Abstract
Copy-number variants (CNVs), in particular rare, small and large ones (< 1% frequency) and those encompassing brain-related genes, have been shown to be associated with neurodevelopmental disorders like autism spectrum disorders (ASDs), attention deficit hyperactivity disorder (ADHD), and intellectual disability (ID). However, the vast majority of CNV findings lack specificity with respect to autistic or developmental-delay phenotypes. Therefore, the aim of the study was to investigate the size and frequency of CNVs in high-functioning ASD (HFA) without ID compared with a random population sample and with published findings in ASD and ID. To investigate the role of CNVs for the “core symptoms” of high-functioning autism, we included in the present exploratory study only patients with HFA without ID. The aim was to test whether HFA have similar large rare (> 1 Mb) CNVs as reported in ASD and ID. We performed high-resolution chromosomal microarray analysis in 108 children and adolescents with HFA without ID. There was no significant difference in the overall number of rare CNVs compared to 124 random population samples. However, patients with HFA carried significantly more frequently CNVs containing brain-related genes. Surprisingly, six HFA patients carried very large CNVs known to be typically present in ID. Our findings provide new evidence that not only small, but also large CNVs affecting several key genes contribute to the genetic etiology/risk of HFA without affecting their intellectual ability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Autism spectrum disorder (ASD) is a neurodevelopmental disorder behaviorally defined by the deficits in reciprocal social interaction and communication as well as presence of restricted and repetitive behaviors. In the DSM-5 and in recent conceptualizations, these two behavioral dimensions represent the core defining features of ASD. Furthermore, frequently associated dimensions, such as language and intellectual disability (ID), contribute significantly to the heterogeneity of ASD phenotype. Individuals with ASD vary greatly in cognitive development, ranging from above average to ID. Multiple family and twin studies with concordance rates for ASD ranging up to 90% in monozygotic twins and up to 10% in dizygotic twins, respectively, showed the major role of heritability in the etiology of ASD (Hallmayer et al. 2011; Rosenberg et al. 2009; Tick et al. 2016; Colvert et al. 2015; Frazier et al. 2014; Sandin et al. 2014). However, the exact genetic mechanisms are not yet completely understood and identifying those genes is challenging (Freitag et al. 2010). In earlier studies, including case–control association, linkage- and genome-wide association studies chromosome regions including 2q (Consortium 2001; Vorstman et al. 2005), 5p (Vorstman et al. 2005; Wang et al. 2009), 7q (Consortium 2001; Chiocchetti et al. 2015), 11q (Vorstman et al. 2005), 15q (Marshall et al. 2008; Depienne et al. 2009), 16p (Consortium 2001; Marshall et al. 2008; Fernandez et al. 2009), and 16q (Vorstman et al. 2005; Wassink et al. 2008) showed significant association to autism (Yingjun et al. 2017).
The role of rare large and small copy-number variations (CNVs) as susceptibility loci in common and complex genetic diseases has been intensively investigated (Pinto et al. 2010; Kaminsky et al. 2011), and large CNVs have been detected in about 10% of patients with ASD (Shishido et al. 2014). It was described in an extensive genome-wide associations study (GWAS) that individuals with ASD carry a significant higher general burden of rare CNVs (1.19 fold), especially affecting loci and genes previously detected in ASD and/or ID (1.69 fold) (Pinto et al. 2010).
Results from Marshall and Scherer (2012) showed that some CNVs are pleiotropic and cause different clinical presentations (Marshall and Scherer 2012). The authors assume that a CNV at a particular locus may affect intelligence quotient (IQ) in individuals with ASD and, e.g., inflexible behavior in obsessive–compulsive disorder (OCD) patients at the same time. Additionally, rare and common variants in genes seem to be associated with synaptic plasticity (Zoghbi 2003) and brain connectivity (Vissers et al. 2012), and are linked to ASD. Moreover, another study showed that rare and large CNVs have been observed in both ASD and ID. However, these variants lack specificity towards ASD in contrast to developmental delay (DD) presentations (Girirajan et al. 2013). Girirajan and colleagues found that as the size of deletions increases, the non-verbal IQ decreased with no further impact on autism severity (Girirajan et al. 2013). In another study, the authors (Girirajan et al. 2011) reported that the frequency of large CNVs (> 1 Mb) was significantly higher in ID-associated phenotypes compared to autism phenotypes. They also concluded that large CNV burden was positively correlated with the ID severity. At the same time, the increase in CNV burden was modest when comparing autistic participants without ID with controls.
Here, we concentrated on a special population of ASD representing the core defining features of ASD including patients with high-functioning autism (HFA) only. HFA is characterized by features like those of Asperger syndrome and autism; however, the patients are cognitively “high functioning” (Chiang et al. 2014). Although there are currently no explicit diagnostic criteria for HFA, the definition is commonly used for autistic children with an IQ above 65–70 (Gillberg 1998). In the DSM-5, these patients are characterized by the specifier “without intellectual impairment” (American Psychiatric Association 2013).
Granted that former studies showed larger CNVs to be mainly associated with ID, we tested whether the frequency of large CNVs in HFA patients will be lower, as well as to investigate the frequency of rare deletions or duplications comparing to controls.
Up to date, there is no CNV analysis in HFA patients exclusively. Schaaf et al. (2011) sequenced several genes known to cause “syndromic autism” and other cognitive disorders only in an ASD population in general by traditional Sanger method and pyrosequencing.
The present work is, to our knowledge, the first genome-wide CNV analysis in a rigorous phenotyped cohort of patients with HFA using high-resolution chromosomal microarray analysis (CMA). By narrowing the broader phenotype spectrum of ASD, this cohort represents the core defining features of ASD without ID; we aimed to increase the knowledge on the pathophysiology and symptomatology of ASD.
Methods
Study sample: children and adolescents with HFA
108 children and adolescents with HFA were recruited at the Departments of Child and Adolescent Psychiatry and Psychotherapy, University Hospitals of Psychiatry Zurich, Switzerland and of the University of Würzburg, Germany.
All HFA patients fulfilled the diagnostic criteria for ASD according to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) (American Psychiatric Association 2013) and for pervasive developmental disorder according to the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) (Dilling et al. 1996).
The diagnosis was confirmed using either the Autism Diagnosis Observation Schedule (ADOS) (Rühl et al. 2004; Lord et al. 2012) or using Autism Diagnosis Interview-Revised (ADI-R) (Bölte et al. 2006; Lord et al. 1994). In 74 patients, SCQ (Rutter et al. 2007; Bölte and Poustka 2006) was additionally available. According to the HFA definition (Gillberg 1998), the inclusion criterion, which we adopted, was an IQ of at least 70 in standardized IQ tests (see below). The choice of different IQ tests was influenced by individual clinical necessities. The following tests were used: Wechsler Intelligence Scale for Children (Petermann and Petermann 2010), Wechsler Intelligence Scale for Adults (Wechsler 1981), Kaufman Assessment Battery for Children (Kaufman et al. 2009), Culture Fair Intelligence Test (Weiss 2006), or the Snijders–Oomen Non-Verbal Intelligence Test 5.5–17 (Tellegen et al. 2003). Patients underwent a psychiatric investigation conducted by a psychologist supervised by a senior or experienced child and adolescent psychiatrist, and were additionally screened for other psychopathologies as described previously (Werling et al. 2015; Nyffeler et al. 2014). The following parameters have been used: Child Behavior Checklist (Döpfner et al. 1998) and the German ADHD rating scale, FBB-HKS (Döpfner and Lehmkuhl 2000). The self-reported ethnicity was Caucasian origin for all subjects.
