Abstract
The anatomy and functional role of the inferior fronto-occipital fascicle (IFOF) remain poorly known. We accurately analyze its course and the anatomical distribution of its frontal terminations. We propose a classification of the IFOF in different subcomponents. Ten hemispheres (5 left, 5 right) were dissected with Klingler’s technique. In addition to the IFOF dissection, we performed a 4-T diffusion tensor imaging study on a single healthy subject. We identified two layers of IFOF. The first one is superficial and antero-superiorly directed, terminating in the inferior frontal gyrus. The second is deeper and consists of three portions: posterior, middle and anterior. The posterior component terminates in the middle frontal gyrus (MFG) and dorso-lateral prefrontal cortex. The middle component terminates in the MFG and lateral orbito-frontal cortex. The anterior one is directed to the orbito-frontal cortex and frontal pole. In vivo tractography study confirmed these anatomical findings. We suggest that the distribution of IFOF fibers within the frontal lobe corresponds to a fine functional segmentation. IFOF can be considered as a “multi-function” bundle, with each anatomical subcomponent subserving different brain processing. The superficial layer and the posterior component of the deep layer, which connects the occipital extrastriate, temporo-basal and inferior frontal cortices, might subserve semantic processing. The middle component of the deep layer could play a role in a multimodal sensory–motor integration. Finally, the anterior component of the deep layer might be involved in emotional and behavioral aspects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The inferior fronto-occipital fascicle (IFOF) is the longest associative bundle connecting occipital cortex, temporo-basal areas and superior parietal lobule to the frontal lobe (Martino et al. 2009). Although the IFOF was described for the first time in the last century by Dejerine (1895) and Curran (1909), its anatomical existence has been debated (Schmahmann and Pandya 2007).
Recent advances in diffusion tensor imaging (DTI) technique, allowing “in vivo” dissection of the human brain white matter (Catani et al. 2002), and more recent anatomical works (Martino et al. 2009) demonstrated its existence and its posterior origin from dorsal parieto-occipital and basal temporo-occipital areas. Nonetheless, although connections with the dorsal premotor and prefrontal cortex have been suggested, the exact anatomo-functional organization of the anterior terminations of the IFOF needs further refinements and investigations (Martino et al. 2009).
In the present work, we provided for the first time to our knowledge, an extensive and detailed anatomical description of the IFOF course from the temporal stem to the frontal cortical terminations. To this end, we performed the fibers dissection according to the Klingler’s (1935) technique in ten post-mortem human hemispheres. We also verified whether anatomical dissection results could be replicated “in vivo” by performing a preliminary DTI study on a single subject using a 4-T MR scanner. On the basis of this original map of the anterior cortical terminations of the IFOF in the frontal lobe, we propose a new anatomical classification of the IFOF components.
Moreover, from a functional point of view, there is still an ongoing debate about the exact role of the IFOF. The most recent evidences provided by functional MRI and intraoperative cortico-subcortical stimulation, eliciting semantic paraphasias all along the course of the IFOF (namely occipito-temporal, sub-insular or frontal part), suggested a role of this bundle in language networks and particularly in semantic processing (Bookheimer 2002; Duffau et al. 2005; Duffau 2008). Furthermore, Plaza et al. (2008) evoked error of judgment during the stimulation of the anterior part of the bundle, above the Broca’s area, supporting the involvement of the distal frontal portion of IFOF near the dorso-lateral prefrontal cortex (DLPFC) in the semantic network. In addition, IFOF seems also to play a role in the awareness and elaboration of visual information for motor planning (Rizzolatti and Matelli 2003) and to be implicated in reading and attention (Catani and Mesulam 2008; Dorrichi et al. 2008; Epelbaum et al. 2008; Fox et al. 2008; Rudrauff et al. 2008).
In lesion studies, IFOF damage has been demonstrated to take part in the pathogenesis of various diseases. It plays a role, for instance, in reading problems of children together with injury of the inferior longitudinal fasciculus (Rollins et al. 2009), as well as in deficit of the combination of affective response with early visual information—generating impairment of recognition of the facial expression of emotion (Philippi et al. 2009). In addition, its disorganization has also been suggested to be a part of the pathological background of obsessive–compulsive disorder and psychotic symptoms (Garibotto et al. 2009; Jacobson et al. 2010).
Based on our results and taking into account the functional role of the cortical sites of terminations of this bundle within the frontal lobe, we propose an original classification of the IFOF fibers in functional subcomponents. The integration of our anatomical dissection data, confirmed by DTI, and discussed in the lights of the most recent evidences from functional neuroimaging as well as from cortico-subcortical studies during awake surgery led us to hypothesize the IFOF as a “multi-task bundle”, with distinct functional subcomponents connecting parallel and distributed subnetworks.
Materials and methods
Anatomical dissection
We fixed ten hemispheres from five fresh specimens in 10% formalin solution for at least 40 days. The hemispheres were frozen at −15°C for 15 days, after removal of vessels, arachnoid and pia-matter. After the anatomical study of the cortical surface, the dissection was performed from the lateral to medial aspect by means of wooden spatulas, according to the Klingler’s (1935) technique. Firstly, we removed the gray matter in the sulci, demonstrating the short U-fibers, following an original method recently reported by Martino et al. (2009). Whenever possible, we left the gray matter just on the top of the gyri to facilitate the identification of the terminal fibers. In all cases, we firstly removed the middle temporal gyrus (MTG), starting from temporal pole and proceeding back, to demonstrate the temporal terminations of arcuate fasciculus (AF). Thereafter, we exposed the indirect and direct (AF) components of superior longitudinal fasciculus (SLF), leaving in situ the dorsal two-third of central lobule and demonstrating the anterior AF terminations in the inferior frontal gyrus (IFG) and middle frontal gyrus (MFG). After removal of insular cortex, exposing the insulo-opercular and claustro-opercular fibers from the extreme and external capsule, we exposed the IFOF stem (under the limen insulae and then entering the frontal lobe) and followed the fibers until their anterior cortical terminations. We cut the IFG, opening two windows of 1.5 cm to follow the deeper fibers: the first one between the pars opercularis and triangularis, to study the possible terminations in DLFPC and MFG; the second one between the pars triangularis and orbitalis to study the existence of most anterior terminations—frontal pole, lateral orbito-frontal and basal orbito-frontal cortices (OFC). Finally, we used metallic forceps for the delicate dissection of thinnest bundles and terminations, putting colored tags (green for IFOF and blue for AF) under these two pathways, to show the fibers direction and crossing. Sequential pictures were taken during the dissection.
Brains were obtained from the Montpellier University of Medicine Anatomy Laboratory. All brains were normal.
