Abstract
The videofluoroscopic dysphagia scale (VDS) was developed as an objective predictor of the prognosis of dysphagia after stroke. We evaluated the clinical validity of the VDS for various diseases. We reviewed the medical records of 1,995 dysphagic patients (1,222 men and 773 women) who underwent videofluoroscopic studies in Seoul National University Hospital from April 2002 through December 2009. Their American Speech–Language–Hearing Association’s National Outcome Measurement System (ASHA NOMS) swallowing scale, clinical dysphagia scale (CDS), and VDS scores were evaluated on the basis of the clinical and/or videofluoroscopic findings by the consensus of two physiatrists. The correlations between the VDS and the other scales were calculated. The VDS displayed significant correlations with the ASHA NOMS swallowing scale and the CDS in every disease group (p < 0.001 in all groups, including central and peripheral nervous system disorders), and these correlations were more apparent for spinal cord injury, peripheral nerve system disorders, and neurodegenerative diseases (correlation coefficients between the VDS and the ASHA NOMS swallowing scale: −0.603, −0.602, and −0.567, respectively). This study demonstrated that the VDS is applicable to dysphagic patients with numerous etiologies that cause dysphagia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dysphagia is a significant clinical problem that can disrupt quality of life and lead to life-threatening conditions such as aspiration pneumonia [1, 2]. Although several clinical bedside tests are widely used [3, 4], the videofluoroscopic swallowing study (VFSS) has been generally accepted as a gold standard in evaluating and managing dysphagia [4–6]. Because the VFSS can evaluate penetration and aspiration in addition to many different abnormalities in the oral, pharyngeal, and esophageal phases, the VFSS has some merit in determining which swallowing therapy should be performed and what type of diet should be prescribed. To measure these VFSS findings as objective quantitative scores, the videofluoroscopic dysphagia scale (VDS), with a sum of 100 points, was created according to the odds ratios of various prognostic factors (Table 1). The VDS is known as a reliable, objective, and quantifiable predictor of long-term persistent dysphagia after stroke: Sensitivity and specificity of the VDS were 0.91 and 0.92. VDS reliability (intraclass correlation coefficient: 0.556) was reported as moderate agreement [6, 7]. Moreover, the VDS can predict aspiration 6 months after stroke (p < 0.05) [6]. However, the clinical applicability of the VDS has not been proven for etiologies other than stroke. Although stroke is the leading cause of dysphagia, other different disorders can provoke dysphagia [1, 8]. Therefore, quantitative measurement of the VFSS for those etiologies is needed, and the VDS might be a good option.
The aim of the present study was to determine the clinical applicability of the VDS in various etiologies.
Methods
Subjects
Data were collected retrospectively for dysphagic patients who underwent a VFSS for the first time in a Seoul National University Hospital between April 2002 and December 2009. The exclusion criterion was inadequate medical records. We obtained clinical data such as sex, age, etiology of dysphagia defined by the clinician who managed the patients, and duration from onset. If the etiology was not identified by the clinician, then the patient was included in the unknown etiology group without identified neurological and structural abnormalities. If the patient and caregiver could not remember the onset of dysphagia, then it was recorded as unknown. The protocol for this study was approved by the Institutional Review Board of Seoul National University Hospital.
Outcome Measure
To quantify the clinical severity of dysphagia, the clinical dysphagia scale (CDS) (Table 2) was scored. The CDS has been confirmed as a quantitative clinical tool that represents the VFSS findings well, and it could be adapted to various patients with dysphagia irrespective of the causal disorders [5]. Also, it showed good reliability (intraclass correlation coefficient: 0.886) [9]. The sensitivity and specificity of the CDS for reporting penetration and aspiration were 81.0, 70.7 % and 78.1, 77.9 %, respectively [10]. The quantified severity of the VFSS findings was calculated as the VDS.
The American Speech–Language–Hearing Association National Outcome Measurement System (ASHA NOMS) swallowing scale (Table 3) was scored [11] using the recommended diet after the VFSS. The ASHA NOMS swallowing scale classifies swallowing function according to the diet limitations of a patient (grade 1: no oral feeding; grade 7: no limitation of the diet). It may be a useful tool for grading the severity of dysphagia, and it has been used in many studies [5, 11, 12].
Procedures
Immediately before the VFSS, a physiatrist first obtained the clinical history (location of lesion, presence of T-cannula, and aspiration symptoms) of the patient and performed a physical exam (lip sealing, mastication, tongue protrusion, laryngeal elevation, and cup drinking), after which the CDS was scored. After scoring the CDS, the VFSS was performed by a physiatrist using the protocol from Logemann’s study [4, 5, 13]. Patients were given 2 or 5 mL of diluted barium (35 % weight/volume), pudding, curd-type yogurt, and boiled rice twice as food for the lateral VFSS view. Diluted barium and curd-type yogurt were given in both the lateral and anteroposterior positions. All test procedures were recorded on a digital video file and analyzed by agreement of two physiatrists. Oral and pharyngeal transit times were measured by using frame-by-frame analysis. These two physiatrists recommended a diet according to the clinical features and VFSS findings of the patient and graded the recommended diet according to the ASHA NOMS swallowing scale. Then, the physiatrist who performed the VFSS scored the VDS using the VFSS findings. All of the outcome scales were rated by the physiatrist who was trained to do so for at least 1 year.
