4 HA unit of vaccine virus, with 1% of pooled chicken RBC. SNT was determined on Vero cells at 100 TCID50 of virus, with a 2-fold dilution of serum. Only virus and positive serum were used as control in both tests. The last dilution, where HA inhibition and the cytopathic effect (CPE) were blocked, was used as HI and SNT titer, respectively.
Results
Safety trial
During the 15-days safety experiment period, no adverse respiratory, gastrointestinal or neurological signs were observed in any of the inoculated animal. These animals maintained similar weight as control mice and remained active during the entire experimental period. Even during the virotherapy experiment, no inadvertent death was noticed in any of the virus-treated immune-deficient mice (data not shown).
Gross tumor growth inhibition
Tumor growth inhibition, based on mean tumor volume, was evident and conspicuous in both treatment groups, compared with that of corresponding mock controls. After starting with the same tumor volume at day one, in both groups, the mean tumor volume in the NDV-I/T group reached a value of 1823.52 mm3, compared with 3123.57 mm3 in the control-I/T group (58% of control). Similarly, the mean tumor volume in the NDV-I/V and control-I/V groups were 1753.06 and 2934.13 mm3 (59.9% of control), respectively, on day 19 (Table 2; Figure 1). The maximum growth inhibition observed with the I/T and I/V route was 43 and 40%, respectively (Figure 2). A significant difference was observed from day 15 and 17 onwards with the I/T and I/V treatments, respectively (Table 2).
As tumor volumes were not the same at the beginning of therapy, the efficiency of treatment was also evaluated by calculating the relative tumor volume (RTV). The efficiency of treatment in the NDV-I/T group was evident: the tumor volume increased very slowly and reached a maximum value of 5.2, with no further increase until day 19, compared with 9.1 in the control group. This was mirrored by an actual inhibition of tumor growth of 57 % in the NDV-I/T group, relative to the control-I/T group. In the I/V groups, at the start of the experiment, the tumor volume of treatment-I/V group was lower than that of the control-I/V group; therefore the therapy efficacy was lower in the treatment-I/V than in the control-I/V group. On day 19, RTV of NDV-I/V group was 6.76, compared with 7.9 in the control-I/V group, hence, the actual inhibition was 16% (Figure 2).
Histopathology of tumor sections
Histopathology analysis of tumors from control mice revealed the uninterrupted tumor growth with continuous mitotic activity. Very little apoptosis/necrosis and immune cells infiltration were observed in these sections. On the other hand, tumors from treatment group mice showed massive areas of necrosis upon histopathological examination. The necrosis was even more pronounced in tumors from the NDV-I/T group than in those from the NDV-I/V group. Upon closer observation, it was evident that the cells were undergoing apoptosis, leading to necrosis (Figure 3, A-D). The necrotic areas were infiltrated with modest numbers of immune cells, including macrophages and lymphocytes.
Antibody response against NDV
During virotherapy, virus-induced antibody production in nude mice was observed with both the intratumoral and the intravenous route of administration. Anti-NDV antibodies were generated at moderate levels, and the maximum antibody titers were 1:32 and 1:64, via the I/T and I/V routes, respectively. The SNT and HI test results are shown in Figure 4.
NDV survival in tumor xenograft
When virus RNA was measured by real-time PCR in the tumor masses, the virus survival was recorded in 2/2 mice on day 10 and in 3/6 mice on day 19, in both treatment groups. However, a very low level of virus load was detected by day 19 (Table 3).
Discussion
Among many candidate oncolytic viruses, NDV has emerged as a promising oncolytic agent [49] because of its efficacy and encouraging safety profile [39]. NDV is a natural pathogen of poultry, with limited pathogenicity in other mammalian species and humans [40, 42]. Safety data of the test NDV strain were also similar to previous reports of pre-clinical and clinical trials [26, 27]. The safety profile observed during virotherapy experiments was especially encouraging based on the lack of adverse effects normally observed with most chemotherapeutic drugs.
The partial but significant tumor growth inhibition observed in this study was in line with reports from other laboratories [35, 43, 46, 47] which used NDV as oncolytic agent and showed a tumor growth inhibition in the range of 30-65%. Similarly, Yan et al. [46] reported a 58.7% and 42.7% growth inhibition with recombinant and unmodified NDV, respectively. In line with these results, 30%, 40% and 60% tumor growth inhibition has been reported when NDV treatment was administered once, twice and three times, respectively [47].