Diffusion tensor imaging
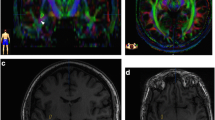
Diffusion-weighted imaging tractography was performed on a 43-year-old right-handed male using a 4-T MR scanner (MedSpec, Bruker). A eight-channel head coil was used both for transmitting and receiving radio frequency. A multislice spin-echo echo-planar sequence with phase correction scans (matrix acquisition: 64 × 64; field of view: 128 × 128 with phase partial fourier 7/8; slice thickness: 2 mm; TR: 7,900 ms; TE: 94 ms; flip angle: 90; bandwidth: 1,565; parallel acquisition acceleration type: GRAPPA; acceleration factor: 2) was used to acquire 50 semi-axial slices positioned parallel to the anterior–posterior commissure plane and covering the entire supratentorial part of the brain. The diffusion preparation was performed using dual bipolar diffusion gradient and a double spin echo, which provides robustness against eddy current artifacts. Each slice was sampled 35 times: 5 times without any diffusion gradient (B0 images) and 30 times applying diffusion-encoding gradients in 30 isotropically distributed orientations, with a diffusion weighting of 1,000 s/mm2 at a maximum strength of 40 mT/m with a rising speed of 200 T/m/s. No cardiac gating was applied so that the total scanning time was 4 min and 36 s. The sequence produced a total of 1,750 images that were combined into 5 B0 and 30 diffusion-weighted volumes, one for each diffusion gradient direction.
A T1-weighted 3D volume with a 1 mm isotropic resolution (MPRAGE; field of view: 256 × 224; TR: 2,700 ms; TE: 4.18 ms; TI: 1,020 ms; flip angle: 7°; acquisition acceleration type: GRAPPA; acceleration factor: 2) was acquired for subsequent fine anatomical region of interest (ROI) localization.
The eddycorrect routine of FMRIB’s Diffusion Toolbox (FDT: http://www.fmrib.ox.ac.uk/analysis/research/fdt) embedded in the FMRIB’s Software Library (FSL: Smith et al. 2004) was used to perform eddy current correction and realignment of diffusion-weighted data using the mean of the B0 volumes as a reference. Diffusion tensor calculation and tracking were performed with Diffusion Toolkit 0.6.1 and TrackVis 0.5.1 (http://trackvis.org; R. Wang and V.J. Wedeen, Athinoula A. Martinos, Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Boston, USA). For tracking we selected the interpolated streamline algorithm, excluding all voxels with a fractional anisotropy value below 0.05 and setting the angle threshold to 45. The T1-weighted volume was coregistered to the fractional anisotropy map obtained after diffusion tensor calculation. The left IFOF was then isolated selecting all streamlines passing through two regions of interest localized in the occipital lobe and in the external/extreme capsule of the left hemisphere as defined by Catani and Thiebaut de Schotten (2008) and excluding all fibers crossing the midline or passing through the temporal pole. To allow a precise definition of the ROIs, the coregistered T1-weighted 3D volume was spatially normalized to the MNI space. Then, the two ROIs for the identification of the IFOF were drawn based on the MNI coordinates provided in the atlas developed by Catani and Thiebaut de Schotten (2008). The ROIs were transformed back to the original anatomical space of the subject applying the inverted spatial transformation used to perform spatial normalization of the T1-weighted volume. The transformed ROIs were then used to perform streamline dissection on the original data. This procedure was adopted to minimize any artifact due to manipulation of original diffusion-weighted data. The superficial portion of the IFOF was further virtually dissected adding a ROI covering the gray and white matter of the triangular and opercular portion the IFG and selecting only those fibers of the previously defined IFOF passing through this ROI. The deep portion of the IFOF was defined subtracting this last group of streamlines from the whole IFOF as previously defined. The deep portion of the IFOF was subsequently subdivided into three portions based on the projection of streamlines to the dorsal frontal gyrus, the MFG and the orbital frontal gyrus.
Rendering of the streamlines was performed using the “tube” render option of TrackVis with a radius of 0.25 and ten sides.
Results
Anatomical dissection
First step: cortical and arcuate fasciculus dissection
In a first step, we performed a careful analysis of the cortical surface to identify any possible anatomical difference among specimens and to draw the anatomical landmarks of our dissection. Especially, we left the dorsal two-thirds of central lobule “in situ” to have a clearer vision, up to the last phases of dissection, of the reciprocal anatomical relationships of the ventral premotor cortex (VPMC), DLPFC and MFG, showing the bundles terminations. Moreover, we left the cortex on the top of the DLPFC, MFG and lateral OFC to obtain a more complete magnification of the terminal fibers.
In all cases, we started by the removal of the MTG exposing the temporal terminations of the C-shaped AF in the middle and superior temporal gyrus (STG) (Fig. 1). When the dissection came to the inferior parietal lobule, we identified the indirect component of SLF (Fig. 2). These long-association fibers run from Geschwind’s territories (inferior parietal lobule) to the IFG and VPMC. The indirect component of SLF is lateral to the AF. Thus, removing it, we demonstrated the complete C-shaped course of the AF (Fig. 3). Underneath the supramarginal gyrus, the fibers from AF turn forward and enter the frontal lobe, terminating in the IFG and MFG.
We exposed the terminations of the C-shaped arcuate fasciculus (AF) in the temporal lobe (red circles) and the terminations of the more superficial indirect posterior component of the superior longitudinal fasciculus (SLF) in the inferior parietal lobule (blue circle) and in the temporal lobe—before reaching the deeper fibers of the inferior fronto-occipital fasciculus (IFOF) above the roof of the temporal horn, which enter in the temporal stem (as described by Martino et al. 2009). (Left hemisphere; a anterior; i inferior; p posterior; s superior)
We removed the inferior parietal lobule (AG and SMG), the posterior-third of the MTG (part of Wernicke’s Area) and the ventral third of the central and post-central gyri to show the course of the indirect posterior (parieto-temporal, from Geshwind to Wernicke’s territories) component of the SLF (red arrow) and the course of the direct anterior (fronto-parietal, from IFG to Geschwind’s territories) of the SLF (blue arrrow). (Right hemisphere; a anterior; i inferior; p posterior; s superior)
The stem of the indirect anterior component (parieto-frontal, from Geschwind to Broca’s territories; blue arrow) of SLF and its terminations in the inferior frontal (IFG; blue circle) and middle frontal gyri (MFG, red circle) are demonstrated. These fibers are separated from the deeper and C-shaped AF fibers (arching between the Broca’s territories-MFG and Wernicke’s territories) by a red tag. (Left hemisphere; a anterior; i inferior; p posterior; s superior)
Once the SLF/AF dissection had been completed, we removed the STG demonstrating the fibers entering in the temporal stem and we lifted the frontal opercula to expose completely the insular cortex.
