Abstract
To evaluate the efficacy of high-frequency repetitive transcranial magnetic stimulation (rTMS) in patients with primary progressive aphasia (PPA). In this randomized, double-blind trial in a single center, patients who were diagnosed with PPA were randomly assigned to receive either real rTMS or sham rTMS treatment. High-frequency rTMS was delivered to the dorsolateral prefrontal cortex (DLPFC). The primary outcome was the change in Boston Naming Test (BNT) score at each follow-up compared to the baseline. The secondary outcomes included change in CAL (Communicative Activity Log) and WAB (Western Aphasia Battery) compared to baseline and neuropsychological assessments. Forty patients (16 with nonfluent, 12 with semantic and 12 with logopenic variant PPA) were enrolled and randomly assigned to the rTMS or sham rTMS group, with 20 patients in each group. Thirty-five patients (87.5%) completed a 6-month follow-up. Compared to the sham rTMS group, the BNT improvement and WAB improvement in the real rTMS group were significantly higher. These significant improvements could be observed throughout the entire 6-month follow-up. At 1 month and 3 months after treatment, CAL improvements of real rTMS were significantly higher than sham rTMS. The improvements in BNT, CAL and WAB did not significantly differ among PPA variants. No significant improvement in neuropsychological assessments was observed. High-frequency rTMS delivered to DLPFC improved language functions in patients with different PPA variants. The efficacy was still observed after 6 months of treatment. Trial registration: NCT04431401 (https://clinicaltrials.gov/ct2/show/NCT04431401).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary progressive aphasia (PPA) is a unique group of neurodegenerative diseases characterized by prominent difficulty with language and isolated language deficits during the early stages of the disease (Montembeault et al. 2018). PPA can be classified into 3 main variants, namely the nonfluent/agrammatic variant (nfvPPA), the semantic variant (svPPA), and the logopenic variant (lvPPA) (Gorno-Tempini et al. 2011). However, subsequent studies have shown that a relatively high proportion of PPA patients cannot be classified into these 3 main variants, and new variants have been suggested (Vandenberghe 2016; Teichmann 2021). The classification of PPA variants was based on the clinical, imaging and/or pathological features of each patient. Unfortunately, no pharmacological therapies are currently available to slow or halt the progression of any PPA variant (Marshall et al. 2018). Recent studies have demonstrated improvements in symptoms following speech and language therapies and noninvasive brain stimulation (NIBS) (Pagnoni et al. 2021; Taylor-Rubin et al. 2021; Sheppard 2022).
NIBS consists of a variety of techniques that aims to transcranially modulate the excitability of specific brain areas and related networks (Boes et al. 2018). Transcranial direct current stimulation (tDCS) and repetitive transcranial magnetic stimulation (rTMS) are two widely used methods of NIBS, which delivered direct or secondary electrical current to the brain to increase or suppress cortical excitability to modulate neuroplasticity (Cirillo et al. 2017; Rajji 2021). An increasing number of studies have shown promising results of NIBS in treatment of neurocognitive disorders, such as Alzheimer’s disease (AD), mild cognitive impairment, and post-stroke aphasia (Ciullo et al. 2021; Lefaucheur et al. 2020; Guan et al. 2017).
The efficacy of NIBS in PPA has also been explored in various studies. A meta-analysis published in 2020 summarized evidence of the efficacy of tDCS or rTMS paired with language therapy in PPA, with 6 tDCS studies and 2 rTMS studies included (Nissim et al. 2020). The results showed that NIBS treatments had a significant and moderate improvement in language functions (mainly naming abilities) over sham, and tDCS yielded greater improvements than rTMS. Several recent publications also demonstrated that tDCS can enhance the efficacy of language therapies in naming abilities and apraxia of speech (Themistocleous et al. 2021; Zhao et al. 2021; Nissim et al. 2022; Sheppard et al. 2022). In addition to language function improvements, several studies showed that rTMS can improve global cognitive function and neuropsychiatric symptoms, which are common manifestations of PPA patients, especially in the advanced stages (Bereau et al. 2016; Pytel et al. 2021).
There is significant heterogeneity across NIBS studies. Part of this heterogeneity is attributed to the heterogeneity of PPA, where the pathophysiology differs between different PPA variants. Another important reason is that the stimulation sites in NIBS studies are various. The left frontal lobe (F7) was the most common stimulation site in tDCS studies, while the dorsal lateral prefrontal cortex (DLPFC) was the most common in rTMS studies (Trebbastoni et al. 2013; Pytel et al. 2021; Margolis et al. 2019; Cotelli et al. 2012; Bereau et al. 2016; Nissim et al. 2020). High-frequency rTMS delivered to DLPFC have shown efficacy to improve language functions in nfvPPA and lvPPA patients (Cotelli et al. 2012; Trebbastoni et al. 2013; Bereau et al. 2016; Neri et al. 2021). A recent randomized, double-blind trial of rTMS selected personalized targets for each patient and left DLPFC rTMS stimulation yielded greater efficacy than other sites in 5 out of 6 svPPA patients (Pytel et al. 2021). Thus, rTMS delivered to DLPFC has the most evidence for the efficacy of the three main PPA variants, which deserves further investigation.
To date, there is only one randomized, double-blind trial with a relatively small sample size that explored the efficacy of rTMS in PPA, with no lvPPA patients included (Pytel et al. 2021). Moreover, patients in most rTMS studies were followed for a short period so that the long-term efficacy of rTMS has not been well studied. Therefore, we did a randomized, double-blind trial to evaluate the short-term and long-term efficacy of rTMS delivered to DFPLC in all 3 variants of PPA patients.
Methods
Study design and participants
This study was a single-center randomized double-blind placebo-controlled trial. Consecutive inpatients and outpatients of Peking Union Medical College Hospital who were diagnosed with PPA and aged 35–75 years were enrolled. In total, 40 patients (16 with nonfluent, 12 with semantic and 12 with logopenic variant PPA) were enrolled in this study, with 20 allocated to the real rTMS group and 20 allocated to the sham rTMS group (Fig. 1). The diagnosis of PPA and its variants were based on the consensus published in 2011 (Gorno-Tempini et al. 2011). Exclusion criteria included: (1) Pattern of deficits could be better explained by other nondegenerative nervous system or medical disorders; (2) cognitive deficits could be explained by other diseases through neuropsychological examination; (3) significant impairments of episodic memory, visual memory and visual perception are observed in early stages; (4) significant behavioral deficits in early stages; (5) history of loss of consciousness due to secondary causes such as epilepsy or encephalitis; (6) disease duration < 6 months. Patients who could not complete assessments and rTMS were also excluded: (1) significant language impairment (hearing impairment or cannot complete assessments due to other causes); (2) severe depression or other psychiatric symptoms (defined as HAMA (Hamilton Anxiety Scale, (Hamilton 1969)) > 14 and HRSD (Hamilton Rating Scale for Depression, (Hamilton 1960)) > 18, respectively) that make language assessment difficult and/or prevent completion of the assessment and treatment process; (3) Mini-mental state examination (MMSE) score ≤ 18 due to global cognitive decline judged by the evaluating physician (Li et al. 2016); (4) presence of contraindications to MRI or rTMS; (5) pregnancy. The study was approved by the Ethics Committee of Clinical Research of Peking Union Medical College Hospital (Beijing, China) and was registered at ClinicalTrials.gov (NCT04431401). Written informed consent was obtained from every participant.