Exclusion criteria: neurological disorders including epilepsy or known genetic diseases linked to autism and ID (IQ < 70) and other severe psychiatric disorders such as psychosis and affective disorders (major depression and mania). The genetic data of the parents of the included patients were not available for analysis.
Control samples
The data of 124 chorionic villi samples of presumably healthy donors from Switzerland of Caucasian origin (76 males and 48 females, chi2 = 17.98, p < 0.0001 compared to the HFA patients) were assessed as previously described (Grünblatt et al. 2017). These chorionic villi samples were collected from pregnant women who decided to have invasive prenatal diagnosis due to advanced maternal age or due to parental wish. They were analyzed in the same manner on the Cytoscan HD Array as the patient samples.
Ethics approval
All procedures were performed with the written informed consent of the parents of all participants and the study was approved by the local ethics committees of the Canton of Zurich (Switzerland, E-36/2009), and of Würzburg (Germany, study numbers 8/06 and 227/09), respectively.
DNA extraction and chromosomal microarray analysis (CMA)
Genomic DNA was extracted from whole blood (EDTA tubes) with the desalting Proteinase K methodology (Miller et al. 1988) in 33 patients and from saliva in 75 patients (Oragene DNA, DNA Genotek Inc., Ontario, Canada) following the manufacturer’s protocol. DNA was analyzed with the Cytoscan HD Array (containing about 750,000 genotype-able SNPs and 1.9 million non-polymorphic probes) (Affymetrix Inc., Santa Clara, CA, USA) at a genome-wide resolution of 50 kb for both duplications and deletions. Array hybridization was performed according to the manufacturer’s protocol. Data were analyzed with Chromosome Analysis Suite (ChAS) software (Affymetrix) for changes of relative intensities. The CNV analyses were based on build 32.1. Genomic coordinates are based on GRCh37/hg19. To exclude common benign CNVs, we used a reference set of 820 in-house controls and 1038 Affymetrix controls in combination with the Database of Genomic Variants (DGV) from the Centre for Applied Genomics (February 2009, hg19). The results derived from the Chromosomal microarray analysis are very stable (Asadollahi et al. 2014). Cases and controls were treated in separate batches and case DNA was extracted from different sources (saliva and blood).
Rare CNVs were defined as aberration in coding sequences of genes that were absent in our in-house and Affymetrix primary control cohort, as well as not found to be reported in the DGV (http://projects.tcag.ca/variation/). The DECIPHER (https://decipher.sanger.ac.uk/) database was used to search for similar rare CNVs found in the current studied sample that occur also in other patients from DECIPHER to compare their phenotypes (last search 19th June 2019).
Brain-related CNVs were defined prior to the analysis if at least one of the genes within CNVs had central nervous system (CNS) expression or link demonstrated in the databases such as GO (Ashburner et al. 2000), Gene Expression Omnibus (GEO) (Edgar et al. 2002), the Genotype-Tissue Expression (GTEx) database (GTEx Consortium 2013), and the Human Protein Atlas database (Uhlen et al. 2015) (see Supplementary Table S1).
Statistical analysis
Frequency analysis was conducted using Chi-square test and Fisher’s exact test. For continuous measures, the Mann–Whitney U test was used.
Statistical analysis was performed with SPSS v.21 (IBM) and StatView v.5.0 (SAS Inst.). The level of significance was α = 0.05.
Results and discussion
Sample
One hundred and eight patients with HFA, 93 males and 15 females, have been enrolled in the study (12 patients with “childhood autism”, 37 with “atypical autism, and 59 with “Asperger syndrome”). The male-to-female ratio of 6.2 is representative for HFA, since the most widely reported male–female ratio for autism prevalence is 4–5.1, however, higher at the high-functioning end (Lai et al. 2015). The mean age ± SD of the patients at investigation was 11.29 ± 3.3 years. Only patients with an IQ of at least 70 in standardized IQ tests were included (IQ range 70–145) (For further details, see Table 1).
Fifty-seven of 108 HFA patients had an additional psychiatric disorder, most often ADHD (38.0%), followed by developmental disorders (21.3%, e.g., specific developmental disorders of scholastic skills, of motor function or mixed specific developmental disorders or phonological disorder) and OCD (3.7%) (for details, see Supplementary Table S2). Thirty-three of the patients received medication (methylphenidate; n = 26) for the treatment of the ADHD symptomatology. For more details about demographic data, see Table 1.
Frequency of rare CNVs in HFA
We detected small and large rare CNVs (mean size = 640.29 kb, SD 1399.17; range 52–8600 kb) in 42 of 108 patients with HFA (38.9%; Supplementary Table S1). There was no significant difference in the overall number of rare CNVs in the HFA sample compared to the control population (n = 39, 31.5%; mean size 273.85 kb, SD 234.04; range 50–1027 kb; chi2 = 1.4, p = 0.24 for details on control population see (Grünblatt et al. 2017)). There was no significant difference in the number of rare deletions between HFA (n = 21, 19.1%) and control population (n = 15, 12.1%; chi2 = 2.601, p = 0.27).
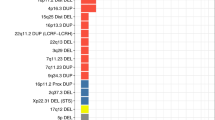
Although there were no significant group differences overall, interestingly, some of the patients with HFA were carriers of unexpected large CNVs, both deletions (cases A114: 2200 kb; A039: 4200 kb; A044: 8600 kb; A10W: 4300 kb) and duplications (cases A40W: 1600 kb; A044: 1600 kb; A092: 1400 kb) in known disease loci (summary in Table 2). In the control group, only one control proband carried a large duplication of unknown significance (0.8%, M40756 1027 kb), while all others carried rather small CNVs (see details on control population in (Grünblatt et al. 2017). Furthermore, there was a significant difference in the number of HFA carrying rare CNVs’ spanning genes involved in synaptic and brain-related processes (n = 28, 25.4%), compared to controls (n = 16, 12.9%; chi2 = 6.02, p = 0.014). This last finding is in line with previous studies on rare CNV, showing that in particularly patients with ASD carry CNVs spanning brain-related gene regions (Belmonte et al. 2004; Gilman et al. 2011).
As the present sample size is rather small, we cannot rule out that the negative results are due to statistical power to detect difference between HFA and controls. However, the current aims of the study were to see whether patients with HFA carry large rare CNVs, as well as large rare CNVs similar to those found previously in patients with ASD with ID or ID alone.
HFA carrying large rare CNVs (> 1 Mb) frequently described in ID
Strikingly, we found in six of our HFA patients very large rare CNVs (> 1 Mb) typically described in ID patients (Phelan and McDermid 2011; Mefford et al. 2012). Therefore, we focused on the medical history and the phenotypical details of each patient (Table 2) and discussed the findings in view of the current literature.
“Patient A10W”
The patient was a 14.9-year-old boy suffering from childhood autism (F84.0; IQ = 115) without any comorbidity. At the time of investigation, he took risperidone for aggressive and impulsive behavior.
We detected a 4.3-Mb large deletion in 22q13.31 (hg19 chr22:46885024–51183767), encompassing 50 genes in total, 31 OMIM-Gene, 11 of which involved in synaptic or brain-related pathways (details see Table 2). According to DECIPHER, overlapping CNVs (hg19 chr22:46885024–51183767) were found in 271 individuals (female n = 119, male n = 110; n = 42 with unknown sex). The ratio between ASD males and females in DECIPHER was 1.6 (female n = 11, male n = 18; n = 8 with unknown sex). Several patients have been reported with this deletion, and the clinical characterization of 22q13 deletion syndrome, known as Phelan–McDermid syndrome (PMS), is well established (Phelan and McDermid 2012). It is a contiguous genetic disorder on the terminal long arm of chromosome 22. These patients show neurological or neurodevelopmental deficits, and 50% of the patients show additionally autism or autistic-like behavior (Phelan and McDermid 2012). To our knowledge, HFA has not been described in patients with Phelan–McDermid syndrome up to now.