We removed the insular gray matter by starting from the arched ridge of the limen insulae. Under it, we found the thick uncinate fasciculus (UF), and more posteriorly and laterally the stem of the IFOF, at the level of the crossing between external capsule and ventral claustrum. Proceeding posteriorly, we removed the cortex of the prominent insular apex, the insular long gyri, the insular central sulcus and the posterior short gyri, revealing the insulo-opercular and claustro-opercular short fibers of extreme, external capsule and dorsal claustrum (postero-superior portion). The gray matter removal continued in the antero-superior direction from the apex revealing the diffuse and islands-like gray matter of ventral claustrum (VC) within the fibers of UF and IFOF narrow and compact stem. Finally, we obtained two triangular-shaped areas. The apex of the posterior one is antero-inferiorly directed and contains (from lateral to medial): the insulo and claustro-opercular fibers, the dorsal claustrum and the dorsal portion of the external capsule (triangle 1; Fig. 4a, b). The apex of the anterior triangular area is postero-inferiorly oriented and contains (from lateral to medial): the VC, UF and IFOF in the ventral portion of external capsule (separating the VC from the putamen) (triangle 2; Fig. 4c).
a After removal of the gray matter of the insular apex and long gyri, central sulcus and short gyri of the insula, we exposed the insulo-opercular and claustro-opercular short fibers of extreme, external capsule and dorsal claustrum (postero-superior portion). We divided this region in two triangles. The posterior one (triangle 1) is antero-inferiorly oriented. The insulo and claustro-opercular fibers, the dorsal claustrum and the dorsal portion of the external capsule are detectable, from lateral to medial, in the triangle 1. The anterior one (triangle 2) is postero-inferiorly oriented and contains (from lateral to medial): the VC, UF and IFOF in the ventral portion of external capsule (EC). b and c The ventral portion of EC is constituted by islands of gray matter (red circles) in the context of the fibers of UF and IFOF and separated the VC from the Putamen. (Left hemisphere; a anterior; i inferior; s superior)
Second step: IFOF dissection from insula to the frontal lobe
In the second step of dissection, we focused on the anterior triangle, particularly on the IFOF, dissecting its fibers in a temporo-frontal direction (from back to front-up). The fibers coming from the temporal radiation of IFOF were previously described upon the roof of the temporal horn (Martino et al. 2009) in a compact stem at the level of the ventral portion of external capsule/claustrum, postero-superior to the UF. As mentioned, the ventral portion of the external capsule/claustrum (anterior triangle) is occupied by the fibers from UF (superficially and ventrally) and from IFOF (deeply and dorsally). Considering the fibers direction, we removed the portion of UF fibers (turning anteriorly and inferiorly to the temporal pole) and we selected, by means of green tags, the narrow and compact stem of the IFOF at the antero-inferior apex of the triangle. The IFOF constitutes, in all specimens, the posterior two-thirds of the ventral external capsule and widens going upward to the frontal lobe, creating a fan-shaped 60° radiation (Fig. 5).
The IFOF fibers constitute the posterior 2/3 of the ventral portion of the external capsule. These fibers enlarge, from the narrow stem at the apex of the anterior triangle 2 (Fig. 4a), in a fun-shaped 60° radiation directed superiorly to the frontal lobe, evidenced in this picture by green tags. The lower tag separates the IFOF stem from the dorsal claustrum and putamen. The other green tags emphasize the IFOF fibers, particularly in this picture the deeper portion (red arrows), until their terminations in the frontal lobe passing under the IFG and inferior frontal sulcus (IFS) to reach the dorso-lateral prefrontal cortex (DLPFC; red circle) and MFG cortices (blue circle). The blue tag evidence the transversal crossing of the AF stem at the level of IFG-inferior frontal sulcus. (Left hemisphere; a anterior; i inferior; s superior)
The fibers constituting this radiation are extremely flattened in comparison to the compact and narrow stem sited at the antero-inferior apex. At the ventral portion of the external capsule, where the fibers are easier to be isolated, we identified two layers of IFOF from the stem and at the fan-shaped radiation: the superficial and the deep layers. The former is characterized by the diffuse and islands-like gray matter of the ventral claustrum; the latter is occupying the ventral portion of the external capsule.
The superficial layer is the most delicate portion of fibers and requires a very careful and fine dissection. It is mainly directed upward and anteriorly to the IFG, with only a small portion of fibers posteriorly oriented (Fig. 4a). These fibers arch medio-laterally at the level of the superior limiting sulcus of the insula, reaching the cortices of the pars triangularis and orbitalis (Fig. 6a, b). In the IFG, these terminations are overlapped by the SLF terminations coming from a horizontal opposed direction. The IFOF terminations, in fact, are directed superiorly and arch medio-laterally, turning slightly down and terminating in the cortex of the IFG (Fig. 6c). Instead, the SLF stem has a transversal postero-anterior course at the level of MFG/inferior frontal sulcus; its terminations branch out in a medio-lateral direction, lying on the IFOF ones and running to the IFG cortex (Fig. 7).
a We demonstrated two layers of IFOF fibers. These are detachable from the stem (green tag) and all along the course of the IFOF radiation (red arrows). The superficial fibers are the most delicate and arch medio-laterally at the level of the superior limiting sulcus of the insula, terminating in the cortices of the IFG (pars opercularis, triangularis and orbitalis, red circle). In these pictures the posterior-third of the IFG has been removed to create a window on the course of deep layer of IFOF fibers. b Thus, we showed the terminations of the more superficial fibers in the cortex of triangular part of IFG and, just posteriorly, the course of the deeper layer under the opercular cortex of the IFG directed to the DLPFC and MFG cortices (red arrows). c The stem of the AF has been cut here (blue circle), at the level of the posterior-third of the IFG, and marked with a blue tag showing the opposite direction of IFOF (red arrows) and AF fibers and their crossing, at the level of inferior frontal sulcus. (Left hemisphere; a anterior; i inferior; s superior)
The IFOF and AF fibers are characterized by a considerable degree of overlap within the frontal region. To show the relations between these two fascicles, we opened a “cortical window” at the level of the middle-posterior third of IFG and MFG. In this picture the IFOF deep fibers, pointed out by green tags, are directed to the MFG with an infero-superior and latero-medial course (red arrows). These fibers are overlapped by the AF fibers (above the blue tags), which have a transversal postero-anterior and more superficial course (blue arrow). (Left hemisphere; a anterior; i inferior; p posterior; s superior)
The fibers of deep layer are identifiable only after complete removal of the superficial layer and of the frontal opercula. Contrary to the superficial layer, we observed that the deep portion of IFOF does not terminate in the IFG but runs superiorly, underneath the IFG. Therefore, in all specimens, we removed progressively a small portion of IFG (approximately 2 cm by step) opening a “window” on the white matter underneath IFG and inferior frontal sulcus (Fig. 6a). We started in all cases by removing the junction between pars opercularis and the VPMC. After cortex and distal terminations removal, we found the compact and thick stem of AF, running in antero-posterior direction. By cutting the stem of AF and opening a deeper “window” (Figs. 6c, 7), we found transversal and deeper fibers, coming from the deep layer of the IFOF fan-radiation and proceeding in infero-superior direction (as showed by the tags in Fig. 7). This portion of IFOF is flattened and the fibers are particularly long and delicate. After the removal of the whole IFG, step by step, we completely analyzed the distribution and the course of the deeper fibers of IFOF.