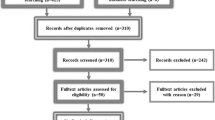
Trial profile. AD Alzheimer’s disease, HAMA Hamilton Anxiety Scale, HRSD Hamilton Rating Scale for Depression, MMSE Mini-mental state examination. a Loss to follow-up because the participant was no longer willing to participate. b Loss to follow-up because the participant was unable to arrive at the hospital at the scheduled follow-up time
Randomization and masking
Participants were randomly allocated (1: 1) to two groups receiving either real rTMS or sham rTMS. Simple randomization without blocks and stratification of any factors were applied. The random allocation sequence was generated by an independent statistician who did not participate in this trial using Random Number Generators of SPSS (version 25.0). The random numbers were sealed in opaque envelopes and a serial number was assigned to each envelope according to the sequence of the randomized number. Then each envelope was opened sequentially at the enrollment of each participant. The participant was allocated to real rTMS or sham rTMS group according to the assigned random number. The randomization and allocation process was supervised by the principal investigator. Two independent groups were, respectively, responsible for the assessments and treatment of patients. RTMS treatment was conducted by physicians unblind to the allocation, while assessments were conducted by independent evaluators blind to the intervention. Participants and their caregivers were also blind to the allocation during the study, since the sham rTMS differed from the real rTMS only in the angle of the coil placement, as described below.
Assessments and outcomes
At the time of study inclusion, all patients were evaluated by a neurologist trained in neuropsychological examination and underwent a 3T brain MRI. We did not perform positron emission tomography imaging or tests for biomarkers and genes. All participants were classified into nfvPPA, svPPA and lvPPA based on the features of speech and language impairment and brain MRI. Patients were follow-up at 1 month (± 1 week), 3 months (± 1 week) and 6 months (± 1 week) after treatment. Speech and language assessments including BNT (Boston Naming Test), CAL (Communicative Activity Log) and WAB (Western Aphasia Battery) were carried out at baseline and each follow-up point (Cheung et al. 2004; Pulvermuller et al. 2001; Wang 1997). These assessments of outcomes will be described in detail below. We planned to test Bilingual Aphasia Test in bilingual patients, but there were no bilingual patients included in our study. Global cognitive function was assessed with MMSE and MoCA (Montreal Cognitive Assessment) at enrollment (Li et al. 2016; Lu et al. 2011). HAMA and HRDS were conducted at baseline and the last follow-up to evaluate anxiety and depression, respectively. We used Edinburgh Handedness Inventory to evaluate handedness (Oldfield 1971). Safety evaluations included monitoring of adverse events, assessment of vital signs and physical and neurological examinations. Complete blood count, liver and kidney function tests and electrocardiogram were performed before treatment, 1 month and 6 months after treatment.
The primary outcome was the change in the BNT score of each follow-up point compared to baseline. BNT is one of the most widely used tests of confrontation naming, which was initially developed for the English-speaking population (Kaplan et al. 1983). We used the Chinese version of BNT which was adapted by Cheung et al. by selecting 30 items from the original English version without replacing and adjusting items (Cheung et al. 2004). This adapted version has been validated and widely used in the Chinese-speaking population (Chen et al. 2014; Li et al. 2022). It has been shown to be valid in differentiating normal from brain-damaged participants (Cheung et al. 2004), and distinguishing between normal individuals, amnestic mild cognitive impairment, and AD patients with aphasia (Guo et al. 2006; Salmon et al. 1995). Thus, it is recommended for screening AD patients for language impairment in China (Tian et al. 2020). It is also applied in studies of vascular cognitive impairment and PPA (Ma et al. 2022; Liu et al. 2015b). The Chinese version of BNT is composed of 30 line drawings of objects and animals. Thirty cards bearing different line-drawing objects or animals were presented, and patients were instructed to name each item depicted on the cards. If the patient named the item correctly, one point was recorded as a “score of spontaneous naming”. If the initial answer was incorrect, a standard semantic cue was provided (e.g., “this is a kind of plant” for “tree”). If the answer after the cue was correct, one point was recorded as “scores after semantic cue”. The total score of BNT was the sum of scores of spontaneous naming and scores after semantic cues, ranging from 0 to 30.
The secondary outcomes included changes in CAL and WAB compared to baseline and total scores of HAMA and HRSD. CAL was developed by Pulvermüller et al. to assess the quantity and quality of the patient's daily communication (Pulvermuller et al. 2001). It was rated by a caregiver of each patient and contains 18 questions (e.g., How frequently would the patient communicate with family members or friends?), each scored on a scale of 0 to 5 (0 = never; 5 = very frequently) with a total score of 90. CAL is a commonly used scale to assess speech function and has been applied in RCTs to evaluate the efficacy of treatment in patients with aphasia (Haro-Martínez et al. 2019; Berthier et al. 2009). CAL has been translated into Chinese and is frequently used in Chinese-speaking patients with post-stroke aphasia to assess speech function and improvement after treatment (Zhou et al. 2014; Xie et al. 2014). WAB was first developed by Kertesz and Poole to determine global language impairment in 1974 (Kertesz and Poole 1974). In 1996, Wang translated the Chinese version of the WAB from the original version and validated the scale in a Chinese population (Wang 1997; Kertesz 2020). The Chinese version of WAB is comprised of several subscales that assess the patients’ language function in terms of spontaneous speech, auditory verbal comprehension, repetition, naming, reading, writing and apraxia and has a total score of 700. This version has also been widely applied in the Chinese-speaking population to evaluate aphasia and assess the improvement of aphasia after treatment (Zhang et al. 2021; Yu et al. 2013; Ren et al. 2019; Liu et al. 2015a). The same versions of scales of language assessment were used at baseline and at each follow-up after treatment.