Although the size of the deletion in patients with PMS can vary, the critical region includes a deletion of SHANK3, encoding for a scaffold protein in the postsynaptic densities of excitatory synapses (Phelan and McDermid 2012). This gene is known to be involved in the functionality of postsynaptic structures of the CNS (Egger et al. 2016). Leblond et al. (2014) claimed SHANK mutations for about 1% of patients with ASD with a specific distribution in terms of the cognitive impairment: SHANK1 were not significantly present in males with normal IQ; SHANK2 were also not significantly detectable and only in patients with mild ID. However, SHANK3 was significantly observable, but in cases with moderate to profound ID. Due to SHANK3´s frequency and impact, the authors advised to screen for mutations in clinical practice in individuals with ASD and ID.
Surprisingly, our patient does not show any of the described symptom characteristics of PMS except for ASD, and although our patient carries a large deletion in this region including the SHANK3 gene, no intellectual impairment was detected. Since we analyzed only one tissue (i.e., blood) in our patient, we cannot exclude that the observed aberration is present in mosaic or even absent in other tissues. Nevertheless, another study investigated 32 patients with PMS, with ascertained SHANK3 deficiency (Soorya et al. 2013). 84% of the aforementioned cases met the diagnostic criteria for ASD and 75% for autistic disorder, indicating that this syndrome is one of the more highly penetrant causes of ASD. Since most of the patients (77%) exhibited severe to profound ID this study provides additional evidence on the severity of intellectual, motor, and speech impairments seen in SHANK3 mutations (Soorya et al. 2013).
Some other genes on the occurred large CNV deletions in patient A10W are discussed: The FAM19A5 (family sequence similarity 19) gene is expressed in the brain and is possibly related to neuropsychological features, like autistic behavior or general DD (Guilherme et al. 2014). The study by van der Zwaag identified BRD1 (Bromodomain-containing protein 1) gene in 22q13.33 region as a plausible novel autism candidate gene within the CNV region (van der Zwaag et al. 2009). A study by Prasad et al. (2012) discovered rare variants on the TYMP (thymidine phosphorylase) gene, which is also located in chromosome 22q13.33 and it associated with ASD. However, no intelligence description of these ASD patients has been provided. Finally, PLXNB2 (Plexin B2) and MAPK8IP2 genes (Mitogen-Activated Protein Kinase 1), both located on 22q13.33, are considered strong candidates for cerebellar phenotypes (Aldinger et al. 2013).
Interestingly, both in patient A10W and in patient A49W, an 8-year-old girl diagnosed with childhood autism (F84.0 according to ICD-10), the gene PIM3 was deleted. PIM3, a proto-oncogene with serine/threonine kinase activity, is located on 22q13.33 and is about 775 kb proximal to the SHANK3 gene. PIM3 participates amongst others in the regulation of the circadian rhythm (Mitz et al. 2018). This could possibly explain sleep disturbances often seen in ASD patients. However, currently, no literature is available describing PIM3 association with ASD or ID.
“Patient A039”
The patient was a 16-year-old boy suffering from atypical autism (F84.1; IQ = 106) without any comorbidity or medication at the time of study participation.
The patient was found to carry a 4.2 Mb large deletion in 3q11.1–q11.2 (hg19 chr3:93519464–97738323) involving 11 genes, 5 OMIM genes. Two genes encompassing the deletion are brain-related genes (Table 2). Furthermore, the patient was carrier of a small 96-kb deletion on 16q24.1 (hg19 chr16:84223309–84319789) encompassing the genes ADAD2 (Adenosine Deaminase Domain Containing 2) and the brain expressed KCNG4 (Table 2).
According to DECIPHER, there were 25 and 36 individuals carrying overlapping CNVs (hg19 chr3:93519464–97738323 and hg19 chr16:84223309–4319789, respectively), amongst them 17/20 male, 4/14 female and 4/2 individuals of unknown sex, respectively. There were more female individuals detected with ID or DD (0/9 females, 5/6 males). In contrast, there were only male individuals detected with autistic symptoms (2/3 males, no females). Despite the heterozygote deletion in the current patient, the gene ARL13B, also known in Joubert syndrome, an autosomal recessive disorder with partial or complete agenesis of the vermis and characterized by neurological and phenotypical symptoms and ID, could be of interest. Recently, loss of ciliary GTPas Arl13b in interneurons showed impairment in interneuronal morphology as synaptic connectivity leading to altered excitatory/inhibitory activity balance (Guo et al. 2017). Indeed, the excitatory/inhibitory imbalance has been postulated to be one of the mechanisms involved in ASD (Uzunova et al. 2016); therefore, this gene might be linked to the phenotype of our patient.
“Patient A40W”
The patient was a 15.3-year-old male adolescent presenting with atypical autism (F84.1; IQ = 78) with a hyperkinetic conduct disorder (F90.1). He was treated with methylphenidate.
We detected a 1.6-Mb duplication on 7q11.23 (hg19 chr7:72659674–74245599), encompassing 31 genes in total, 24 OMIM genes. Four genes are brain-related (Table 2). The duplication encompasses the Williams–Beuren syndrome (WBS) region, a well-described microdeletion syndrome (Pober 2010b). In contrast, the clinical phenotype caused by the reciprocal duplication is less documented and only few studies to date report duplication of the WBS region (WBCR) (Berg et al. 2007). According to DECIPHER database, 210 individuals were reported to carry overlapping CNVs (hg19 chr7:72659674–74245599), in which 80 were females and 119 were males (n = 11 unknown sex). Only seven conferred autistic behavior with more males individuals (male n = 5, female n = 2), while 92 conferred with ID/DD (male n = 53, female n = 36, n = 3 unknown sex).
In regard to the duplication syndrome, different studies detected children with speech delay including autistic symptoms (Berg et al. 2007b; Van der Aa et al. 2009; Sanders et al. 2011) or without autistic symptoms (Torniero et al. 2007) as well as cognitive dysfunction ranging from ID to normal cognitive abilities (Somerville et al. 2005) or general DD (Depienne et al. 2009). Interestingly, WBS is characterized mostly by a highly social and empathic personality (Pober 2010a), which contrasts the autistic symptoms observed in the patients with duplications. Our patient fits well into the described phenotypic spectrum of HFA with the autistic presentation and absent cognitive impairment.
“Patient A092”
The patient, a 10-year-old boy, was diagnosed with Asperger syndrome (F84.5; IQ = 124) without any comorbidity, but showed some ADHD symptoms without fulfilling the full diagnosis for ADHD. The patient did not take any medication.
We detected a 1.4-Mb large duplication in 16p13.11 (hg19 chr16:14927356–16328781), encompassing 22 genes in total, 10 OMIM genes. Five were brain-related (Table 2). According to DECIPHER, 437 individuals carry overlapping CNVs, in which 39 individuals with autistic symptoms were found (male n = 24, female n = 10, n = 2 known sex), while ID/DD consisted of 62 females and 85 males (n = 11 unknown sex).
Various studies showed that deletions within chr16p13.11 are associated with a variety of neuropsychiatric disorders such as DD and behavioral abnormalities, like ADHD and ASD (Nagamani et al. 2011; Fujitani et al. 2017). Ramalingam and colleagues (Ramalingam et al. 2011) detected duplications within chr16p13.11 in patients with ID and autistic symptoms. In another study, patients with duplications in this region were found with clinical features including difficulties with social interactions, which were comparable with autistic symptoms (Nagamani et al. 2011). Duplication in this region has also been previously described in patients with speech delay and learning difficulties (Hannes et al. 2009).