The deep layer is composed of three portions, with posterior, middle and anterior terminations:
-
The posterior portion of the deep layer proceeds superiorly, underneath the superficial layer of IFOF, and crosses under the thick and compact stem of AF at the level of inferior frontal sulcus (Fig. 8). At the level of IFG and inferior frontal sulcus, these fibers arch slightly backward to reach the cortex of MFG and DLPFC. We found IFOF terminations in the MFG and DLPFC in all specimens, even if in the left hemispheres these terminations seem more consistent and more posteriorly arched (Fig. 9a–d).
Fig. 8 The deep IFOF terminations are distributed within the frontal region in three directions: posterior, middle and anterior. At the level of the inferior frontal sulcus, fibers with a posterior orientation (green tags; red arrows) are crossed above by AF fibers (blue tags; blue arrow). AF fibers were interrupted and removed (segmented part of the blue arrow) at the level of the posterior-third of the IFG (pars opercularis) and were separated by means of blue tags to show the underneath IFOF fibers supero-posteriorly directed (red arrows). (Left hemisphere; a anterior; i inferior; s superior)
Fig. 9 a A fine dissection reveals the existence of a deeper layer of IFOF fibers directed beyond the IFG (red arrows). The blue tags are posed beneath the stem of AF/SLF (postero-anteriorly directed) (blue circle), cut at the level of posterior-third of IFG, showing the crossing of AF/SLF (superficial) and IFOF (deeper) fibers. b and c In these two left hemispheres, the green tags mark the course of the posterior portion of the deep fibers of IFOF (red arrows). Terminations are directed to the MFG and the DLPFC (green circle). Interestingly, during their course, these fibers take a slight posterior arcuate orientation, especially in the left hemispheres. d We detected, in fact, the same terminations also within all right specimens, but it should be noted that at this side the fibers appears less thick and curved. (a–c left hemisphere; d right hemisphere; a anterior; i inferior; p posterior; s superior)
-
The middle portion has a supero-anterior course, underneath the middle-third of IFG (pars triangularis) and the ventral portion of the OFC, terminating in the MFG and lateral OFC (Fig. 10a–c).
Fig. 10 After fine dissection for the deeper portion of IFOF fibers, green tags were inserted under the narrow stem, postero-laterally to the limen insulae and under the fun-shaped radiation, constituted by three subcomponents: posterior, middle and anterior portions of deep fibers. a The middle-third of the IFG was taken up (red circle) by a pince to show the course of the middle portion (red arrows) of the IFOF deep layer in the frontal lobe. b The course of the middle portion of fibers of the deep IFOF is shown, passing underneath the pars triangularis toward the MFG cortices in a left hemisphere (red arrows). c Other fibers, still referable to the middle portion, are followed more anteriorly to the lateral orbito-frontal cortex (blue arrow). (Left hemisphere; a anterior; i inferior; s superior)
-
The anterior portion has a straight anterior course passing underneath and antero-inferiorly to the pars orbitalis, terminating in the frontal pole and basal orbito-frontal cortex (partially overlapping by the UF terminations) (Fig. 11a–d).
Fig. 11 In these pictures we removed just the apex and the short insular gyri, leaving “in situ” the central sulcus and long insular gyri (red circle). We dissected just the stem of IFOF and UF at the level of the limen insulae following the course of the anterior portion of IFOF deeper fibers. a The removal of the anterior third of the IFG (pars orbitalis) allows recognizing a further portion of the IFOF, which runs toward the more anterior region of the frontal lobe (red arrows). b Particularly, we demonstrated a fiber portion with a straight course to the frontal pole (red arrows). c Other fibers curve antero-inferiorly at the level of limen insulae, reaching the basal orbito-frontal cortex and partially overlapping the UF fibers (blue arrow). d At the level of the limen insulae the identification of the anterior portion of IFOF deeper fibers and the separation from the UF fibers is not easy. Thus, we put a red tag separating the more anterior fibers of the UF from the posterior IFOF fibers, to differentiate IFOF and UF fibers terminations within the basal orbito-frontal region (Left hemisphere; a anterior; i inferior; p posterior; s superior)
Finally, we studied the relationship of SLF and IFOF terminations. The fibers from SLF were more superficial and horizontally oriented with regard to the deeper and vertical course of IFOF fibers, especially in the more posterior part. The IFOF fibers, going to the DLPFC and MFG, pass under the pars opercularis and triangularis and cross under the SLF at the level of the inferior frontal sulcus. We demonstrated this crossing by putting tags under these bundles (blue for SLF and green for IFOF), with particular interest to the overlapping of the respective terminations in DLPFC, MFG and IFG (Fig. 12).
We isolated with green tags the course of the IFOF fibers from the narrow stem at the limen insulae to the frontal lobe. Also the AF/SLF is evidenced by blue tags. We opened a window at the level of middle-posterior thirds of IFG, cutting also the AF/SLF stem. The overlapping between the terminations of the superficial layer of IFOF (green arrows) and AF/SLF at the level of IFG is showed (blue circle). The IFOF fibers of the deep portion (red arrows) arches medio-laterally at the level of the superior limiting sulcus of insula and terminates in the IFG cortices, contiguous and inferiorly to the AF/SLF terminations as showed at the level of the anterior blue tag. (Left hemisphere; a anterior; i inferior; s superior)
Diffusion tensor imaging
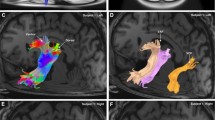
In this work, we also present the virtual dissection of the left hemisphere IFOF of a single subject. Figure 13 displays the whole IFOF with all subcomponents color-coded in the same way as they are depicted in Figs. 14 (superficial component) and 15 (deep component). Figure 14 displays the superficial component as defined by the part of the IFOF directed to the IFG. Figure 15 displays the IFOF without the superficial component.