rTMS protocol
The rTMS intervention was conducted using a Magstim stimulator (Magstim, London, UK) connected to a figure-of-8 coil. The motor threshold (MT) of the abductor digiti minimi muscle was determined for each patient before treatment, which was defined as the lowest intensity capable of eliciting at least 5 motor-evoked potentials of at least 50 mV in 10 consecutive stimulations when single-pulse TMS was delivered to the contralateral M1 cortex (Siebner et al. 2022). RTMS was delivered to the left DLPFC for right-handed patients and the right DLPFC for left-handed patients. The localization of DLPFC was determined using an elastic TMS location cap based on the international 10–20 electroencephalography system (Jasper 1958) and DLPFC corresponded to the F3 or F4 electroencephalography electrode (Fig. 2). This method of localizing DLPFC using TMS location cap has been validated using neuronavigation (Herwig et al. 2003) and applied in various TMS studies (Zhao et al. 2020; Li et al. 2020). Participants were randomly assigned to receive real or sham rTMS. The whole course of treatment lasted for 4 weeks, participants received rTMS of a session per day on 5 consecutive days in each week (20 sessions in total). A daily session of stimulation consisted of 50 trains of 10 Hz rTMS (20 pulses per train with 2-s inter-train intervals, 1000 pulses per session) at an intensity of 120% MT. The protocol was similar to our previous study on acute stroke (Guan et al. 2017) and fulfilled the safety norms recommended for this technique (Rossi et al. 2009, 2021). In the real rTMS treatment group, coils were placed tangentially to the scalp, with the tangent point being the F3 or F4 electrode. While sham rTMS was delivered using the same parameters, with the coils placed perpendicular to the scalp to produce tactile sensation and noise without applying magnetic fields to the brain (Fig. 2). This is because the magnetic field the coils generate is perpendicular to the plane of the coils. The methodology of sham rTMS was identical to several previous studies (Shi et al. 2021; Khedr et al. 2019; Jansen et al. 2019). The TMS coil was held steady with a customized coil holder to prevent coil rotation during treatment (Fig. 2). Our protocol did not include any speech and language therapy, and no patients received speech or language training outside the study. Patients were allowed to continue their previous treatment with cholinesterase inhibitors.
Site of stimulation and examples of coil placement. A Stimulation was delivered to the left DLPFC for right-handed patients. B Left DLPFC correspond to the F3 electrode based on the international 10–20 electroencephalography system. C Real rTMS treatment, coils were placed tangent tangentially to the scalp. D Sham rTMS treatment, coils placed perpendicular to the scalp
Sample size and statistical analysis
The sample size calculation was based on a superiority test. In our pilot study, the mean score of objects naming in WAB in PPA patients was 13.2, with a standard variation of 1.4. A conservative improvement of 10% of the point between groups was used to calculate the sample size. The significance level was set at 0.05, and the power was set at 0.80. The analysis revealed the smallest sample size of 40 participants in total (20 per group). The lost to follow-up rate was anticipated to be 10%, thus a final sample size of 44 participants (22 per group) was determined.
Numerical variables were shown as mean ± standard deviation (SD) for normal distributed data or median (Inter quartile range, IQR) for skewed distributed data. Baseline characteristics were compared using t-test or Wilcoxon rank sum test for numerical variables and chi-square test for categorical variables.
The primary and secondary outcomes were examined using mixed two-way repeated measures analyses of variance (ANOVA). For changes in BNT, CAL and WAB scores compared to baseline, treatment (real or sham rTMS) was the between-subjects factor and time (1, 3 and 6 months after treatment) was the within-subjects factor. For HAMA and HRSD scores, treatment (real or sham rTMS) was the between-subjects factor and time (baseline and 6 months after treatment) was the within-subjects factor. Furthermore, a mixed three-way repeated measures ANOVA was used to analyze whether the effects of rTMS differ across PPA variants, with treatment (real or sham rTMS) and PPA variants (nfvPPA, svPPA or lvPPA) as between-subjects factors, and time (same as the two-way repeated measures ANOVA) as a within-subjects factor.
Before the repeated measures ANOVAs were conducted, the normality of data was tested with a normal quantile plot (Q-Q plot). Mauchly’s test was used to test for sphericity and Levene’s test was used to test the homogeneity of variances. The Greenhouse–Geisser correction was used for nonspherical data to adjust the degree of freedom. P values were adjusted with the Bonferroni correction for multiple post hoc comparisons. Partial eta-squared (η2p) values were provided to demonstrate effect size. The significance level was set at a two-sided 0.05. Statistical analyses were performed using IBM SPSS (version 25.0).
Results
Baseline Characteristics
Between July 2020 and December 2021, 62 patients were assessed for eligibility, of whom 40 (16 with nonfluent, 12 with semantic and 12 with logopenic variant PPA) were randomly assigned to each group. Twenty patients received real rTMS and 20 patients received sham rTMS (Fig. 1). Of the 40 treated patients, 18 patients in the real rTMS group and 17 patients in the sham rTMS group completed the study of 6 months follow-up, with 5 (12.5%) dropped out. Three participants were lost to follow-up because they were unable to arrive at the hospital at the scheduled follow-up time. Meanwhile, two participants were no longer willing to participate during the follow-up (Fig. 1).
Baseline characteristics of patients are shown in Table 1. Median disease duration of all participants was 24.0 (12.0–36.0) months, and the sham rTMS group had a longer disease duration than real rTMS group [35.5 ± 27.9 vs 24.0 (6.8–24.0) months, p = 0.043]. Two treatment groups were generally well matched regarding other characteristics including gender, cholinesterase inhibitor usage, PPA variants and neuropsychological assessments. No significant differences were observed between the baseline characteristics of the patients who were lost to follow-up and those who did not (Table S1 and S2 provided as Online Resource). Among patients who completed the 6-month follow-up, there were no significant differences in baseline outcome variables between the real and sham rTMS group (Table 2), but the sham rTMS group had a longer duration of disease and higher MMSE and MoCA scores (Table S1 provided as Online Resource). One patient in the real rTMS group who finished the 6-month follow-up was left-handed and received rTMS delivered to the right DLPFC, while all other patients were right-handed. No patients were receiving any speech or language therapy during the study.
Primary outcome
The primary outcome was the change in the BNT score. The BNT scores and changes from baseline are shown in Table 2 and Fig. 3. For BNT improvement compared to baseline, since the data fitted with normal distributions and Levene’s test indicated homogenous variances, subsequent repeated measures ANOVA was conducted (Table 3). Mauchly’s test revealed that the assumption of sphericity was violated (p = 0.043), thus Greenhouse–Geisser correction was used to adjust the degree of freedom.