In the literature similar results have been published with colon cancer cell lines. One study reported 68% inhibition of tumor growth in HT-29 colon carcinoma cells [35] and another group found that 2/10 mice showed appreciable response to NDV treatment against CT-26 colon cancer cells [43]. These colon xenografts were also found to be susceptible to oncolytic viruses other than NDV [9], which underscores the growing importance of oncolytic virotherapy.
Partial tumor regression of higher magnitude was reported with tumors other than colorectal carcinomas [6, 44, 45]. In sharp contrast to the present work, complete or near-complete regression were achieved in these study models with non-recombinant NDV strains [29, 30, 35, 36] and with different recombinant NDVs [3, 10, 28, 37, 43, 48]. These results may be due to the use of higher and multiple doses, as well as to the use of purified viruses [5, 6, 45]. An oncolytic response, although modest, was observed in SW-620 cells with the I/V route of administration of NDV treatment, which was encouraging. Earlier studies with mice models [35, 43, 45] and clinical trials [11, 18, 27, 34] have reported the utility and efficacy of the I/V route for virotherapy.
According to our literature search, this is the first in vivo study of tumor lytic potential of NDV (and possibly with any oncolytic virus) in India. Previous reports were limited to in vitro oncolysis evaluation only. Our work strongly suggests the inherent oncolytic potential of NDV Mukteshwar strain towards colon cancer cells. Further research and experiments, using higher doses of virus and virus modifications, are warranted to improve our understanding of this topic.
Histopathological evaluation of the tumors was expected to reveal massive areas of necrosis [3, 29, 30, 34], preceded by apoptosis [6, 47] with an increase in cell fusion [3]. As part of the host response, infiltration of immune cells, development of granulation tissue and complete replacement of tumor tissue with normal tissue were observed in previous studies [3, 18, 29, 37, 43, 45]. Necrosis is considered a hallmark of viral activity against tumor cells. Necrosis must be preceded by apoptosis or syncytia formation. Apoptosis with chromatin condensation and fragmentation were seen in many areas of the tumor mass, however, syncytia formations were not easily observed. A likely explanation for this is that SW-620 tumor cells may lack fusion-stimulating proteins [3, 31] or the syncytia had been converted into necrotic areas. The low level of tumor cell infiltration is in agreement with Reichard et al. [36] but several other studies showed higher levels of immune cell infiltration and suggested they were an oncolytic factor [48].
In nude mice, antibody response was observed by both the I/T and I/V routes of treatment. As previously reported, nude mice can also generate some antibody response [4]. Most of the earlier reports on mice experiments with NDV or with a few other oncolytic viruses did not report the occurrence of an antibody response. Hence, we compared the results from our work with those from the few available reports of mice studies with other oncolytic viruses [12, 17, 21, 32] and with NDV-based clinical trials [11, 18, 27, 34].
Very high titers had been obtained in clinical trials or mice studies with vaccinia virus and vesicular stomatitis virus (VSV). The high titer observed in human trials may be due to the immunocompetent host and the high dose of NDV used (up to 1 x 1012 PFU). The exceptional immunogenicity of vaccinia virus and VSV can explain the high titer observed in mice experiments. However, in our opinion, a moderate level of immunogenicity may be a useful feature of NDV in virotherapy.
In the present work, the oncolytic activity of NDV was sustained for a few days with antibodies (two weeks post-infection), however, subsequently, the virus was cleared by the immune system. Clinical trials revealed that the virus can be detected, even though antibody responses and tumor lysis were more efficient after generation of anti-NDV antibodies [34]. Contrary to this notion, a few studies showed that VSV and vaccinia virus were cleared as soon as the antibody response was generated [12, 17, 21, 32].
It is thought that oncolytic activity will last until the virus remains in the tumor micro-environment. In line with the present work, two [1, 9, 19] to three weeks [22, 32] viral survival was observed with different techniques and virus/tumor models. Some studies reported a very short survival period (5 days), whereas we observed a longer survival time [21]. Longer virus survival was reported in mice studies with other recombinant viruses [14, 15] and is linked to better tumor lysis activity. The type and dose of virus, the immune status of the host and tumor burden may be important factors for the final outcome and should be considered when devising a therapeutic protocol. Based on the survival data and immune clearance results, the NDV virotherapeutic agent could survive in the tumor mass for at least 3 weeks; its levels were probably reduced after week 2, which might be due to immune clearance of the virus. The safety profile, intrinsic oncolysis potential, ability to survive in the tumor mass and moderate immunogenicity are thought to be highly useful features of the R2B Mukteshwar strain of NDV and make the virus a promising oncolytic candidate. Further research and experiments with higher viral doses and genetic modifications of the virus may lead to its use in the clinical setting.