In this picture all subcomponents color-coded of IFOF are shown with DTI. The superficial layer of IFOF bundle is displayed in red color and the three portions of deep layer, posterior, middle and anterior are colored in green, orange and blue, respectively. a axial view; b sagittal view; c coronal view
DTI reconstruction of the deep layer after removal of the superficial layer. The anterior component is displayed in blue, the middle in orange and the posterior one in green. The DTI results confirm, once again, the course and terminations territories demonstrated in the cadavers dissections. a axial view; b sagittal view; c coronal view
To simplify the description of DTI results, we will assume a postero-anterior course of the IFOF with a posterior origin and an anterior termination. Similarly to what described by Lawes et al. (2008), we found three main apparent origins of the IFOF located in the occipital inferior gyrus, in inferior portion of the occipital middle gyrus and to a minimal extent within the lingual gyrus. Within the occipital inferior gyrus, it is possible to identify two apparently separate stems: one located in the most anterior and lateral part at the border with the inferior temporal gyrus, the other located in a more medial and posterior region at the border with the fusiform gyrus. From these regions the streamlines follow a path that closely resemble the one described in the anatomical dissection both in the paper by Lawes et al. (2008) and in that of Martino et al. (2009). Streamlines converge and pass through the extreme capsule where the whole tract appears to be compacted. The terminations of the streamlines at the frontal lobe closely match the ones previously described in the anatomical dissection, i.e., they reach four distinct areas: the IFG, the lateral OFC, the medial portion of the MFG and the posterior portion of the DLPFC. Based on the anatomical dissection results, we selected the part of IFOF terminating in the IFG to verify its course and location (Fig. 14a–c). This part should correspond to what, based on the anatomical dissection, has been labeled as the superficial portion. We found that it originates from the antero-lateral portion of the occipital inferior gyrus and from the antero-inferior portion of the occipital middle gyrus with a minimal component originating from the lingual gyrus. After passing in the most inferior and lateral portion of the extreme capsule, it turns lateral to terminate in the IFG, closely resembling the anatomical dissection path. Once this superficial component was removed, it was possible to explore the remaining part of the IFOF that should match the deep portion as described in the anatomical dissection (Fig. 15a–c). Notably, immediately after passing through the extreme capsule, where it appears located medially and superiorly to the superficial portion (Fig. 13a–c), it divides into three fan-shaped clusters of streamlines directed, respectively, to the lateral OFC, to the MFG and to the DLPFC (Fig. 15a–c). Following backwards the occipital origin of these three bundles, we found that orbito-frontal part originates mostly from the medial posterior portion of the inferior occipital gyrus at the border with the fusiform gyrus with few streamlines coming from the postero-lateral stem in the inferior occipital gyrus. The other two bundles of streamlines directed to the MFG and to the DLPFC apparently originate almost only from the stem located in the antero-lateral portion of the inferior occipital gyrus at the border with the posterior part of the inferior temporal gyrus.
Notably, since we constrained the IFOF to those streamlines passing through the ROIs defined by Catani and Thiebaut de Schotten (2008), which do not encompass any portion of the parietal lobe white matter, we did not observe any portion of the IFOF within the parietal lobe.
Discussion
General considerations
The interest in IFOF anatomy, distribution and functional role has recently been renewed. The fascinating DTI “in vivo” reconstructions of IFOF, especially with the seminal work of Catani et al. (2002), and Catani and Ffytche (2005) and followed by the creation of DTI atlases of human white matter (Catani and Thiebaut de Schotten 2008), confirmed the fibers distribution described in the anatomical works of the last century (Dejerine 1895). However, although DTI is a valid tool for the visualization of the stems of the main white matter pathways, explaining why we adopted this imaging to confirm our dissection findings, its reliability in the identification of the crossing and “kissing” fibers, as well as in the determination of cortical terminations is still debated (Catani et al. 2002). Thus, nowadays the anatomical fibers dissection according to the Klingler’s (1935) technique represents a valuable way for the visualization of human white matter and to improve the knowledge on brain connectivity.
Recently, Martino et al. (2009) reported an original description of the posterior terminations of IFOF, proposing a new categorization of IFOF fibers in a superficial and a deep layer, and describing new cortical terminations (i.e., superior parietal lobule and postero-basal temporal areas) in addition to the occipital cortex. These anatomical evidences of IFOF terminations in areas involved in verbal semantic network and the data provided by the intraoperative direct stimulation of this bundle (eliciting semantic paraphasias along its entire course) (Duffau et al. 2005), led us to propose new concept with regard to the IFOF role. Indeed, this pathway has been proposed to underly the “ventral” semantic stream, connecting the posterior brain areas to the IFG, lateral OFC and DLPFC. However, its anterior terminations within the frontal cortex have never been accurately described.
Here, our dissection work has detailed, for the first time to our knowledge, the anterior organization of the IFOF fibers in two main components, superficial and deep, according to the previous description of the bundle in its posterior part (Martino et al. 2009). Interestingly, the frontal terminations of the superficial layer (i.e., IFG, pars triangularis and orbitalis) are distinct from the frontal termination of the deep layer (MFG, DLPFC, lateral OFC).
In addition, we combined our dissection results with DTI study. Of note, the organization of the anterior part of our DTI reconstruction is similar to what we found during the anatomical dissection. Indeed, it was possible to separate a superficial portion directed to the IFG and a deep portion divided in three discrete fan-shaped bundles directed to the orbital, middle and dorsal frontal gyri. Surprisingly, most of DTI reconstruction of the IFOF in the literature showed only one anterior termination within the OFC (Catani et al. 2002; Catani and Thiebaut de Schotten 2008; Lawes et al. 2008). It is nonetheless worth nothing that our DTI results do not fully match the dissection data concerning the posterior terminations (Martino et al. 2009). It is likely due to the fact that we applied a combination of the two-ROIs approach proposed by Catani et al. (2002), and Catani and Thiebaut de Schotten (2008) with a multi-ROIs operation as proposed by Mori et al. (2005) to refine the tract of interest. The whole IFOF was defined using two ROIs localized in the MNI space covering the occipital lobe from the lingual and fusiform gyrus (Z = −9) up to few millimeters above the inferior border of the splenium (Z = 15). This restriction explains why we did not observe any part of the IFOF within the parietal lobe, a termination fully described by Martino et al. (2009) and clearly visible also in the Mori et al. (2005) DTI atlas, where the posterior ROI constraining the IFOF was much larger than the one we used here (it also included the parietal lobe).
By correlating our anatomical data to the functional evidences provided by direct cortico-subcortical stimulation (Duffau et al. 2005; Henry et al. 2004; Martino et al. 2009) and by functional neuroimaging studies, we hypothesize that IFOF might be a “multi-task bundle”, with subcomponents involved in distinct functions according to their cortical terminations. Our aim is to discuss about the role of each subpart of the IFOF.
Anatomical and functional considerations on the superficial layer
The results of our anatomical study demonstrate that the two components of IFOF (superficial and deep) previously described (Martino et al. 2009, 2010), are also visible at the fronto-temporal junction and within the frontal lobe. After the common narrow stem (posterior to the limen insulae and postero-superior to the UF), the IFOF enlarge in a fan-shaped 60° radiation of fibers entering in the frontal lobe. Here we identified a superficial and a deep layer too.