Language assessments. Box plots with all the data points shown. BNT Boston Naming Test, CAL Communicative Activity Log, WAB Western Aphasia Battery. *Significant main effect of treatment without significant interaction between treatment and time in repeated measures ANOVA. **Significant simple effect of treatment when a significant treatment-by-time interaction exists
There were significant main effects of treatment (F (1, 33) = 5.125, p = 0.030, η2p = 0.134) and time (F (1.70, 55.99) = 16.815, p < 0.001, η2p = 0.338). No significant interaction between treatment and time was found. Post hoc analysis showed that the average improvements of BNT in the real and sham rTMS group were 2.9 points (95% CI 2.1, 3.6) and 1.7 points (95% CI 0.9, 2.4), respectively. The improvement of BNT in the real rTMS group was 1.2 points (95% CI 0.1, 2.3, p = 0.030) higher than the sham rTMS group. This result indicated that real rTMS could provide significantly higher improvement in BNT scores than sham rTMS, and this effect was stable in 6 months after treatment due to the absence of significant interaction.
Secondary outcomes
The secondary outcomes are displayed in Table 2 and Fig. 3. These outcomes were all normally distributed for each combination of three factors according to Q–Q plots. Levene’s test demonstrated the equality of variance for all the outcomes. For outcomes including changes in CAL and WAB scores compared to baseline, Mauchly’s tests for sphericity were not significant (p = 0.189 and 0.263, respectively). For HAMA and HRSD, since the within-subjects factor was two-level (i.e., baseline and 6 months), Mauchly’s test could not be conducted. The results of two- way repeated measures ANOVA for every outcome are displayed as follows (Table 3).
CAL improvement
The repeated measures ANOVA yielded significant main effects of treatment (F (1, 33) = 4.545, p = 0.041, η2p = 0.121) and time (F (2, 66) = 24.243, p < 0.001, η2p = 0.424). Since there was a significant treatment × time interaction (F (2, 66) = 3.662, p = 0.031, η2p = 0.100), simple effects of treatment at each time point need to be analyzed. At 1 month and 3 months after treatment, CAL improvements of real rTMS were significantly higher than sham rTMS, the mean differences were 4.7 (95% CI 1.2, 8.1, p = 0.010) and 2.7 (0.6, 5.5, p = 0.033), after Bonferroni correction (Table 2). The results indicated that rTMS could significantly improve CAL score at 1 and 3 months after treatment, but this effect did not persist at 6 months.
WAB improvement
For WAB improvement, there were significant main effects of treatment (F (1, 33) = 4.861, p = 0.035, η2p = 0.128) and time (F (2, 66) = 19.165, p < 0.001, η2p = 0.367). There was no significant interaction between treatment and time (F (2, 66) = 0.285, p = 0.753, η2p = 0.009). Post hoc analysis showed that the average improvements of WAB in the real and sham rTMS group were 25.3 points (95% CI 22.3, 28.2) and 20.6 points (95% CI 17.6, 23.7), respectively. The improvement of WAB in the real rTMS group was 4.6 points (95% CI 0.4, 8.9, p = 0.035) higher than the sham rTMS group. The results suggested that rTMS could significantly improve WAB scores in PPA patients, and the effect was stable in 6 months after treatment due to the absence of significant interaction.
HAMA and HRSD
For HAMA, the main effect of time was significant (F (1, 33) = 32.856, p < 0.001, η2p = 0.499). However, the main effect of treatment and the interaction between time and treatment did not reach significance (Table 3). For HRSD, no significant main effect was found. The effect of treatment × time interaction was significant (F (1, 29) = 4.921, p = 0.027, η2p = 0.140). However, post hoc analysis showed that there were no significant differences between the sham and the real rTMS group at 6 months after treatment (p = 0.431).
The effect of PPA variants on outcomes
A mixed three-way repeated measures ANOVA of outcomes with PPA variant included as a between-subject factor was conducted (Table S3 provided as an Online Resource). The results showed that no main effect of the PPA variant and its interaction with other factors were significant. This indicated that the effect of rTMS on all the outcomes did not differ across different PPA variants.
Discussion
In this randomized double-blind sham-controlled trial, we found that high-frequency rTMS delivered to DLPFC improved language function in PPA patients. We used changes in BNT, CAL, and WAB scores to assess improvements in naming, quantity and quality of daily communication, and overall language function, respectively. The significant improvement of BNT and WAB scores could be observed throughout the entire 6-month follow-up, while the significant improvement of CAL scores could only be observed at 1 and 3 months after treatment. The effect of rTMS on all these outcomes did not differ across different PPA variants.
Our findings validate the results of several previous non-randomized controlled studies with relatively small sample sizes. Naming function is an important outcome assessment in the vast majority of rTMS studies for PPA. Several studies have shown that rTMS improves naming function, including action naming and object naming, mainly in nfvPPA and lvPPA patients (Cotelli et al. 2012; Bereau et al. 2016; Pytel et al. 2021; Margolis et al. 2019). In addition, we found that rTMS could also improve naming function in svPPA patients. Furthermore, the results of a previous RCT showed that rTMS improved clinical symptoms reported by PPA patients and caregivers (Pytel et al. 2021). Likewise, we found that rTMS improved CAL scores, which reflected caregiver-reported improvements in language function. The same study also reported improvements in neuropsychiatric symptoms including apathy and depression after rTMS treatment, and a tendency of improvement of anxiety (p = 0.05). However, our study also evaluated anxiety and depression by measuring HAMA and HRSD scores at baseline and 6 months but did not see significant improvements (Pytel et al. 2021). This may be explained because we did not monitor the scores of neuropsychiatric symptoms in the short term after treatment, and the improvement of neuropsychiatric symptoms by rTMS does not last for up to 6 months. This needs to be further investigated in future studies.
Another interesting point is that in our study, the sham rTMS group also showed improvements in including BNT, CAL and WAB (Table 2 and Fig. 2). We assume that this may be due to placebo effects or practice effects. Previous unblinded studies with small sample sizes of rTMS showed no placebo or practice effect for sham rTMS in PPA patients (Bereau et al. 2016; Cotelli et al. 2012). However, a recent randomized controlled trial showed that sham rTMS improved spontaneous speech, reading efficiency, and apathy in nearly half of the patients (Pytel et al. 2021). In addition, placebo effects or practice effects appear to be more common in studies of tDCS for PPA. In the study by Nissim et al., sham tDCS delivered to the left frontotemporal region improved WAB-Aphasia quotient (Nissim et al. 2022). Moreover, Zhao et.al found that sham tDCS delivered to the left frontal lobe also improved letter accuracy on trained items (Zhao et al. 2021). Both studies were similar to ours in that the same set of scales was used for assessment before and after NIBS, and showed a similar placebo effect or practice effect, indicating possible practicing effects when testing untrained items.