References
Adelfinger M, Bessler S, Frentzen A, Cecil A, Langbein-Laugwitz J, Gentschev I, Szalay AA (2015) Preclinical testing oncolytic vaccinia virus strain GLV-5b451 expressing an anti-VEGF single-chain antibody for canine cancer therapy. Viruses 7:4075–4092
Ahamed T, Hossain KM, Billah MM, Islam KMD, Ahasan MM, Islam ME (2004) Adaptation of Newcastle disease virus (NDV) on Vero cell line. Int J Poultry Sc 3:153–156
Altomonte J, Marozin S, Schmid RM, Ebert O (2010) Engineered Newcastle disease virus as an improved oncolytic agent against hepatocellular carcinoma. Mol Ther 18:275–284
Belizário JE (2009) Immunodeficient mouse models: an overview. Open Immunol J 2:79–85
Buijs P, van Nieuwkoop S, Vaes V, Fouchier R, van Eijck C, van den Hoogen B (2015) Recombinant immunomodulating lentogenic or mesogenic oncolytic Newcastle disease virus for treatment of pancreatic adenocarcinoma. Viruses 7:2980–2998
Chai Z, Zhang P, Fu F, Zhang X, Liu Y, Hu L, Li X (2014) Oncolytic therapy of a recombinant Newcastle disease virus D90 strain for lung cancer. J Virol 11:84–92
Cody JJ, Hurst DR (2015) Promising oncolytic agents for metastatic breast cancer treatment. Oncol Virotherap 4:63–73
Dey S, Chellappa MM, Gaikwad S, Kataria JM, Vakharia VN (2014) Genotype characterization of commonly used Newcastle Disease Virus vaccine strains of India. PLoS One 9:e98869. doi:10.1371/journal.pone.0098869
Ehrig K, Kilinc MO, Chen NG, Stritzker J, Buckel L, Zhang Q, Szalay AA (2013) Growth inhibition of different human colorectal cancer xenografts after a single intravenous injection of oncolytic vaccinia virus GLV-1h68. J Transl Med 11:79. doi:10.1186/1479-5876-11-79
Elankumaran S, Chavan V, Qiao D, Shobana R, Moorkanat G, Biswas M, Samal SK (2010) Type I interferon-sensitive recombinant Newcastle disease virus for oncolytic virotherapy. J Virol 84:3835–3844
Freeman AI, Zakay-Rones Z, Gomori JM, Linetsky E, Rasooly L, Greenbaum E, Rozenman-Yair S, Panet A, Libson E, Irving CS, Galun E, Siegal T (2006) Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol Ther 13:221–228
Galivo F, Diaz RM, Wongthida P, Thompson J, Kottke T, Barber G, Melcher A, Vile R (2010) Single-cycle viral gene expression, rather than progressive replication and oncolysis, is required for VSV therapy of B16 melanoma. Gene Ther 17:158–170
Ganar K, Das M, Sinha S, Kumar S (2014) Newcastle disease virus: Current status and our understanding. Virus Res 184:71–81
Gentschev I, Adelfinger M, Josupiet R, Ruldoph S, Ehrig K, Donat U, Weibel S, Chen NG, Yu YA, Zhang Q, Heisig M, Thamm D, Stritzker J, MacNeill A, Szalay AA (2012) Preclinical evaluation of oncolytic vaccinia virus for therapy of canine soft tissue sarcoma. PLoS One. 7:e37239 (Available from http://journals.plos.org/plosone/article/citation?id=10.1371/journal.pone.0037239)
Gentschev I, Ehrig K, Donat U, Hess M, Rudolph S, Chen N, Weibel S, Chen NG, Yu YA, Zhang Q, Heisig M, Thamm D, Stritzker J, MacNeill A, Szalay AA (2010) Significant growth inhibition of canine mammary carcinoma xenografts following treatment with oncolytic vaccinia virus GLV-1h68. J Oncol. doi:10.1155/2010/736907). (http://www.hindawi.com/journals/jo/2010/736907/)
Gentschev I, Patil SS, Petrov I, Cappello J, Adelfinger M, Szalay AA (2014) Oncolytic virotherapy of canine and feline cancer. Viruses 6:2122–2137
Hastie E, Grdzelishvili VZ (2012) Vesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancer. J Gen Virol 93:2529–2545
Hotte SJ, Lorence RM, Hirte HW, Polawski SR, Bamat MK, O’Neil JD, Roberts MS, Groene WS, Major PP (2007) An optimized clinical regimen for the oncolytic virus PV701. Clin Cancer Res 13:977–985
Huang TG, Ebert O, Shinozaki K, Gracia-Sastre A, Woo SLC (2003) Oncolysis of hepatic metastasis of colorectal cancer by recombinant vesicular stomatitis virus in immune –competent mice. Mole Ther 8:434–440