The former (superficial), passing through the ventral claustrum and terminating in the antero-inferior IFG (pars triangularis and orbitalis), is characterized by an arch-shaped course through the extreme and external capsules until the IFG cortices, where these fibers terminate contiguous and inferiorly to the SLF terminations (Fig. 12). This portion of fibers can be easily detected after removal of the insular cortex and subcortical insular fibers (insulo- and claustro-opercular fibers) starting from the insular apex. The DTI reconstruction is also in agreement with the direction and disposition of the fibers we dissected, especially concerning the frontal termination and the subdivision into a superficial and deep portion at the level of the extreme/external capsule (Figs. 13, 14, 15). Interestingly, the data provided by subcortical stimulation of IFOF, evoking semantic paraphasia along its entire course and especially below the anterior third of the insula (Duffau et al. 2005; Duffau 2008), are in line with our dissection and DTI results.
Moreover, the terminations we described in the IFG (i.e., pars triangularis and orbitalis) (Fig. 13) are also in line with the semantic role of the IFOF. Indeed, the involvement of the antero-inferior portion of the IFG in semantic processing has previously been proposed (Garibotto et al. 2009; Sakai 2005; Schmahmann and Pandya 2007) and confirmed by brain stimulation and functional imaging studies (Duffau 2008; Vigneau et al. 2006). Direct cortical stimulation of the pars orbitalis and pars triangularis elicited semantic paraphasia in awake patients (Duffau et al. 2005). Moreover, a meta-analysis of functional MRI works demonstrated activations during language semantics within the IFG (Vigneau et al. 2006). Other functional imaging studies suggested an involvement of the anterior portion of the IFG in the semantic aspects of language (Rizzolatti and Matelli 2003), especially in the semantic working memory (WM) (Sakai 2005; Vigneau et al. 2006). Moreover, left IFG has also been proposed to have a crucial role in selection and integration of semantic information, for both signed and spoken language, concerning higher level of lexico-semantic processing (Sakai 2005).
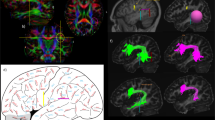
In summary, our anatomical description of the superficial layer of IFOF and its terminations within the IFG provides a strong anatomical background supporting the hypothesis of the IFOF as a main pathway which sub-serves the semantic system: the IFOF represents a “three-regions network” connecting the occipital extra-striate cortex, temporo-basal region and IFG (Fig. 16).
Schematic representation of the superficial layer of IFOF fibers, connecting the superior parietal lobule, the occipital extra-striate cortex, the Wernicke’s territories and Fusa (fusiform area at the occipito-temporal junction) to the IFG (pars triangularis and opercularis) is given. This portion of the bundle might be involved in the semantic elaboration of the language according to the evidences provided by the direct subcortical stimulation of the bundle in awake patients during denomination task. Moreover, the frontal sites of terminations of the superficial layer (i.e., IFG, pars triangularis and pars orbitalis) have been demonstrated to be largely involved in the semantic elaboration of the language
Anatomical and functional considerations on the deep layer
The dissection of the deep layer was technically more difficult, due to flattened and delicate fibers. It was nonetheless possible to demonstrate that this bundle enlarged in three distinct components, terminating, respectively, to: (1) the DLPFC and MFG passing under the IFG/inferior frontal sulcus; (2) the most anterior part of the MFG and the lateral OFC; (3) the fronto-polar and basal OFC. Their courses have been followed from the narrow stem under the insular apex, by removing the entire IFG and passing below the inferior frontal sulcus, until their cortical terminations. The terminations from the first (posterior) portion, directed to the DLPFC/MFG, have a vertical course, going upward from the IFOF stem and passing under the operculo-triangularis junction. These fibers tilt slightly back at the level of the inferior frontal sulcus to reach the DLPFC and posterior-MFG. They are deeper and cross the transversal fibers coming from the distal stem of the AF/SLF, which terminates contiguously and partially overlapped by the IFOF terminations in the DLPFC.
Data provided by functional studies are in agreement with our anatomical description of the IFOF deep fiber course. Indeed, the DLPFC has been demonstrated to play a role in the semantic elaboration of the language (Sakai 2005). In particular, semantic disturbances and judgment errors have been evoked by the subcortical stimulation of this area (Plaza et al. 2008). Moreover, the evidence regarding the terminations of IFOF in the DLPFC and MFG, could be an anatomical confirmation of the direct connection previously hypothesized (Petrides and Pandya 2006), between the frontal cortex and the occipital posterior associative extra-striate cortex and temporo-basal area, involved in visual recognition and conceptualization. These results are in line with the effect produced by the direct stimulation of DLPFC/posterior-MFG (frontal sites of terminations of IFOF fibers) and along the course (i.e., at the level of inferior frontal sulcus and lateral and anterior to the head of caudate) of the fibers described above (i.e., posterior deep portion) (Duffau et al. 2005).
Our anatomical findings lead to suggest a “multi-functional” segmentation of IFOF, which could be involved in both language and non-language processing (Figs. 16, 17).
The deep layer of IFOF fibers is represented in this scheme. Green the posterior portion of deep fibers (connecting the superior parietal lobule/occipital extra-striate cortex/Fusa to the DLPFC/MFG) is involved in the semantic elaboration of the language and in the visual recognition and conceptualization. Orange the middle portion of the deep fibers (connecting the superior parietal lobule to the MFG/lateral OFC) is involved in the integration of the multimodal sensory inputs and in the motor planning functions (prefrontal cortex). Blue the anterior portion of the deep fibers (connecting the occipital extra-striate/Fusa to the basal OFC and partially overlapped with the UF), which could be involved in emotional and behavioral aspects
Indeed, we identified a middle (second) deep portion of fibers, passing under the pars triangularis and proceeding upward and slightly anteriorly to the MFG and lateral OFC. Considering the posterior terminations previously described in the superior parietal lobule (Martino et al. 2009), the middle and posterior frontal deep terminations of IFOF could constitute an anatomical link between the frontal cortex and the superior parietal lobule. This subcomponent of the IFOF could allow an involvement of the prefrontal cortex in the integration of the multimodal sensory inputs and motor planning functions (Petrides and Pandya 2006). Processing of visual coordinates in association with information coming from the sensory–motor areas is essential for the perception of complex movements, visual concentration toward areas of interest and planning of visually guided movements (Aralamask et al. 2006; Cabeza and Nyberg 2000).
Therefore, we suggest that IFOF might play a role in a “multimodal integration” among brain regions distant from each other. This hypothesis is supported by functional neuroimaging studies (Cabeza and Nyberg 2000; Ernst et al. 2002; Lawrence et al. 2009; Rizzolatti and Matelli 2003), which analyzed cortical brain regions involved in decision-making process. Activations demonstrated a network of several areas, including the OFC, DLPC, ventrolateral prefrontal cortex, anterior cingulate cortex, insula, parietal cortex, thalamus and cerebellum, implicated in the evaluation of stimuli and in the outputs selection. Interestingly, different lateralization of the functional subcomponents has also been reported: adaptive aspects and planning/working memory are mainly right-sided; emotional, sensory and motor components are left-sided; informed memory and motor control result bilaterally represented. These evidences are in agreement with our anatomical findings, which showed a bilateral representation of IFOF fibers.