Previous studies of NIBS have had relatively short follow-up periods, including the recently published randomized controlled trials (Coemans et al. 2021; Themistocleous et al. 2021; Zhao et al. 2021; Nissim et al. 2022; Sheppard et al. 2022; Pytel et al. 2021). One strength of our study is that we proved long-term improvements in language function after rTMS treatment. Our results suggest that improvements in BNT and WAB scores are still observed 6 months after treatment, although the improvement is greater at 1 month and 3 months. The mechanism by which short-term treatment of NIBS produces long-term effects is still unclear. Experimental studies suggest that the mechanism of long-term effects may involve gene regulation, protein expression, morphological changes, modified network properties and glial function (Cirillo et al. 2017; Ciullo et al. 2021). Since no effective disease-modifying therapy is available for PPA, we speculate that multiple courses of tDCS or rTMS treatment are promising to improve symptoms and maintain language function in the long term. Therefore, further studies are needed to explore the duration of the efficacy of NIBS and its mechanism to design a multi-course treatment plan.
We demonstrated that high-frequency rTMS delivered to DLPFC could improve language function in 3 PPA variants. DLPFC is traditionally considered to be mainly associated with general executive control functions (Hertrich et al. 2021). However, studies of intracranial brain mapping and anatomy have shown that DLPFC is connected with both ventral and dorsal language pathways (Sarubbo et al. 2020, 2016, 2013). Hagoort’s “Memory, Unification, Control” model of language processing assigned DLPFC to the control component (Hagoort 2013). Thus, damage to DLPFC might affect the connection with these pathways related to language. Meanwhile, rTMS studies have proven that stimulation delivered to DPLFC can improve naming function and sentence processing in healthy people or Alzheimer’s disease patients (Klaus and Schutter 2018; Cotelli et al. 2011; Fertonani et al. 2010). Cotelli et al. demonstrated that high-frequency rTMS delivered to DLPFC could enhance action naming in nfvPPA, but no improvement in svPPA was observed (Cotelli et al. 2012). Subsequent studies have shown that rTMS delivered to DLPFC could also enhance language function in both nfvPPA and lvPPA patients (Trebbastoni et al. 2013; Margolis et al. 2019; Bereau et al. 2016). A recent randomized, double-blind trial of rTMS selected personalized targets for each patient and left DLPFC rTMS stimulation yielded greater efficacy than other sites in 5 out of 6 svPPA patients (Pytel et al. 2021). Thus, DLPFC rTMS has been proven to be effective in all 3 main PPA variants, which is similar to our study. Although the mechanism of rTMS delivered to DLPFC to improve the language function of PPA patients is not clear, a hypothesis has been proposed that DLPFC is also involved in PPA patients (Teichmann 2021). Therefore, DLPFC might be an important treatment target for PPA and deserved further investigation.
The following limitations should be considered. First, we did not apply a neuronavigation system to localize the stimulation site because of the limitation of the device. Considering that the efficacy of TMS may decline without neuronavigation (Nguyen et al. 2018), further studies using neuronavigation are needed to validate our results. Second, no functional MRI or nuclear medicine imagining was performed in our study. Previous studies have combined NIBS and imaging techniques to explore the mechanisms of NIBS and predictive factors of response to treatment (Coemans et al. 2021; Harris et al. 2019; Nissim et al. 2022; Bereau et al. 2016; Sheppard 2022). Third, we did not evaluate neuropsychiatric symptoms at 1 month and 3 months after treatment, such that neuropsychiatry symptoms improvement shortly after rTMS might be neglected. However, improvement in neuropsychiatric symptoms in short term has been demonstrated in other study (Pytel et al. 2021).
In conclusion, our study suggests that high-frequency rTMS delivered to DLPFC improves language functions in patients with nfvPPA, svPPA and lvPPA, and the efficacy was still observed after 6 months of treatment. Our study shows that the effects of rTMS could be sustained over a relatively long period of time, therefore, provides evidence for designing multiple courses of rTMS treatment for long-term improvement in patients with PPA. We look forward to future studies to provide more evidence on the efficacy and the duration of the treatment effect of rTMS on PPA patients. And hopefully, PPA patients would have access to management strategies of high-quality evidence and a better chance of favorable outcomes in the future.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Bereau M, Magnin E, Nicolier M, Berthet L, Dariel E, Ferreira S, Sylvestre G, Monnin J, Chopard G, Bouladour H, Vandel P, Haffen E (2016) Left prefrontal repetitive transcranial magnetic stimulation in a logopenic variant of primary progressive aphasia: a case report. Eur Neurol 76(1–2):12–18. https://doi.org/10.1159/000447399
Berthier ML, Green C, Lara JP, Higueras C, Barbancho MA, Dávila G, Pulvermüller F (2009) Memantine and constraint-induced aphasia therapy in chronic poststroke aphasia. Ann Neurol 65(5):577–585. https://doi.org/10.1002/ana.21597
Boes AD, Kelly MS, Trapp NT, Stern AP, Press DZ, Pascual-Leone A (2018) Noninvasive brain stimulation: challenges and opportunities for a new clinical specialty. J Neuropsychiatr Clin Neurosci 30(3):173–179. https://doi.org/10.1176/appi.neuropsych.17110262
Chen TB, Lin CY, Lin KN, Yeh YC, Chen WT, Wang KS, Wang PN (2014) Culture qualitatively but not quantitatively influences performance in the Boston naming test in a Chinese-speaking population. Dement Geriatr Cognit Disord Extra 4(1):86–94. https://doi.org/10.1159/000360695