Iikow CS, Swift SL, Bell JC, Diallo JS (2014) From scourage to cure: Tumour selective viral pathogenesis as a new strategy against cancer. PloS Pathog 10:e1003836 (http://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1003836)
Janelle V, Langlois M, Lapierre P, Charpentier T, Poliquin L, Lamarre A (2013) The strength of the T cell response against a surrogate tumor antigen induced by oncolytic VSV therapy does not correlate with tumor control. Mole Ther 22:1198–1210
Kooby DA, Carew JF, Halterman M, Mack W, Jonathan E, Bertino JR, Blumgart LH, Federoff HJ, Fong Y (1999) Oncolytic viral therapy for human colorectal cancer and liver metastases using a multi-mutated herpes simplex virus type-1 (G207). FASEBJ 13:1325–1334
Kournikakis B, Fildes J (1988) Titration of avirulent Newcastle disease virus by plaque assay method. J Virol Methods 20:185–193
Kumar R, Tiwari AK, Chaturvedi U, Kumar GR, Sahoo AP, Rajmani RS, Saxena L, Saxena S, Tiwari S, Kumar S (2012) Velogenic Newcastle disease virus as an oncolytic virotherapeutics: in vitro characterization. Appl Biochem Biotechnol 167:2005–2022
Kumar U, Kumar S (2015) Molecular characterization of an apoptotic strain of Newcastle disease virus isolated from an outbreak in India. Can Gene Ther 22:402–409
Lam HY, Yeap SK, Rasoli M, Omar AR, Yusouff K, Suraini AA (2011) Safety and clinical uses of Newcastle disease virus in cancer therapy. J Biomed Biotechnol. doi:10.1155/2011/718710
Laurie SA, Bell JC, Atkins HL, Roach J, Bamat MK, O’Neil JD, Roberts MS, Groene WS, Lorence RM (2006) A phase 1 clinical study of intravenous administration of PV701, an oncolytic virus, using two-step desensitization. Clin Cancer Res 12:2555–2562
Li P, Chen CH, Li S, Givi B, Yu Z, Zamarin D, Palese P, Fong Y, Wong RJ (2011) Therapeutic effects of a fusogenic Newcastle disease virus in treating head and neck cancer. Head Neck 33:1394–1399
Lorence RM, Katubig BB, Reichard KW, Reyes HM, Phuangsab A, Sassetti MD, Walter RJ, Peeples ME (1994) Complete regression of human fibrosarcoma xenografts after local Newcastle disease virus therapy. Cancer Res 54:6017–6021
Lorence RM, Reichard KW, Katubig BB, Reyes HM, Phuangsab A, Mitchell BR, Cascino CJ, Walter RJ, Peeples ME (1994) Complete regression of human neuroblastoma xenografts in athymic mice after local Newcastle disease virus therapy. J Natl Cancer Inst 86:1228–1233
Maveeva OV, Guo ZS, Shabalina SA, Chumakov PM (2015) Oncolysis by paramyxoviruses: multiple mechanisms contribute to therapeutic efficiency. Mole Ther Oncolytics 2:15011. doi:10.1038/mto.2015.11
Mukherjee S, Haenel T, Himbeck R, Scott B, Ramshaw I, Lake RA, Harnett G, Phillips P, Morey S, Smith D, Davidson JA, Musk AW, Robinson B (2000) Replication-restricted vaccinia as a cytokine gene therapy vector in cancer: persistent transgene expression despite antibody generation. Cancer Gene Ther 7:663–670
Patil SS, Gentschev I, Noite I, Oglive G, Szalaly AA (2012) Oncolytic virotherapy in veterinary medicine: current status and future prospects for canine patients. J Trans Med. doi:10.1186/1479-5876-10-3
Pecora AL, Rizvi N, Cohen GI, Meropol NJ, Sterman D, Marshall JL, Goldberg S, O’Neil JD, Groene WS, Roberts S, Rabin H, Bamat MK, Lorence RM (2002) Phase I trial of intravenous administration of PV701, an oncolytic virus, in patients with advanced solid cancers. J Clin Oncol 20:2251–2266
Phuangsab A, Lorence RM, Reichard KW, Peeples ME, Walter RJ (2001) Newcastle disease virus therapy of human tumor xenografts: antitumor effects of local or systemic administration. Cancer Lett 172:27–36
Reichard KW, Lorance RM, Cascino CJ, Peeples ME, Waller RJ, Fernando MB, Reyes HM, Greager JA (1992) Newcastle disease virus selectively kills human tumor cells. J Surg Res 52:448–453