Data Analysis
Spearman’s correlation coefficient between the VDS and other scores was calculated. We also analyzed the etiology and age group of the patients. SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA) was used for all statistical procedures. A p value of <0.05 was considered significant.
Results
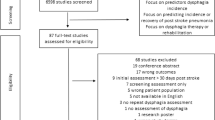
A total of 2,164 dysphagic patients who had undergone the VFSS were identified. In total, 169 patients were excluded (there was no medical record of the etiology for 2 patients, and the description of the VFSS findings was imperfect for 167 patients). Data for the remaining 1,995 patients (1,222 men) were analyzed in this study.
The characteristics of the patients are given in Table 4. The average age of the patients was 58.7 ± 19.3 years. Excluding 197 patients who could not remember the onset of dysphagia, the average duration between the onset of dysphagia and the time of evaluation for 1,798 patients was 243.5 ± 711.6 days (range = 0–30 years, median = 41 days). The three most common causal disorders of dysphagia were (1) central nervous system disorders (e.g., stroke, brain tumor, neurodegenerative disease, traumatic brain injury, and spinal cord injury), (2) local structural lesions involving the head and neck (e.g., tumors of the oral cavity, pharynx, and esophagus; postoperative anatomical alteration; anterior cervical osteophyte; and corrosive esophageal stricture), and (3) poor general medical condition due to other medical or surgical problems. The rates of incidence of these three causal disorders were 58.0, 14.0, and 13.0 %, respectively.
The values of the ASHA NOMS, the CDS, and the VDS of various etiologies are given in Table 5. For the ASHA NOMS, local structural lesions involving the head and neck had lowest value (3.6 ± 2.4), while unknown etiology had the highest (5.4 ± 2.2). For the CDS, the lowest value was for unknown etiology (15.3 ± 19.7) and the highest for peripheral neuropathy (28.3 ± 22.1). For the VDS, the lowest value was for unknown etiology (18.3 ± 18.8) and the highest for neuromuscular junction disorders or myopathy (37.6 ± 19.1).
In every disease group there was a significant correlation between the VDS and the ASHA NOMS swallowing scale (p < 0.001 in all groups). Most of the disease groups showed at least moderate strength of correlation, particularly spinal cord injury, peripheral neuropathy, and neurodegenerative disease (correlation coefficients between the VDS and the ASHA NOMS swallowing scale of these disease groups: −0.603, −0.602, and −0.567, respectively). Furthermore, for every etiology, there was a significant correlation with at least moderate strength between the VDS and CDS scores (p < 0.001 in all groups) (Table 6).
In addition, the data revealed significant correlations in all age groups between the VDS and the other scales (p < 0.001 in all decades; Table 7).
Discussion
The VDS displayed significant correlations with the ASHA NOMS swallowing scale and the CDS scores, regardless of the etiology and age of the patients. Therefore, a higher VDS score indicates greater diet limitations and more severe dysphagia.
Originally, the VDS was created to quantify the severity of dysphagia of patients who had a stroke [6], but there were also statistically significant correlations for the rest of the etiologies included in this study. Moreover, for many etiologies (i.e., spinal cord injury, peripheral neuropathy, neurodegenerative disease, traumatic brain injury, brain tumor, poor general medical condition, and local structural lesions involving the head and neck), the correlation between the VDS and the ASHA NOMS swallowing scale was stronger than that for stroke. Thus, the VDS also can be applied to describe quantitatively the severity of dysphagia in conditions other than stroke.
However, there were no significant differences of the ASHA NOMS, the CDS, and the VDS between “stronger” and “weaker” correlated etiologies. For instance, if the clinician could not find the cause of dysphagia, we classified that as unknown etiology. It means that the cause of dysphagia might not be severe in some patients with somatization disorder and laryngopharyngeal reflux. All three scales showed that the dysphagia of the patients with unknown etiology is the least severe. However, this cannot explain why the strongest correlation was found for unknown etiology. Maybe these differences resulted from the characteristics of each etiology. For example, the diet recommendation for the acute stroke patients could be more conservative, although it could be more lenient for the chronic stroke patients. This tendency might result in the relatively low correlation found in stroke etiology. However, this assumption cannot be generalized to other disease categories. Further study will be required to answer this question.
Significant correlations also were found in every age group. In particular, a correlation was also evident for those who were younger than 10 years (although the correlation coefficient was lower than that for other age groups, the p value was significant). Even though the VFSS can be performed with children [14], the evidence obtained for adults cannot be generalized to children [15]. The VDS also could be clinically useful for quantitatively describing the severity of dysphagia in children. In addition, the VDS could be a helpful reference for choosing the diet for dysphagic children. However, the correlation coefficient was lower than that for other age groups. Therefore, the VDS should not be the criterion and the diet should be determined carefully using all clinical information.