Cheung RW, Cheung MC, Chan AS (2004) Confrontation naming in Chinese patients with left, right or bilateral brain damage. J Int Neuropsychol Soc 10(1):46–53. https://doi.org/10.1017/S1355617704101069
Cirillo G, Di Pino G, Capone F, Ranieri F, Florio L, Todisco V, Tedeschi G, Funke K, Di Lazzaro V (2017) Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul 10(1):1–18. https://doi.org/10.1016/j.brs.2016.11.009
Ciullo V, Spalletta G, Caltagirone C, Banaj N, Vecchio D, Piras F, Piras F (2021) Transcranial direct current stimulation and cognition in neuropsychiatric disorders: systematic review of the evidence and future directions. Neuroscientist 27(3):285–309. https://doi.org/10.1177/1073858420936167
Coemans S, Struys E, Vandenborre D, Wilssens I, Engelborghs S, Paquier P, Tsapkini K, Keulen S (2021) A Systematic review of transcranial direct current stimulation in primary progressive aphasia: methodological considerations. Front Aging Neurosci 13:710818. https://doi.org/10.3389/fnagi.2021.710818
Cotelli M, Calabria M, Manenti R, Rosini S, Zanetti O, Cappa SF, Miniussi C (2011) Improved language performance in Alzheimer disease following brain stimulation. J Neurol Neurosurg Psychiatr 82(7):794–797. https://doi.org/10.1136/jnnp.2009.197848
Cotelli M, Manenti R, Alberici A, Brambilla M, Cosseddu M, Zanetti O, Miozzo A, Padovani A, Miniussi C, Borroni B (2012) Prefrontal cortex rTMS enhances action naming in progressive non-fluent aphasia. Eur J Neurol 19(11):1404–1412. https://doi.org/10.1111/j.1468-1331.2012.03699.x
Fertonani A, Rosini S, Cotelli M, Rossini PM, Miniussi C (2010) Naming facilitation induced by transcranial direct current stimulation. Behav Brain Res 208(2):311–318. https://doi.org/10.1016/j.bbr.2009.10.030
Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M (2011) Classification of primary progressive aphasia and its variants. Neurology 76(11):1006–1014. https://doi.org/10.1212/WNL.0b013e31821103e6
Guan YZ, Li J, Zhang XW, Wu S, Du H, Cui LY, Zhang WH (2017) Effectiveness of repetitive transcranial magnetic stimulation (rTMS) after acute stroke: a one-year longitudinal randomized trial. CNS Neurosci Ther 23(12):940–946. https://doi.org/10.1111/cns.12762
Guo Q, Hong Z, Shi W, Sun Y, Lv C (2006) Boston naming test in Chinese Elderly patient with mild cognitive impairment and Alzheimer’s dementia. Chin Ment Health J 20(2):81–84
Hagoort P (2013) MUC (Memory, Unification, Control) and beyond. Front Psychol 4:416. https://doi.org/10.3389/fpsyg.2013.00416
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatr 23(1):56–62. https://doi.org/10.1136/jnnp.23.1.56
Hamilton M (1969) Diagnosis and ratings of anxiety. Br J Psychiatry 3:76–79
Haro-Martínez AM, Lubrini G, Madero-Jarabo R, Díez-Tejedor E, Fuentes B (2019) Melodic intonation therapy in post-stroke nonfluent aphasia: a randomized pilot trial. Clin Rehabil 33(1):44–53. https://doi.org/10.1177/0269215518791004
Harris AD, Wang Z, Ficek B, Webster K, Edden RA, Tsapkini K (2019) Reductions in GABA following a tDCS-language intervention for primary progressive aphasia. Neurobiol Aging 79:75–82. https://doi.org/10.1016/j.neurobiolaging.2019.03.011
Hertrich I, Dietrich S, Blum C, Ackermann H (2021) The role of the dorsolateral prefrontal cortex for speech and language processing. Front Hum Neurosci 15:645209. https://doi.org/10.3389/fnhum.2021.645209
Herwig U, Satrapi P, Schonfeldt-Lecuona C (2003) Using the international 10–20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr 16(2):95–99. https://doi.org/10.1023/b:brat.0000006333.93597.9d
Jansen JM, van den Heuvel OA, van der Werf YD, de Wit SJ, Veltman DJ, van den Brink W, Goudriaan AE (2019) The effect of high-frequency repetitive transcranial magnetic stimulation on emotion processing, reappraisal, and craving in alcohol use disorder patients and healthy controls: a functional magnetic resonance imaging study. Front Psychiatr 10:272. https://doi.org/10.3389/fpsyt.2019.00272
Jasper HH (1958) The ten-twenty electrode system of the International Federation. Electroencephalogr Clin Neurophysiol 10:370–375
Kaplan E, Goodglass H, Weintraub S (1983) The Boston naming test, 2nd edn. Lea & Febiger, Philadelphia
Kertesz A (2020) The western aphasia battery: a systematic review of research and clinical applications. Aphasiology 36(1):21–50. https://doi.org/10.1080/02687038.2020.1852002
Kertesz A, Poole E (1974) The aphasia quotient: the taxonomic approach to measurement of aphasic disability. Can J Neurol Sci 1(1):7–16
Khedr EM, Mohamed KO, Soliman RK, Hassan AMM, Rothwell JC (2019) the effect of high-frequency repetitive transcranial magnetic stimulation on advancing parkinson’s disease with dysphagia: double blind randomized clinical trial. Neurorehabil Neural Repair 33(6):442–452. https://doi.org/10.1177/1545968319847968
Klaus J, Schutter D (2018) The role of left dorsolateral prefrontal cortex in language processing. Neuroscience 377:197–205. https://doi.org/10.1016/j.neuroscience.2018.03.002
Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, Filipovic SR, Grefkes C, Hasan A, Hummel FC, Jaaskelainen SK, Langguth B, Leocani L, Londero A, Nardone R, Nguyen JP, Nyffeler T, Oliveira-Maia AJ, Oliviero A, Padberg F, Palm U, Paulus W, Poulet E, Quartarone A, Rachid F, Rektorova I, Rossi S, Sahlsten H, Schecklmann M, Szekely D, Ziemann U (2020) Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin Neurophysiol 131(2):474–528. https://doi.org/10.1016/j.clinph.2019.11.002
Li H, Jia J, Yang Z (2016) Mini-mental state examination in elderly Chinese: a population-based normative study. J Alzheimer Dis 53(2):487–496. https://doi.org/10.3233/JAD-160119
Li Y, Luo H, Yu Q, Yin L, Li K, Li Y, Fu J (2020) Cerebral functional manipulation of repetitive transcranial magnetic stimulation in cognitive impairment patients after stroke: an fMRI study. Front Neurol 11:977. https://doi.org/10.3389/fneur.2020.00977