Silberhumer GR, Brader P, Wong J, Serganova IS, Gönen M, Gonzalez SJ, Blasberg R, Zamarin D, Fong Y (2010) Genetically engineered oncolytic Newcastle disease virus effectively induces sustained remission of malignant pleural mesothelioma. Mol Cancer Ther 9:2761–2769
Singh H, Jadon NS, Tiwari AK, Pandey P (2013) Apoptotic effect of VP3 gene of chicken anaemia virus on HELA cells. Ind J Vet Surg 34:9–12
Schirrmacher V (2016) Fifty years of clinical application of Newcastle disease virus: time to celebrate! Biomedicines 4:16. doi:10.3390/biomedicines4030016
Swayne DE, King DJ (2003) Avian influenza and Newcastle disease. J Am Vet Med Assoc 222:1534–1540
Tayeb S, Zakay-Rones Z, Panet A (2015) Therapeutic potential of oncolytic Newcastle disease virus: a critical review. Oncol Virotherap 4:49–62
The world organization for animal health (2012) Newcastle disease. Manual of diagnostic tests and vaccines for terrestrial animals. OIE, Paris, pp 1–19
Vigil A, Martinez O, Chua MA, Xiao S, Cros JF, Martínez-Sobrido L, Woo SL, Grarcia-Sastre A (2007) Use of reverse genetics to enhance the oncolytic properties of Newcastle disease virus. Cancer Res 67:8285–8292
Wei D, Sun N, Nan G, Wang Y, Liu HQ, Peeters B (2012) Construction of recombinant Newcastle disease virus Italien strain for oncolytic virotherapy of tumors. Hum Gene Ther 23:700–710
Yaacov B, Eliahoo E, Lazar I, Ben-Shlomo M, Greenbaum I, Panet A, Zakay-Rones Z (2008) Selective oncolytic effect of an attenuated Newcastle disease virus (NDV-HUJ) in lung tumors. Cancer Gene Ther 15:795–807
Yan Y, Jia L, Zhang J, Liu Y, Bu X (2014) Effect of recombinant Newcastle disease virus transfection on lung adenocarcinoma A549 cells in vivo. Oncol Lett 8:2569–2576
Zaher KS, El-Zahed HM, Amin AH (2013) In-vivo and In-vitro oncolytic effect of Newcastle disease virus. Acad J Cancer Res 6:74–78
Zamarin D, Holmgaard RB, Subudhi, SK, Park JS, Mansour M, Palese P, Merghoub T, Wolchok JD, Allison JP (2014) Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med 6(226):226ra32. (http://stm.sciencemag.org/content/6/226/226ra32.long)
Zamarin D, Palese P (2012) Oncolytic Newcastle disease virus for cancer therapy: old challenges and new directions. Fut Microbiol 7:347–367
Acknowledgements
The authors acknowledge Dr. Mukul Jain for granting permission for work in the Zydus Research Centre (ZRC); Dr. Krishnaroop for help with cell culture at ZRC, Ahmedabad; Dr.Gaurav M. Pandya, Dr.Ummed V. Ramani and Dr. Mamta Janmeda for carrying out real-time PCR work; the staff at ZRC, Ahemdabad, during the mice trial. We further acknowledge the funds and other support provided by Principal, Veterinary College and University Authorities, Navsari Agricultural University, Navsari, as well as ZRC, Ahmedabad.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Government of Gujarat’s program entitled “Strengthening the Vanbandhu College of Veterinary Science and Animal Husbandry, Navsari phase–V” (Budget Head, 12404) and Zydus Research Centre, Ahmadabad.
Conflict of interest
All authors declare that there lies no conflict of interest.
Ethical approval
All applicable guidelines were followed regarding housing, care and animal experimentation during the study. Necessary prior permission was obtained from institutional ethics committee of Vanbandhu College of Veterinary Science and animal husbandry, Navsari (No-013-VCN-VMC-2014) and Zydus Research Centre, Ahmadabad (No-ZRC/PH/BP/002/12-2K14) to carry out animal experiments.
Rights and permissions
About this article
Cite this article
Sharma, K.K., Kalyani, I.H., Mohapatra, J. et al. Evaluation of the oncolytic potential of R2B Mukteshwar vaccine strain of Newcastle disease virus (NDV) in a colon cancer cell line (SW-620). Arch Virol 162, 2705–2713 (2017). https://doi.org/10.1007/s00705-017-3411-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-017-3411-4