Interestingly, we found in our patient the same duplication with similar location (chromosome 16p13.11) as Gazzeloone et al. reported in a pediatric patient who suffered from OCD (Gazzellone et al. 2016). However, the duplication in his study was smaller (783 kb) than in our patient. This locus has been associated with neurocognitive disorders like autism and OCD (Gazzellone et al. 2016). Despite our rather small study sample, we found another patient (A044) with overlapping duplication as found in A092 (Table 2). The clinical manifestations of our two patients associated with 16p13.11 duplications are in agreement with the clinical description in previous studies and suggests pathogenicity in the context of ASD (Allach El Khattabi et al. 2018).
“Patient A044”
The 10-year-old boy was diagnosed with Asperger syndrome (F84.5; IQ = 87; details Table 2) with a comorbid OCD and congenital hypothyroidism at the time of investigation. The patient was medicated with methylphenidate and levothyroxine.
Beside a 1.6-Mb duplication on chr16p13.11 (hg19 chr16: 14899277–16494783), very similar to the one observed in the patient A092 and discussed above, we detected an additional 8.6-Mb large deletion on 8q24.21–q24.3 (hg19 chr8:131409413–140033208), encompassing 23 genes in total and 14 OMIM genes. Four genes are brain-related.
A case report with a similar deletion described a patient with multiple congenital malformations, mental delay, and seizures (Verheij et al. 2009). According to DECIPHER, 75 individuals were found to have overlapping gene variants similar to hg19 chr8:131409413–140033208 (34 males, 28 females, 13 of unknown sex). There was a predominance of males showing autistic behavior (males n = 5; females n = 0), or ID or DD (males n = 13; females n = 7).
A recent genome-wide study performed a quantitative linkage analysis to the autism endophenotype (social responsiveness) and identified two loci on chromosome 8 (Lowe et al. 2015). They detected a peak SNP on chr8q24.22, where ASAP1 is located. Interestingly, this gene is deleted in our patient as well. Another gene, the KCNQ3 (Potassium Channel, Voltage-Gated KQT-Like Subfamily Q, Member 3), a brain-related gene, was deleted in our patient A044. In the study by Rauch and colleagues (2012), aberrations involving KCNQ3 in a few families with ID were reported (Rauch et al. 2012). Moreover, this gene was found to be involved in epilepsy (Miceli et al. 1993). Interestingly, our patient had once a seizure at the age of 12 months that did not reoccur since (till age 15). Curry et al. illustrated two unrelated patients with ID and large homozygous deletions (> 150 kb). One patient with ID showed a deletion in 8q24.2 (Curry et al. 2008). Furthermore, FAM135B and COL22A1 (Tsang et al. 2013), present in the deleted region of our patient, were identified as candidate genes for ASD in some studies, but were not investigated particularly in HFA patients up to now.
“Patient A114”
The patient is a 9-year-old boy presenting with atypical autism (F84; IQ = 87) with comorbid ADHD (F90.0) medicated with methylphenidate, comorbid transient tic disorder (F95.0), and a combined reading and spelling disorder (F81.0).
We detected a 2.2-Mb deletion on 2q37.2 (hg19 chr2:240633456-242783384), encompassing 40 genes in total and 23 OMIM genes. Eight genes were brain-related (Table 2). According to the DECIPHER, 113 individuals conferred with gene variations at the same position as hg19 chr2:240633456-242783384 (male n = 46, female n = 52, n = 15 unknown sex). Nine had autism (male n = 2, female n = 3, n = 4 unknown sex), while 50 conferred ID/DD with ratio of 0.7 between sexes (male n = 18, female n = 27, n = 5 unknown sex).
This deleted region encompasses the 2q37 deletion syndrome characterized by hypotonia, mild-to-severe ID, DD, and other facial or physical abnormalities and sometimes kidney tumor (Wilms tumor) (Doherty and Lacbawan 1993). The study by Felder et al. (2009) described a patient with 2q37 deletion syndrome (features of Albright Hereditary Osteodystrophy). The deleted region included the following genes FARP2, HDLBP, and PASK (Felder et al. 2009) (which were deleted in our patient, too), whose expression analyses performed on lymphoblastoid cell lines showed a considerably downregulation. They hypothesized that haploinsufficiency of these genes are possibly responsible for the patient’s phenotype (Felder et al. 2009). In our patient, all three genes were affected in the deletion that could explain ASD.
Several genes deleted in the patients A114 have been linked to ASD, ID and/or DD. For example, Wheeler et al. claimed that the deleted region contains next to the coding sequence of HDAC4 two uncharacterized non-coding RNA sequences like LOC150935 (contained in the deletion our patient carries). They concluded that haploinsufficiency of HDAC4 does not cause ID in their patients (Wheeler et al. 2014).
In the study by Imitola et al. (2015), the deleted region was identified in a patient fulfilling the criteria for this above-mentioned syndrome with DD. This deletion contains those genes which are also affected in our patient: DTYMK, SEPT2, THAP4, PPP1R7, and STK25, whereas network analysis revealed that STK25 was associated with neural development (Imitola et al. 2015). Puffenberger et al. (2012) performed an exome sequencing on two children from the Wisconsin sibship and revealed that the PRR21 variant cannot be causative for the general DD and ASD of these patients (Puffenberger et al. 2012). The case report by Devillard et al. (2010) described a boy with autism and a deletion of the distal breakpoint at 2q37.3. He showed a cognitive delay (IQ 46–50). High-resolution SNP microarray confirmed the deletion of the gene OTOS and C2orf54 located at 2q37.3 (Devillard et al. 2010). Smith et al. (2001) evaluated four genes mapping in the 2q37.2 region, whereas GPC1 is the most likely candidate gene for autism (Smith et al. 2001). The patient mentioned in Smith’s work showed average score in the intelligence test. The study by Leroy and colleagues (2013) described 14 intellectually deficient patients with a 2.6–8.8 Mb large 2q37 deletion (Leroy et al. 2013). Next to the ID, the patient displayed with morphological and behavioral problems like ASD. They identified candidate genes like ATG4B, PASK, HDLBP, and FARP2, which were detected in our patient, too (Leroy et al. 2013).
Conclusion
Rare large CNVs have emerged as the major pathogenic factors, amongst others for ASD and ID. To find etiologically relevant factors for social interaction and communication deficits together with restricted and repetitive behaviors, the core defining features of ASD - HFA - was assumed to represent a very specific phenotype for these ASD features. Here we present the results of a high-resolution chromosomal microarray CNV analysis of children and adolescents with HFA, often understudied in ASD genetic studies. Previous studies demonstrated large chromosomal aberrations in ASD to be associated with ID.
In the present study, the patients suffered from high-functioning ASD without ID. Surprisingly, we detected in six patients large CNVs, up to now associated with ID and additional features. A limitation of this study is the fact that only one tissue type per patient was analyzed (peripheral blood, or saliva). Therefore, we cannot exclude that the abnormalities observed are in reality mosaics, a feature that might explain the absent of ID and of other malformations in our patients. Another limitation of this study is that no genetic data from the parents were available and it was not possible to assess whether the CNVs are de novo or inherited.
Comparable to other studies conducted in early onset OCD patients (Grünblatt et al. 2017) and HFA (Gilman et al. 2011), in the present investigation, brain-associated CNVs were significantly more often seen in HFA compared to controls and thus confirm the previous findings. Our detailed discussion of the individual findings further illustrates the phenotypes associated with such CNVs and helps to improve genetic counseling in affected families.
In summary, this study suggests that large CNVs can be associated with autistic symptoms seen in HFA. Thus, our results indicate that a large number of structural variants like CNVs might still be unreported in psychiatric disorders in general and especially for HFA.