Li Y, Qiao Y, Wang F, Wei C, Wang R, Jin H, Xie B, You J, Jia J, Zhou A (2022) Culture effects on the Chinese version boston naming test performance and the normative data in the native Chinese-speaking elders in mainland China. Front Neurol 13:866261. https://doi.org/10.3389/fneur.2022.866261
Liu L, Luo XG, Dy CL, Ren Y, Feng Y, Yu HM, Shang H, He ZY (2015a) Characteristics of language impairment in Parkinson’s disease and its influencing factors. Transl Neurodegener 4(1):2. https://doi.org/10.1186/2047-9158-4-2
Liu S, Shi Z, Cai L, Liu S, Lu H, Han T, Wang Y, Zhou Y, Gao S, Ji Y (2015b) Clinical and neuroimaging characteristics of patients with primary progressive aphasia. Chin J Neurol 48(8):681–686. https://doi.org/10.3760/cma.j.issn.1006-7876.2015.08.011
Lu J, Li D, Li F, Zhou A, Wang F, Zuo X, Jia XF, Song H, Jia J (2011) Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: a population-based study. J Geriatr Psychiatry Neurol 24(4):184–190. https://doi.org/10.1177/0891988711422528
Ma J, Zhang Y, Guo Q, Hua X, Zheng M, Wu J, Xu J (2022) Correlation of naming function and other cognitive functions in patients with vascular cognitive impairment-no dementia. China J Tradit Chin Med Pharm 37(5):2702–2706
Margolis SA, Festa EK, Papandonatos GD, Korthauer LE, Gonsalves MA, Oberman L, Heindel WC, Ott BR (2019) A pilot study of repetitive transcranial magnetic stimulation in primary progressive aphasia. Brain Stimul 12(5):1340–1342. https://doi.org/10.1016/j.brs.2019.06.001
Marshall CR, Hardy CJD, Volkmer A, Russell LL, Bond RL, Fletcher PD, Clark CN, Mummery CJ, Schott JM, Rossor MN, Fox NC, Crutch SJ, Rohrer JD, Warren JD (2018) Primary progressive aphasia: a clinical approach. J Neurol 265(6):1474–1490. https://doi.org/10.1007/s00415-018-8762-6
Montembeault M, Brambati SM, Gorno-Tempini ML, Migliaccio R (2018) Clinical, anatomical, and pathological features in the three variants of primary progressive aphasia: a review. Front Neurol 9:692. https://doi.org/10.3389/fneur.2018.00692
Neri F, Romanella SM, TomaiPitinca ML, Taddei S, Monti L, Benocci S, Santarnecchi E, Cappa SF, Rossi S (2021) rTMS-induced language improvement and brain connectivity changes in logopenic/phonological variant of Primary progressive aphasia. Clin Neurophysiol 132(10):2481–2484. https://doi.org/10.1016/j.clinph.2021.07.017
Nguyen JP, Suarez A, Le Saout E, Meignier M, Nizard J, Lefaucheur JP (2018) Combining cognitive training and multi-site rTMS to improve cognitive functions in Alzheimer’s disease. Brain Stimul 11(3):651–652. https://doi.org/10.1016/j.brs.2018.02.013
Nissim NR, Moberg PJ, Hamilton RH (2020) Efficacy of noninvasive brain stimulation (tDCS or TMS) paired with language therapy in the treatment of primary progressive aphasia: an exploratory meta-analysis. Brain Sci 10(9):597. https://doi.org/10.3390/brainsci10090597
Nissim NR, Harvey DY, Haslam C, Friedman L, Bharne P, Litz G, Phillips JS, Cousins KAQ, Xie SX, Grossman M, Hamilton RH (2022) Through thick and thin: baseline cortical volume and thickness predict performance and response to transcranial direct current stimulation in primary progressive aphasia. Front Hum Neurosci 16:907425. https://doi.org/10.3389/fnhum.2022.907425
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9(1):97–113. https://doi.org/10.1016/0028-3932(71)90067-4
Pagnoni I, Gobbi E, Premi E, Borroni B, Binetti G, Cotelli M, Manenti R (2021) Language training for oral and written naming impairment in primary progressive aphasia: a review. Transl Neurodegener 10(1):24. https://doi.org/10.1186/s40035-021-00248-z
Pulvermuller F, Neininger B, Elbert T, Mohr B, Rockstroh B, Koebbel P, Taub E (2001) Constraint-induced therapy of chronic aphasia after stroke. Stroke 32(7):1621–1626. https://doi.org/10.1161/01.str.32.7.1621
Pytel V, Cabrera-Martín MN, Delgado-Álvarez A, Ayala JL, Balugo P, Delgado-Alonso C, Yus M, Carreras MT, Carreras JL, Matías-Guiu J, Matías-Guiu JA (2021) Personalized repetitive transcranial magnetic stimulation for primary progressive aphasia. J Alzheimer Dis 84(1):151–167. https://doi.org/10.3233/jad-210566
Rajji TK (2021) Noninvasive brain stimulation for the treatment of neurocognitive disorders: right for prime time? Curr Opin Psychiatry 34(2):129–135. https://doi.org/10.1097/YCO.0000000000000686
Ren C, Zhang G, Xu X, Hao J, Fang H, Chen P, Li Z, Ji Y, Cai Q, Gao F (2019) The Effect of rTMS over the different targets on language recovery in stroke patients with global aphasia: a randomized sham-controlled study. Biomed Res Int 2019:4589056. https://doi.org/10.1155/2019/4589056
Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of TMSCG (2009) Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120(12):2008–2039. https://doi.org/10.1016/j.clinph.2009.08.016
Rossi S, Antal A, Bestmann S, Bikson M, Brewer C, Brockmoller J, Carpenter LL, Cincotta M, Chen R, Daskalakis JD, Di Lazzaro V, Fox MD, George MS, Gilbert D, Kimiskidis VK, Koch G, Ilmoniemi RJ, Lefaucheur JP, Leocani L, Lisanby SH, Miniussi C, Padberg F, Pascual-Leone A, Paulus W, Peterchev AV, Quartarone A, Rotenberg A, Rothwell J, Rossini PM, Santarnecchi E, Shafi MM, Siebner HR, Ugawa Y, Wassermann EM, Zangen A, Ziemann U, Hallett M, Basis of this article began with a Consensus Statement from the Ifcn Workshop on "Present FoTMSSEGSOutA (2021) Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin Neurophysiol 132(1):269–306. https://doi.org/10.1016/j.clinph.2020.10.003
Salmon DP, Jin H, Zhang M, Grant I, Yu E (1995) Neuropsychological assessment of chinese elderly in the Shanghai dementia survey. Clin Neuropsychol 9(2):159–168. https://doi.org/10.1080/13854049508401598
Sarubbo S, De Benedictis A, Maldonado IL, Basso G, Duffau H (2013) Frontal terminations for the inferior fronto-occipital fascicle: anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain Struct Funct 218(1):21–37. https://doi.org/10.1007/s00429-011-0372-3