Lesion studies also support our hypothesis. For instance, a recent work specifically dedicated to disorders of the facial recognition of six basic emotional expressions in 104 brain-damaged subjects (Philippi et al. 2009), demonstrated an alteration in the connectivity underlain by the right IFOF. This evidence pleads in favor of the fact that this bundle, owing to its connections with emotion-related cortical regions (i.e., orbito-frontal cortex), constitutes a crucial component of the network which sub-serves the combination of affective responses with early visual information (and thus which allows recognition of facial expressions and emotions). In the same vein, a selective neurodegeneration of the frontal IFOF fibers has been demonstrated by DTI studies in progressive supranuclear palsy patients (Kvickström et al. 2011), that is, a fatal neurodegenerative-operative disorder characterized by progressive motor, cognitive and behavioral symptoms. Similarly, investigation on the alterations of the connectivity underlying dyslexia and dyscalculia confirmed the specific involvement of IFOF in lecture and calculation, respectively. In particular, Rollins et al. (2009) showed the existence of differences in the structure of white matter between dyslexic and normal subjects. Thus, study of the developmental dyscalculia, based on a network-analysis approach using DTI (Rykhlevskaia et al. 2009), revealed that this disorder resulted from a multiple circuits dysfunction and that connections between the right fusiform gyrus and the temporo-parietal area, including IFOF, represented a critical point in the pathogenesis of the disease. These data also support the involvement of IFOF in visuo-spatial and numerical operations, crucial in mathematical processing.
Finally, the third (anterior) portion of the deep layer of the IFOF has an anterior and deep course under the pars orbitalis (IFG), running to the fronto-polar cortex and basal OFC. These terminations partially overlapped with the UF terminations, especially in the basal OFC. This subcomponent might be involved in emotional and behavioral aspects, by processing visual emotional stimuli and by retrieving emotional information. Indeed, the disorganization of the more anterior part of the IFOF has also been suggested to participate in the pathogenesis of symptoms of the obsessive–compulsive disorder. In a recent study (Garibotto et al. 2009), the patterns of organization and directionality of the major fiber bundles were evaluated in a subpopulation of 15 patients with obsessive–compulsive disorder and 16 control subjects. A correlation was noted between the amount of alteration concerning the quantity and the course of the fibers, the severity of the symptoms, and the decision-making and visuo-spatial performances. These changes were found specifically in the part IFOF underlying the OFC.
However, we have to acknowledge the fact that the IFOF is likely not the sole “multi-functional” pathway in the human brain.
Furthermore, it is worth noting that the inferior fronto-occipital fasciculus has never been evidenced in the rhesus monkey (Schmahmann and Pandya 2006, 2007; Schmahmann et al. 2007). Schmahmann and Pandya (2006) suggested that observation of such IFOF may be due to a DTI artifact explained by the proximity of the inferior longitudinal fasciculus with the uncinate facisculus and the extreme capsule. This interpretation is nonetheless in conflict with anatomic dissection data widely reported in humans (Ludwig and Klingler 1956; Heimer 1995; Martino et al. 2009), based on the fact that the IFOF runs in the roof of the sphenoidal horn of the ventricle, while the inferior longitudinal fasciculus runs below this temporal horn (Martino et al. 2009). In the present study, both dissection and DTI findings also support the existence of an IFOF in humans. As a consequence, we may suggest that such a long-association pathway which directly connects the frontal lobe with the posterior cortices, and which seems to be involved in language semantic processing (Duffau et al. 2005), appeared in humans while not existing in primates. Comparative studies focusing on IFOF should be performed to better understand how the human brain developed from monkeys.
Limitations of the study
White matter blunt dissection according to the Klingler’s technique is widely considered as an accurate and reliable method for the study of brain connectivity, and thus as a useful tool for validation of DTI data. Blunt dissection can nevertheless be particularly difficult, especially for the dissection of crossing fibers as well as at the level of regions with overlapping of different fiber terminations. The dissection of some fiber pathways may result in the disruption of other fibers and the complex relationships between different tracts is sometimes difficult to demonstrate. Moreover, blunt dissection requires a specific expertise in the preparation of the specimens, in the Klingler’s method, and requires a good knowledge of the human white matter. Finally, a further limitation of this technique is the inability to convert flat pictures of brain structures in three-dimensional images. For these reasons, we used DTI-MRI to confirm our anatomical evidences—even if we have to acknowledge that only one brain was studied. Indeed, DT-MRI recently renewed the interest in the study of white matter connectivity in humans. However, this method also suffers from several limitations. Despite recent technical improvements (Lawes et al. 2008), the study of the cortical terminations of the main fiber pathways as well as the study of intersecting and kissing tracts remains difficult (Schmahmann et al. 2007). Of note, histological technique, such as modified myelin staining with microscopic mapping, has recently been proposed as an additional tool to obtain a more precise delineation and spatial resolution of fiber tracts, particularly at the level of the most critical sites (Burgel et al. 2006). Such alternative technique could be combined with blunt dissection and a large data set of DTI in the next future, a work now in process in our institution.
As a consequence, our original findings on the IFOF course and terminations with the frontal lobe should be confirmed by other studies.
Conclusions
On the basis of our original findings, we hypothesize that the IFOF might be a complex “multi-function bundle”, connecting distant and distributed brain areas (superior parietal, occipito-extrastriate, temporo-basal and frontal areas) and subserving language and non-language brain functions.
These results, supported by DTI, give further arguments in favor of the current concept of the distributed anatomo-functional organization of the central nervous system. According to this “hodotopic” model, the brain processing is organized in multimodal, large-scale, parallel, integrated and plastic networks. Our data are in line with this model. Indeed, we demonstrated that the IFOF fibers reached at least seven frontal territories (the pars orbitalis, triangularis and opercularis of the IFG, the MFG, DLPFC, OFC, PFC) involved in different brain networks. We also showed that the IFOF fibers had a functional segregated organization. Moreover, the distribution of the cortical over-specialized sub-regions and the hypotheses on the IFOF functional roles demonstrated by previous studies are in line with the anatomical evidences we reported. Of note, the terminations of the two main streams of language, phonological and semantic (underlain by AF/SLF and IFOF, respectively), are partially overlapped and interconnected in the frontal lobe, likely explaining its integrative role.
However, further anatomo-functional studies are needed to confirm this model and the role of each subcomponent of the IFOF.