Availability of data and materials
The data sets generated and/or analyzed during the current study are not publicly available due to limits in consents, but are available from the corresponding author on reasonable request.
Abbreviations
- ADAD2 :
-
Adenosine deaminase domain containing 2
- ADCY9 :
-
Adenylate cyclase 8
- ADHD:
-
Attention deficit hyperactivity disorder
- AHI1 :
-
Abelson helper integration site 1
- ARL13B :
-
ADP-ribosylation factor-like 13B
- ARL6 :
-
ADP-riboylation factor-like 6
- ASAP1 :
-
ArfGAP with SH3 domain, ankyrin repeat, and PH domain 1
- ASD:
-
Autism spectrum disorder
- BBS:
-
Bardet–Biedl syndrome
- BRD1:
-
Bromodomain-containing protein 1
- CBCL:
-
Child behavior checklist
- CC2D2A :
-
Coiled-coil and C2 domain containing 2A
- Celsr1 :
-
Cadherin, EGF LAG seven-pass G-type receptor 1
- CEP290 :
-
Centrosomal protein 290
- ChAS:
-
Chromosome analysis suite
- CMA:
-
Chromosomal microarray analysis
- CNS:
-
Central nervous system
- CNV:
-
Copy-number variant
- COL22A1 :
-
Collagen, Type XXII, alpha 1
- CRYBG3 :
-
Crystallin beta-gamma domain containing 3
- DD:
-
Developmental delay
- DGV:
-
Database of genomic variants
- DHFRL1 :
-
Dihydrofolate reductase-like 1
- DNA:
-
Deoxyribonucleic acid
- EPHA6 :
-
EPH Receptor A6
- FAM135B :
-
C8orfK32, family with sequence similarity 135 member B
- FAM19A5 :
-
Family sequence similarity 19
- FARP2 :
-
FERM, RhoGEF, and pleckstrin domain-containing protein
- GABRR3 :
-
Gamma-aminobutyric acid (GABA) A receptor, Rho 3
- GRAMD4 :
-
GRAM domain containing 4
- GWAS:
-
Genome-wide association study
- HDAC4 :
-
Histone deacetylase 4
- HDLBP :
-
High-density lipoprotein binding protein
- HFA:
-
High-functioning autism
- ID:
-
Intellectual disability
- INPP5E :
-
Inositol polyphosphate-5-phosphatase E
- IQ:
-
Intelligence quotient
- Kb:
-
Kilobyte
- KCNG4 :
-
Potassium voltage-gated channel subfamily KQT member 4
- KCNQ3 :
-
Potassium channel, voltage-gated KQT-like subfamily Q, member 3
- KHDRBS3:
-
KH domain containing, RNA binding, signal transduction associated 3
- LOC150935 :
-
Uncharacterized LOC150935
- MAPK8IP2 :
-
Mitogen-activated protein kinase 8 interacting protein 2
- Mb:
-
Megabyte
- MINA :
-
MYC-induced nuclear antigen
- NPHP1 :
-
Nephrocystin-1
- NRXN1:
-
Neurexin-1-alpha
- NSUN3 :
-
NOP2/sun domain family, member 3
- OCD:
-
Obsessive-compulsive disorder
- PASK :
-
Proline-alanine-rich STE2 0-related kinase
- PLXNB2 :
-
Plexin B2
- PROS1 :
-
Protein S
- PTCHD1:
-
Patched domain containing 1
- RPGRIP1L :
-
Retinitis pigmentosa GTPase regulator interacting protein 1 like
- SCO2 :
-
SCO2 cytochrome c oxidase assembly
- SCQ:
-
Social communication questionnaire
- SHANK2:
-
SH3 and multiple ankyrin repeat domains protein 2
- SHANK3:
-
SH3 and multiple ankyrin repeat domains protein 3
- STX19 :
-
Syntaxin 19
- TBC1D22A :
-
TBC1 domain family, member 22A
- TMEM67/MKS3 :
-
Transmembrane protein-67
- TTLL8 :
-
Tubulin tyrosine ligase-like family member 8
- TUBGCP6 :
-
Tubulin, gamma complex associated protein 6
- TYMP :
-
Thymidine phosphorylase
- VSTM4 :
-
V-Set and Transmembrane Domain Containing 4
- WBCR:
-
Williams–Beuren critical region
- WBS:
-
Williams–Beuren syndrome
References
Aldinger KA, Kogan J, Kimonis V, Fernandez B, Horn D, Klopocki E, Chung B, Toutain A, Weksberg R, Millen KJ, Barkovich AJ, Dobyns WB (2013) Cerebellar and posterior fossa malformations in patients with autism-associated chromosome 22q13 terminal deletion. Am J Med Genet A 161(1):131–136. https://doi.org/10.1002/ajmg.a.35700
Allach El Khattabi L, Heide S, Caberg J-H, Andrieux J, Doco Fenzy M, Vincent-Delorme C, Callier P, Chantot-Bastaraud S, Afenjar A, Boute-Benejean O, Pierre Cordier M, Faivre L, Francannet C, Gerard M, Goldenberg A, Masurel-Paulet A, Mosca-Boidron A-L, Marle N, Anne M, Pipiras E (2018) 16p13.11 microduplication in 45 new patients: refined clinical significance and genotype-phenotype correlations. J Med Genet. https://doi.org/10.1136/jmedgenet-2018-105389
American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), 5th edn. APA, Washington DC
Asadollahi R, Oneda B, Joset P, Azzarello-Burri S, Bartholdi D, Steindl K, Vincent M, Cobilanschi J, Sticht H, Baldinger R, Reissmann R, Sudholt I, Thiel CT, Ekici AB, Reis A, Bijlsma EK, Andrieux J, Dieux A, FitzPatrick D, Ritter S, Baumer A, Latal B, Plecko B, Jenni OG, Rauch A (2014) The clinical significance of small copy number variants in neurodevelopmental disorders. J Med Genet 51(10):677–688. https://doi.org/10.1136/jmedgenet-2014-102588
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G (2000) Gene ontology: tool for the unification of biology. The gene ontology consortium. Nat Genet 25(1):25–29. https://doi.org/10.1038/75556
Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ (2004) Autism and abnormal development of brain connectivity. J Neurosci 24(42):9228–9231. https://doi.org/10.1523/JNEUROSCI.3340-04.2004
Berg JS, Brunetti-Pierri N, Peters SU, Kang S-HL, Fong C-T, Salamone J, Freedenberg D, Hannig VL, Prock LA, Miller DT, Raffalli P, Harris DJ, Erickson RP, Cunniff C, Clark GD, Blazo MA, Peiffer DA, Gunderson KL, Sahoo T, Patel A, Lupski JR, Beaudet AL, Cheung SW (2007) Speech delay and autism spectrum behaviors are frequently associated with duplication of the 7q11.23 Williams-Beuren syndrome region. Genet Med 9(7):427–441
Bölte S, Poustka F (2006) Fragebogen zur Sozialen Kommunikation - Autismus-Screening; Deutsche Fassung des Social Communication Questionnaire (SCQ). Verlag Hans Huber, Bern
Bölte S, Crecelius K, Poustka F (2000) Der Fragebogen über Verhalten und soziale Kommunikation (VSK). https://doi.org/10.1026//0012-1924.46.3.149
Bölte S, Le Couteur A, Lord C, Rutter M (2006) Diagnostisches Interview für Autismus-revidiert: ADI-R; deutsche Fassung des Autism diagnostic interview-revised (ADI-R) von Michael Rutter, Ann Le Couteur und Catherine Lord; Manual. Huber