The VDS can produce numerical data regarding swallowing function through the use of comprehensive VFSS findings [6]. Therefore, clinicians can more precisely understand and explain dysphagia and delineate the aggravation and improvement of dysphagia in detail as opposed to only describing the presence of penetration or aspiration. Moreover, because the VDS is determined by VFSS, it can be a more objective tool than other clinical evaluations despite the need for the fluoroscopic room.
The CDS, which was developed to screen dysphagia after stroke using clinical findings such as clinical history and bedside test data, displayed correlation with the VFSS findings in various disease groups [5]. In the current study, correlations between the CDS and the VDS were evident for all disease and age groups, indicating that the VDS correlates with the clinical findings of dysphagic patients irrespective of the causal disorder(s) or age of the patients. Accordingly, clinicians can use the CDS and the VDS for dysphagic patients for clinical or academic purposes to quantify the severity of dysphagia.
This study has some limitations. First, some information was not obtainable such as the duration of dysphagia from onset to study participation for some patients. However, this did not affect the results of the study regarding the correlations between the scores. Second, this study was a retrospective single-center study, which could result in bias and loss of data (e.g., level of spinal cord injury, type of operation on head and neck area, level of cognition). Third, the raters of the VDS were not blinded to the clinical findings which could be a limitation. Fourth, only one physiatrist scored the VDS for one patient. If two or more physiatrists scored the VDS, then the data might be more reliable. Further prospective multicenter studies with more data such as follow-up prognosis will be required to solve these issues.
Conclusions
The VDS is a useful scale for quantifying the severity of dysphagia in various disease and age groups. The VDS can be a useful tool in clinical settings and studies to measure the findings of the VFSS.
References
McHorney CA, Robbins J, Lomax K, Rosenbek JC, Chignell K, Kramer AE, Earl Bricker D. The SWAL–QOL and SWAL–CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia. 2002;17:97–114.
Roth E. Medical complications encountered in stroke rehabilitation. Phys Med Rehabil Clin North Am. 1991;2:563–77.
Trapl M, Enderle P, Nowotny M, Teuschl Y, Matz K, Dachenhausen A, Brainin M. Dysphagia bedside screening for acute-stroke patients: the Gugging swallowing screen. Stroke. 2007;38:2948–52.
Han TR, Paik NJ, Park JW. The clinical functional scale for dysphagia in stroke patients. Korean J Stroke. 2001;3:153–7.
Jung SH, Lee KJ, Hong JB, Han TR. Validation of clinical dysphagia scale: based on videofluoroscopic swallowing study. J Korean Acad Rehabil Med. 2005;29(4):343–50.
Han TR, Paik NJ, Park JW, Kwon BS. The prediction of persistent dysphagia beyond six months after stroke. Dysphagia. 2007;23:59–64.
Kim DH, Choi KH, Kim HM, Koo JH, Kim BR, Kim TW, Ryu JS, Im S, Choi IS, Pyun SB. Inter-rater reliability of videofluoroscopic dysphagia scale. Ann Rehabil Med. 2012;36:791–6.
Kuhlemeier KV. Epidemiology and dysphagia. Dysphagia. 1994;9:209–17.
Chun SW, Lee SA, Jung IY, Beom J, Han TR, Oh BM. Inter-rater agreement for the clinical dysphagia scale. Ann Rehabil Med. 2011;35:470–6.
Han TR, Paik NJ, Park JW. The functional dysphagia scale using videofluoroscopic swallowing study in stroke patients. J Korean Acad Rehabil Med. 1999;23:1118–26.
American Speech–Language–Hearing Association. National Outcomes Measurement System (NOMS): adult speech–language pathology user’s guide. Rockville: American Speech–Language–Hearing Association; 2003.
Paik NJ, Kim IS, Kim JH, Oh BM, Han TR. Clinical validity of the functional dysphagia scale based on videofluoroscopic swallowing study. J Korean Acad Rehabil Med. 2005;29:43–9.
Logemann JA. Evaluation and treatment of swallowing disorders. San Diego: College Hill Press; 1998.
Zerilli K, Stefans V, DiPietro M. Protocol for the use of videofluoroscopy in pediatric swallowing dysfunction. Am J Occup Ther. 1990;44:441.
DeMatteo C, Matovich D, Hjartarson A. Comparison of clinical and videofluoroscopic evaluation of children with feeding and swallowing difficulties. Dev Med Child Neurol. 2005;47:149–57.
Acknowledgments
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (A101272).
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J., Oh, BM., Kim, J.Y. et al. Validation of the Videofluoroscopic Dysphagia Scale in Various Etiologies. Dysphagia 29, 438–443 (2014). https://doi.org/10.1007/s00455-014-9524-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-014-9524-y