References
Aralamask A, Ulmer JL, Kocak M, Salvan CV, Hillis AE, Youssen DM (2006) Association, commissural, and projection pathways and their functional deficit reported in literature. J Comput Assist Tomogr 30:695–715
Bookheimer S (2002) Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Ann Rev Neurosci 25:151–188
Burgel U, Amunts K, Hoemke L, Mohlberg H, Gilsbach JM, Zilles K (2006) White matter fiber tracts of the human brain: three dimensional mapping at microscopic resolution, topography and intersubject variability. Neuroimage 29:1092–1105
Cabeza R, Nyberg L (2000) Imaging cognition II: an empirical review of 275 pet and fMRI studies. J Cogn Neurosci 12:1–47
Catani M, Ffytche DH (2005) The rises and falls of disconnection syndromes. Brain 128:2224–2239
Catani M, Mesulam M (2008) The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex 44:953–961
Catani M, Thiebaut de Schotten M (2008) A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44:1105–1132
Catani M, Howard RJ, Pajevic S, Jones DK (2002) Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 17:77–94
Curran EJ (1909) A new association fiber tract in the cerebrum. J Comp Neurol 19:645–656
Dejerine JJ (1895) Anatomie des Centres Nerveux. Rueff et Cie, Paris
Dorrichi F, Thiebaut De Schotten M, Tomaiuolo F, Bartolomeo P (2008) White matter (dis)connections and gray matter (dys)functions in visual neglect: gaining insights into the brain networks of spatial awareness. Cortex 44:983–985
Duffau H (2008) The anatomo-functional connectivity of language revisited: new insights provided by electrostimulation and tractography. Neuropsychologia 46:927–934
Duffau H, Gatignol P, Mandonnet E, Peruzzi P, Tzourio-Mazoyer N, Capelle L (2005) New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain 128:797–810
Epelbaum S, Pinel P, Gaillard R, Delmaire C, Perrin M, Dupont S, Dehaene S, Cohen L (2008) Pure alexia as a disconnection syndrome. New diffusion imaging evidence for an old concept. Cortex 44:962–974
Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, London ED (2002) Decision-making in a risk-taking task: a pet study. Neuropsychopharmacology 26:682–691
Fox CJ, Iara G, Barton JJS (2008) Disconnection in prosopagnosia and face processing. Cortex 44:996–1009
Garibotto V, Scifo P, Gorini A, Alonso CR, Brambati S, Bellodi L, Perani D (2009) Disorganization of anatomical connectivity in obsessive compulsive disorder: a multi-parameter diffusion tensor imaging study in a subpopulation of patients. Neurobiol Dis 37:468–476
Heimer I (1995) The human brain and spinal cord: functional neuroanatomy and dissection guide, 2nd edn. Springer, New York
Henry RG, Berman JI, Nagarajan SS, Mukherjee P, Berger MS (2004) Subcortical pathways serving cortical language sites: initial experience with diffusion tensor imaging fiber tracking combined with intraoperative language mapping. Neuroimage 21:616–622
Jacobson S, Kelleher I, Harley M, Murtagh A, Clarke M, Blanchard M, Connolly C, O’hanlon E, Garavan H, Cannon M (2010) Structural and functional brain correlates of subclinical psychotic symptoms in 11–13 year old schoolchildren. Neuroimage 49:1875–1885
Klingler J (1935) Erleichterung der makroskopischen Präparation des Gehirns 78 durch den Gefrierprozess. Schweiz Arch Neurol Psychiatr 36:247–256
Kvickström P, Eriksson B, Van Westen D, Lätt J, Elfgren C, Nilsson C (2011) Selective frontal neurodegeneration of the inferior fronto-occipital fasciculus in progressive supranuclear palsy (PSP) demonstrated by diffusion tensor tractography. BMC Neurol 11:13
Lawes IN, Barrick TR, Murugam V, Spierings N, Evans DR, Song M, Clark CA (2008) Atlas-based segmentation of white matter tracts of the human brain using diffusion tensor tractography and comparison with classical dissection. Neuroimage 39:62–79
Lawrence NS, Jollant F, O’daly O, Zelaya F, Phillips ML (2009) Distinct roles of prefrontal cortical subregions in the Iowa gambling task. Cereb Cortex 19:1134–1143
Ludwig E, Klingler J (1956) Atlas cerebri humani. The inner structure of the brain demonstrated on the basis of macroscopical preparations. Little Brown, Boston
Martino J, Brogna C, Robles SG, Vergani F, Duffau H (2009) Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex 46:691–699
Martino J, Vergani F, Robles SG, Duffau H (2010) New insights into the anatomic dissection of the temporal stem with special emphasis on the inferior fronto-occipital fasciculus: implications in surgical approach to left mesiotemporal and temporoinsular structures. Neurosurgery 66:4–12
Mori S, Wakana S, Nagae-Poetscher LM, Van Zijl PCM (2005) MRI atlas of human white matter. Elsevier, Amsterdam
Petrides M, Pandya DN (2006) Efferent association pathways originating in the caudal prefrontal cortex in the macaque monkey. J Comp Neurol 498:227–251
Philippi CL, Mehta S, Grabowski T, Adolphs R, Rudrauf D (2009) Damage to association fiber tracts impairs recognition of the facial expression of emotion. J Neurosci 29:15089–15099
Plaza M, Gatignol P, Cohen H, Berger B, Duffau H (2008) A discrete area within the left dorsolateral prefrontal cortex involved in visual–verbal incongruence judgment. Cereb Cortex 18:1253–1259
Rizzolatti G, Matelli M (2003) Two different streams form the dorsal visual system: anatomy and functions. Exp Brain Res 153:146–157
Rollins NK, Vachha B, Srinivasan P, Chia J, Pickering J, Hughes CW, Gimi B (2009) Simple developmental dyslexia in children: alterations in diffusion-tensor metrics of white matter tracts at 3 T. Radiology 251:882–891
Rudrauff D, Mehta S, Grabowski T (2008) Disconnection’s renaissance takes shape: format incorporation in group-level lesion studies. Cortex 44:1084–1096
Rykhlevskaia E, Uddin LQ, Kondos L, Menon V (2009) Neuroanatomical correlates of developmental dyscalculia: combined evidence from morphometry and tractography. Front Hum Neurosci 3:51
Sakai KL (2005) Language acquisition and brain development. Science 310:815–819
Schmahmann JD, Pandya DN (2006) Fiber pathways of the brain. Oxford University Press, New York
Schmahmann JD, Pandya DN (2007) The complex history of the fronto-occipital fasciculus. J Hist Neurosci 16:362–377
Schmahmann JD, Pandya DN, Wang R, Dai G, D’Arceuil HE, de Crespigny AJ, Wedeen VJ (2007) Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain 130:630–653
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy R, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:208–219
Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, Mazoyer B, Tzourio-Mazoyer N (2006) Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage 30:1414–1432
Acknowledgments
We would like to express our heartfelt thanks to cadaver donors, who gave their own bodies to the University of Montpellier School of Medicine for medical research. We also want to thank Professor François Canovas for his administrative assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarubbo, S., De Benedictis, A., Maldonado, I.L. et al. Frontal terminations for the inferior fronto-occipital fascicle: anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain Struct Funct 218, 21–37 (2013). https://doi.org/10.1007/s00429-011-0372-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-011-0372-3