Bonaglia MC, Giorda R, Borgatti R, Felisari G, Gagliardi C, Selicorni A, Zuffardi O (2001) Disruption of the ProSAP2 gene in a t(12;22)(q24.1;q13.3) is associated with the 22q13.3 deletion syndrome. Am J Hum Genet 69(2):261–268. https://doi.org/10.1086/321293
Chiang H-M, Tsai LY, Cheung YK, Brown A, Li H (2014) A meta-analysis of differences in IQ profiles between individuals with asperger’s disorder and high-functioning autism. J Autism Dev Disord 44(7):1577–1596. https://doi.org/10.1007/s10803-013-2025-2
Chiocchetti AG, Kopp M, Waltes R, Haslinger D, Duketis E, Jarczok TA, Poustka F, Voran A, Graab U, Meyer J, Klauck SM, Fulda S, Freitag CM (2015) Variants of the CNTNAP2 5’ promoter as risk factors for autism spectrum disorders: a genetic and functional approach. Mol Psychiatry 20(7):839–849. https://doi.org/10.1038/mp.2014.103
Colvert E, Tick B, McEwen F, Stewart C, Curran SR, Woodhouse E, Gillan N, Hallett V, Lietz S, Garnett T, Ronald A, Plomin R, Rijsdijk F, Happe F, Bolton P (2015) Heritability of autism spectrum disorder in a UK population-based twin sample. JAMA Psychiatry 72(5):415–423. https://doi.org/10.1001/jamapsychiatry.2014.3028
Consortium IMGSoA (2001) A genome-wide screen for autism: strong evidence for linkage to chromosomes 2q, 7q, and 16p. Am J Hum Genet 69(3):570–581
Curry CJ, Mao R, Aston E, Mongia SK, Treisman T, Procter M, Chou B, Whitby H, South ST, Brothman AR (2008) Homozygous deletions of a copy number change detected by array CGH: a new cause for mental retardation? Am J Med Genet A 146A(15):1903–1910. https://doi.org/10.1002/ajmg.a.32450
Depienne C, Moreno-De-Luca D, Heron D, Bouteiller D, Gennetier A, Delorme R, Chaste P, Siffroi JP, Chantot-Bastaraud S, Benyahia B, Trouillard O, Nygren G, Kopp S, Johansson M, Rastam M, Burglen L, Leguern E, Verloes A, Leboyer M, Brice A, Gillberg C, Betancur C (2009) Screening for genomic rearrangements and methylation abnormalities of the 15q11-q13 region in autism spectrum disorders. Biol Psychiatry 66(4):349–359. https://doi.org/10.1016/j.biopsych.2009.01.025
Devillard F, Guinchat V, Moreno-De-Luca D, Tabet A-C, Gruchy N, Guillem P, Nguyen Morel M-A, Leporrier N, Leboyer M, Jouk P-S, Lespinasse J, Betancur C (2010) Paracentric inversion of chromosome 2 associated with cryptic duplication of 2q14 and deletion of 2q37 in a patient with autism. Am J Med Genet A 152A(9):2346–2354. https://doi.org/10.1002/ajmg.a.33601
Dilling H, Freyberger HJ, Stieglitz RD (1996) ICD-10 field trial of the diagnostic criteria for research in German-speaking countries. Introduction. Psychopathology 29(5):258–259
Doherty ES, Lacbawan FL (1993) 2q37 Microdeletion Syndrome. In: Pagon RA, Adam MP, Ardinger HH et al. (eds) GeneReviews (R). University of Washington, Seattle All rights reserved., Seattle (WA)
Döpfner M, Lehmkuhl G (2000) FBB-HKS [Rating-scale for hyperkinetic disorder from the diagnostic system for mental disorders in childhood and adolescence according to ICD-10 and DSM-IV (DISYPS-KJ)], 2nd edn. Hofgrefe: Göttingen, Germany
Döpfner M, Plück J, Bölte S, Lenz K, Melchers P, Heim K (eds) (1998) Arbeitsgruppe Kinder- und Jugendlichen- und Familiendiagnostik: Child Behavior Checklist (CBCL); Elternfragebogen über das Verhalten von Kindern und Jugendlichen, 2nd edn. Arbeitsgruppe Kinder-, Jugend- und Familiendiagnostik KJFD, Köln
Edgar R, Domrachev M, Lash AE (2002) Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30(1):207–210. https://doi.org/10.1093/nar/30.1.207
Egger JI, Zwanenburg RJ, van Ravenswaaij-Arts CM, Kleefstra T, Verhoeven WM (2016) Neuropsychological phenotype and psychopathology in seven adult patients with Phelan-McDermid syndrome: implications for treatment strategy. Genes Brain Behav. https://doi.org/10.1111/gbb.12285
Felder B, Radlwimmer B, Benner A, Mincheva A, Tödt G, Beyer KS, Schuster C, Bölte S, Schmötzer G, Klauck SM, Poustka F, Lichter P, Poustka A (2009) FARP2, HDLBP and PASK are downregulated in a patient with autism and 2q37.3 deletion syndrome. Am J Med Genet A 149A(5):952–959. https://doi.org/10.1002/ajmg.a.32779
Fernandez BA, Roberts W, Chung B, Weksberg R, Meyn S, Szatmari P, Joseph-George AM, MacKay S, Whitten K, Noble B (2009) Phenotypic spectrum associated with de novo and inherited deletions and duplications at 16p11. 2 in individuals ascertained for diagnosis of autism spectrum disorder. J Med Genet 47(3):195–203. https://doi.org/10.1136/jmg.2009.069369
Frazier TW, Thompson L, Youngstrom EA, Law P, Hardan AY, Eng C, Morris N (2014) A twin study of heritable and shared environmental contributions to autism. J Autism Dev Disord 44(8):2013–2025. https://doi.org/10.1007/s10803-014-2081-2
Freitag CM, Staal W, Klauck SM, Duketis E, Waltes R (2010) Genetics of autistic disorders: review and clinical implications. Eur Child Adolesc Psychiatry 19(3):169–178. https://doi.org/10.1007/s00787-009-0076-x
Fujitani M, Zhang S, Fujiki R, Fujihara Y, Yamashita T (2017) A chromosome 16p13.11 microduplication causes hyperactivity through dysregulation of miR-484/protocadherin-19 signaling. Mol Psychiatry 22(3):364–374. https://doi.org/10.1038/mp.2016.106
Gazzellone MJ, Zarrei M, Burton CL, Walker S, Uddin M, Shaheen SM, Coste J, Rajendram R, Schachter RJ, Colasanto M, Hanna GL, Rosenberg DR, Soreni N, Fitzgerald KD, Marshall CR, Buchanan JA, Merico D, Arnold PD, Scherer SW (2016) Uncovering obsessive-compulsive disorder risk genes in a pediatric cohort by high-resolution analysis of copy number variation. J Neurodev Disord 8:36. https://doi.org/10.1186/s11689-016-9170-9
Gillberg C (1998) Asperger syndrome and high-functioning autism. Br J Psychiatry 172(3):200–209. https://doi.org/10.1192/bjp.172.3.200
Gilman SR, Iossifov I, Levy D, Ronemus M, Wigler M, Vitkup D (2011) Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron 70(5):898–907. https://doi.org/10.1016/j.neuron.2011.05.021
Girirajan S, Brkanac Z, Coe BP, Baker C, Vives L, Vu TH, Shafer N, Bernier R, Ferrero GB, Silengo M (2011) Relative burden of large CNVs on a range of neurodevelopmental phenotypes. PLoS Genet 7(11):e1002334