Sarubbo S, De Benedictis A, Merler S, Mandonnet E, Barbareschi M, Dallabona M, Chioffi F, Duffau H (2016) Structural and functional integration between dorsal and ventral language streams as revealed by blunt dissection and direct electrical stimulation. Hum Brain Mapp 37(11):3858–3872. https://doi.org/10.1002/hbm.23281
Sarubbo S, Tate M, De Benedictis A, Merler S, Moritz-Gasser S, Herbet G, Duffau H (2020) Mapping critical cortical hubs and white matter pathways by direct electrical stimulation: an original functional atlas of the human brain. Neuroimage 205:116237. https://doi.org/10.1016/j.neuroimage.2019.116237
Sheppard SM (2022) Noninvasive brain stimulation to augment language therapy for primary progressive aphasia. Handb Clin Neurol 185:251–260. https://doi.org/10.1016/b978-0-12-823384-9.00018-9
Sheppard SM, Goldberg EB, Sebastian R, Walker A, Meier EL, Hillis AE (2022) Transcranial direct current stimulation paired with verb network strengthening treatment improves verb naming in primary progressive aphasia: a case series. Am J Speech Lang Pathol 31(4):1736–1754. https://doi.org/10.1044/2022_ajslp-21-00272
Shi Y, Song R, Wang Z, Zhang H, Zhu J, Yue Y, Zhao Y, Zhang Z (2021) Potential clinical value of circular RNAs as peripheral biomarkers for the diagnosis and treatment of major depressive disorder. EBioMedicine 66:103337. https://doi.org/10.1016/j.ebiom.2021.103337
Siebner HR, Funke K, Aberra AS, Antal A, Bestmann S, Chen R, Classen J, Davare M, Di Lazzaro V, Fox PT, Hallett M, Karabanov AN, Kesselheim J, Beck MM, Koch G, Liebetanz D, Meunier S, Miniussi C, Paulus W, Peterchev AV, Popa T, Ridding MC, Thielscher A, Ziemann U, Rothwell JC, Ugawa Y (2022) Transcranial magnetic stimulation of the brain: what is stimulated? A consensus and critical position paper. Clin Neurophysiol 140:59–97. https://doi.org/10.1016/j.clinph.2022.04.022
Taylor-Rubin C, Croot K, Nickels L (2021) Speech and language therapy in primary progressive aphasia: a critical review of current practice. Expert Rev Neurother 21(4):419–430. https://doi.org/10.1080/14737175.2021.1897253
Teichmann M (2021) The current international consensus criteria can lead to under and over-diagnosis of primary progressive aphasia variants. Revue Neurologique 177(4):370–375. https://doi.org/10.1016/j.neurol.2020.12.001
Themistocleous C, Webster K, Tsapkini K (2021) Effects of tDCS on sound duration in patients with apraxia of speech in primary progressive aphasia. Brain Sci 11(3):335. https://doi.org/10.3390/brainsci11030335
Tian J, Xie H, Wang L, Wang Y, Wang H, Shi J, Qin B, Fan D, Ni J, Sun Y, (ADC) tGPoAsDC (2020) Chinese guideline for the diagnosis and treatment of Alzheimer’s disease dementia (2020). Chin J Geriatr 40(3):269–283. https://doi.org/10.3760/cma.j.issn.0254-9026.2021.03.001
Trebbastoni A, Raccah R, de Lena C, Zangen A, Inghilleri M (2013) Repetitive deep transcranial magnetic stimulation improves verbal fluency and written language in a patient with primary progressive aphasia-logopenic variant (LPPA). Brain Stimul 6(4):545–553. https://doi.org/10.1016/j.brs.2012.09.014
Vandenberghe R (2016) Classification of the primary progressive aphasias: principles and review of progress since 2011. Alzheimer Res Ther 8(1):16. https://doi.org/10.1186/s13195-016-0185-y
Wang YH (1997) The introduction of western aphasia battery (WAB). Zhongguo Kangfu Lilun Yu Shijian Ia:87–89
Xie Y, Liu H, Wu C, Li X, Zheng P, Chen H, Li X (2014) Effect of constraint-induced aphasia therapy on chronic aphasia after stroke. Chin J Rehabilitat Theory Pract 20(11):1011–1013. https://doi.org/10.3969/j.issn.1006-9771.2014.11.004
Yu ZZ, Jiang SJ, Bi S, Li J, Lei D, Sun LL (2013) Relationship between linguistic functions and cognitive functions in a clinical study of Chinese patients with post-stroke aphasia. Chinese Med J-Peking 126(7):1252–1256. https://doi.org/10.3760/cma.j.issn.0366-6999.20121463
Zhang B, Chang J, Park J, Tan Z, Tang L, Lyu T, Han Y, Fan R, Gao Y, Kong J (2021) Uncinate fasciculus and its cortical terminals in aphasia after subcortical stroke: a multi-modal MRI study. NeuroImage Clin 30:102597. https://doi.org/10.1016/j.nicl.2021.102597
Zhao D, Li Y, Liu T, Voon V, Yuan TF (2020) Twice-daily theta burst stimulation of the dorsolateral prefrontal cortex reduces methamphetamine craving: a pilot study. Front Neurosci 14:208. https://doi.org/10.3389/fnins.2020.00208
Zhao Y, Ficek B, Webster K, Frangakis C, Caffo B, Hillis AE, Faria A, Tsapkini K (2021) White matter integrity predicts electrical stimulation (tDCS) and language therapy effects in primary progressive aphasia. Neurorehabil Neural Repair 35(1):44–57. https://doi.org/10.1177/1545968320971741
Zhou Q, Cong F, Shen Y, Yin Z, Zhang W, Ye Q, Chen W, Shan C (2014) Effectiveness of constraint induced language therapy combined with low frequency repetitive transcranial magnetic stimulation for non-fluent aphasia. Chin J Rehabilitat 29(5):325–327. https://doi.org/10.3870/zgkf.2014.05.001
Funding
This work was supported by the Non-profit Central Research Institute Fund of the Chinese Academy of Medical Sciences (2019XK320039) and National High-Level Hospital Clinical Research Funding (2022-PUMCH-B-017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
Approval was obtained from the Ethics Committee of Clinical Research of Peking Union Medical College Hospital (Beijing, China). The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Informed consent
Written informed consent was obtained from every participant.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Y., Tan, Y., Hao, H. et al. Treatment of primary progressive aphasia by repetitive transcranial magnetic stimulation: a randomized, double-blind, placebo-controlled study. J Neural Transm 130, 111–123 (2023). https://doi.org/10.1007/s00702-023-02594-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-023-02594-w