Girirajan S, Dennis MY, Baker C, Malig M, Coe BP, Campbell CD, Mark K, Vu TH, Alkan C, Cheng Z (2013) Refinement and discovery of new hotspots of copy-number variation associated with autism spectrum disorder. Am J Hum Genet 92(2):221–237
Grünblatt E, Oneda B, Ekici AB, Ball J, Geissler J, Uebe S, Romanos M, Rauch A, Walitza S (2017) High resolution chromosomal microarray analysis in paediatric obsessive-compulsive disorder. BMC Med Genom 10(1):68. https://doi.org/10.1186/s12920-017-0299-5
GTEx Consortium (2013) The Genotype-tissue expression (GTEx) project. Nat Genet 45(6):580–585. https://doi.org/10.1038/ng.2653
Guilherme RS, Soares KC, Simioni M, Vieira TP, Gil-da-Silva-Lopes VL, Kim CA, Brunoni D, Spinner NB, Conlin LK, Christofolini DM, Kulikowski LD, Steiner CE, Melaragno MI (2014) Clinical, cytogenetic, and molecular characterization of six patients with ring chromosomes 22, including one with concomitant 22q11.2 deletion. Am J Med Genet A 164(7):1659–1665. https://doi.org/10.1002/ajmg.a.36512
Guo J, Otis JM, Higginbotham H, Monckton C, Cheng J, Asokan A, Mykytyn K, Caspary T, Stuber GD, Anton ES (2017) Primary Cilia Signaling Shapes the Development of Interneuronal Connectivity. Dev Cell 42(3):286–300. https://doi.org/10.1016/j.devcel.2017.07.010
Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Miller J, Fedele A, Collins J, Smith K (2011) Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 68(11):1095–1102
Hannes FD, Sharp AJ, Mefford HC, de Ravel T, Ruivenkamp CA, Breuning MH, Fryns JP, Devriendt K, Van Buggenhout G, Vogels A, Stewart H, Hennekam RC, Cooper GM, Regan R, Knight SJ, Eichler EE, Vermeesch JR (2009) Recurrent reciprocal deletions and duplications of 16p13.11: the deletion is a risk factor for MR/MCA while the duplication may be a rare benign variant. J Med Genet 46(4):223–232. https://doi.org/10.1136/jmg.2007.055202
Imitola J, Khurana DS, Teplyuk NM, Zucker M, Jethva R, Legido A, Krichevsky AM, Frangieh M, Walsh CA, Carvalho KS (2015) A novel 2q37 microdeletion containing human neural progenitors genes including STK25 results in severe developmental delay, epilepsy, and microcephaly. Am J Med Genet A 167(11):2808–2816. https://doi.org/10.1002/ajmg.a.37268
Kaminsky EB, Kaul V, Paschall J, Church DM, Bunke B, Kunig D, Moreno-De-Luca D, Moreno-De-Luca A, Mulle JG, Warren ST (2011) An evidence-based approach to establish the functional and clinical significance of copy number variants in intellectual and developmental disabilities. Genet Med 13(9):777–784
Kaufman AS, Kaufman NL, Melchers P, Preuss U (2009) Kaufman Assessment Battery for Children, Deutsche Version. Individualtest zur Messung von Intelligenz und Fertigkeit bei Kindern. Hogrefe
Lai M-C, Lombardo MV, Auyeung B, Chakrabarti B, Baron-Cohen S (2015) Sex/gender differences and autism: setting the scene for future research. J Am Acad Child Adolesc Psychiatry 54(1):11–24. https://doi.org/10.1016/j.jaac.2014.10.003
Leblond CS, Nava C, Polge A, Gauthier J, Huguet G, Lumbroso S, Giuliano F, Stordeur C, Depienne C, Mouzat K, Pinto D, Howe J, Lemiere N, Durand CM, Guibert J, Ey E, Toro R, Peyre H, Mathieu A, Amsellem F, Rastam M, Gillberg IC, Rappold GA, Holt R, Monaco AP, Maestrini E, Galan P, Heron D, Jacquette A, Afenjar A, Rastetter A, Brice A, Devillard F, Assouline B, Laffargue F, Lespinasse J, Chiesa J, Rivier F, Bonneau D, Regnault B, Zelenika D, Delepine M, Lathrop M, Sanlaville D, Schluth-Bolard C, Edery P, Perrin L, Tabet AC, Schmeisser MJ, Boeckers TM, Coleman M, Sato D, Szatmari P, Scherer SW, Rouleau GA, Betancur C, Leboyer M, Gillberg C, Delorme R, Bourgeron T (2014) Meta-analysis of SHANK Mutations in Autism Spectrum Disorders: a gradient of severity in cognitive impairments. PLoS Genet 10(9):e1004580. https://doi.org/10.1371/journal.pgen.1004580
Leroy C, Landais E, Briault S, David A, Tassy O, Gruchy N, Delobel B, Gregoire M-J, Leheup B, Taine L, Lacombe D, Delrue M-A, Toutain A, Paubel A, Mugneret F, Thauvin-Robinet C, Arpin S, Le Caignec C, Jonveaux P, Beri M, Leporrier N, Motte J, Fiquet C, Brichet O, Mozelle-Nivoix M, Sabouraud P, Golovkine N, Bednarek N, Gaillard D, Doco-Fenzy M (2013) The 2q37-deletion syndrome: an update of the clinical spectrum including overweight, brachydactyly and behavioural features in 14 new patients. Eur J Hum Genet 21(6):602–612. https://doi.org/10.1038/ejhg.2012.230
Lord C, Rutter M, Le Couteur A (1994) Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24(5):659–685
Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop S (2012) Autism diagnostic observation schedule: ADOS-2. Western Psychological Services Los Angeles
Lowe JK, Werling DM, Constantino JN, Cantor RM, Geschwind DH (2015) Quantitative linkage analysis to the autism endophenotype social responsiveness identifies genome-wide significant linkage to two regions on chromosome 8. Am J Psychiatry 172(3):266–275. https://doi.org/10.1176/appi.ajp.2014.14050576
Marshall CR, Scherer SW (2012) Detection and characterization of copy number variation in autism spectrum disorder. Methods Mol Biol 838:115–135. https://doi.org/10.1007/978-1-61779-507-7_5
Marshall CR, Noor A, Vincent JB, Lionel AC, Feuk L, Skaug J, Shago M, Moessner R, Pinto D, Ren Y, Thiruvahindrapduram B, Fiebig A, Schreiber S, Friedman J, Ketelaars CE, Vos YJ, Ficicioglu C, Kirkpatrick S, Nicolson R, Sloman L, Summers A, Gibbons CA, Teebi A, Chitayat D, Weksberg R, Thompson A, Vardy C, Crosbie V, Luscombe S, Baatjes R, Zwaigenbaum L, Roberts W, Fernandez B, Szatmari P, Scherer SW (2008) Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet 82(2):477–488. https://doi.org/10.1016/j.ajhg.2007.12.009
Mefford HC, Batshaw ML, Hoffman EP (2012) Genomics, intellectual disability, and autism. N Engl J Med 366(8):733–743. https://doi.org/10.1056/NEJMra1114194
Miceli F, Soldovieri MV, Joshi N, Weckhuysen S, Cooper EC, Taglialatela M (1993) KCNQ3-Related Disorders. In: Adam MP, Ardinger HH, Pagon RA et al. (eds) GeneReviews((R)). University of Washington, Seattle All rights reserved., Seattle (WA)
Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16(3):1215
Mitz AR, Philyaw TJ, Boccuto L, Shcheglovitov A, Sarasua SM, Kaufmann WE, Thurm A (2018) Identification of 22q13 genes most likely to contribute to Phelan McDermid syndrome. Eur J Hum Genet 26(3):293–302. https://doi.org/10.1038/s41431-017-0042-x
Nagamani SC, Erez A, Bader P, Lalani SR, Scott DA, Scaglia F, Plon SE, Tsai CH, Reimschisel T, Roeder E, Malphrus AD, Eng PA, Hixson PM, Kang SH, Stankiewicz P, Patel A, Cheung SW (2011) Phenotypic manifestations of copy number variation in chromosome 16p13.11. Eur J Hum Genet 19(3):280–286. https://doi.org/10.1038/ejhg.2010.184
Nyffeler J, Walitza S, Bobrowski E, Gundelfinger R, Grünblatt E (2014) Association study in siblings and case-controls of serotonin- and oxytocin-related genes with high functioning autism. J Mol Psychiatry 2(1):1. https://doi.org/10.1186/2049-9256-2-1
Petermann F, Petermann U (2010) HAWIK-IV (3. erweiterte Auflage). Huber, Bern
Phelan K, McDermid HE (2012) The 22q133 deletion syndrome (Phelan-McDermid Syndrome). Mol Syndromol 2(3–5):186–201
Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R, Conroy J, Magalhaes TR, Correia C, Abrahams BS, Almeida J, Bacchelli E, Bader GD, Bailey AJ, Baird G, Battaglia A, Berney T, Bolshakova N, Bolte S, Bolton PF, Bourgeron T, Brennan S, Brian J, Bryson SE, Carson AR, Casallo G, Casey J, Chung BH, Cochrane L, Corsello C, Crawford EL, Crossett A, Cytrynbaum C, Dawson G, de Jonge M, Delorme R, Drmic I, Duketis E, Duque F, Estes A, Farrar P, Fernandez BA, Folstein SE, Fombonne E, Freitag CM, Gilbert J, Gillberg C, Glessner JT, Goldberg J, Green A, Green J, Guter SJ, Hakonarson H, Heron EA, Hill M, Holt R, Howe JL, Hughes G, Hus V, Igliozzi R, Kim C, Klauck SM, Kolevzon A, Korvatska O, Kustanovich V, Lajonchere CM, Lamb JA, Laskawiec M, Leboyer M, Le Couteur A, Leventhal BL, Lionel AC, Liu XQ, Lord C, Lotspeich L, Lund SC, Maestrini E, Mahoney W, Mantoulan C, Marshall CR, McConachie H, McDougle CJ, McGrath J, McMahon WM, Merikangas A, Migita O, Minshew NJ, Mirza GK, Munson J, Nelson SF, Noakes C, Noor A, Nygren G, Oliveira G, Papanikolaou K, Parr JR, Parrini B, Paton T, Pickles A, Pilorge M, Piven J, Ponting CP, Posey DJ, Poustka A, Poustka F, Prasad A, Ragoussis J, Renshaw K, Rickaby J, Roberts W, Roeder K, Roge B, Rutter ML, Bierut LJ, Rice JP, Salt J, Sansom K, Sato D, Segurado R, Sequeira AF, Senman L, Shah N, Sheffield VC, Soorya L, Sousa I, Stein O, Sykes N, Stoppioni V, Strawbridge C, Tancredi R, Tansey K, Thiruvahindrapduram B, Thompson AP, Thomson S, Tryfon A, Tsiantis J, Van Engeland H, Vincent JB, Volkmar F, Wallace S, Wang K, Wang Z, Wassink TH, Webber C, Weksberg R, Wing K, Wittemeyer K, Wood S, Wu J, Yaspan BL, Zurawiecki D, Zwaigenbaum L, Buxbaum JD, Cantor RM, Cook EH, Coon H, Cuccaro ML, Devlin B, Ennis S, Gallagher L, Geschwind DH, Gill M, Haines JL, Hallmayer J, Miller J, Monaco AP, Nurnberger JI Jr, Paterson AD, Pericak-Vance MA, Schellenberg GD, Szatmari P, Vicente AM, Vieland VJ, Wijsman EM, Scherer SW, Sutcliffe JS, Betancur C (2010) Functional impact of global rare copy number variation in autism spectrum disorders. Nature 466(7304):368–372. https://doi.org/10.1038/nature09146
Pober BR (2010) Williams-Beuren syndrome. N Engl J Med 362(3):239–252. https://doi.org/10.1056/NEJMra0903074
Prasad A, Merico D, Thiruvahindrapuram B, Wei J, Lionel AC, Sato D, Rickaby J, Lu C, Szatmari P, Roberts W, Fernandez BA, Marshall CR, Hatchwell E, Eis PS, Scherer SW (2012) A discovery resource of rare copy number variations in individuals with autism spectrum disorder. G3 (Bethesda) 2(12):1665–1685. https://doi.org/10.1534/g3.112.004689
Puffenberger EG, Jinks RN, Wang H, Xin B, Fiorentini C, Sherman EA, Degrazio D, Shaw C, Sougnez C, Cibulskis K (2012) A homozygous missense mutation in HERC2 associated with global developmental delay and autism spectrum disorder. Hum Mutat 33(12):1639–1646
Ramalingam A, Zhou XG, Fiedler SD, Brawner SJ, Joyce JM, Liu HY, Yu S (2011) 16p13.11 duplication is a risk factor for a wide spectrum of neuropsychiatric disorders. J Hum Genet 56(7):541–544. https://doi.org/10.1038/jhg.2011.42
Rauch A, Wieczorek D, Graf E, Wieland T, Endele S, Schwarzmayr T, Albrecht B, Bartholdi D, Beygo J, Di Donato N, Dufke A, Cremer K, Hempel M, Horn D, Hoyer J, Joset P, Ropke A, Moog U, Riess A, Thiel CT, Tzschach A, Wiesener A, Wohlleber E, Zweier C, Ekici AB, Zink AM, Rump A, Meisinger C, Grallert H, Sticht H, Schenck A, Engels H, Rappold G, Schrock E, Wieacker P, Riess O, Meitinger T, Reis A, Strom TM (2012) Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet 380(9854):1674–1682. https://doi.org/10.1016/S0140-6736(12)61480-9
Rosenberg RE, Law JK, Yenokyan G, McGready J, Kaufmann WE, Law PA (2009) Characteristics and concordance of autism spectrum disorders among 277 twin pairs. Arch Pediatr Adolesc Med 163(10):907–914
Rühl D, Bölte S, Feineis-Matthews S, Poustka F (2004) Diagnostische Beobachtungsskala für Autistische Störungen; Deutsche Fassung der Autism Diagnostic Observation Schedule (ADOS). Verlag Hans Huber, Switzerland
Rutter M, Bailey A, Lord C, Cianchetti C, Fancello GS (2007) SCQ: Social Communication Questionnaire: Manuale. Giunti OS
Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, Chu SH, Moreau MP, Gupta AR, Thomson SA, Mason CE, Bilguvar K, Celestino-Soper PB, Choi M, Crawford EL, Davis L, Wright NR, Dhodapkar RM, DiCola M, DiLullo NM, Fernandez TV, Fielding-Singh V, Fishman DO, Frahm S, Garagaloyan R, Goh GS, Kammela S, Klei L, Lowe JK, Lund SC, McGrew AD, Meyer KA, Moffat WJ, Murdoch JD, O’Roak BJ, Ober GT, Pottenger RS, Raubeson MJ, Song Y, Wang Q, Yaspan BL, Yu TW, Yurkiewicz IR, Beaudet AL, Cantor RM, Curland M, Grice DE, Gunel M, Lifton RP, Mane SM, Martin DM, Shaw CA, Sheldon M, Tischfield JA, Walsh CA, Morrow EM, Ledbetter DH, Fombonne E, Lord C, Martin CL, Brooks AI, Sutcliffe JS, Cook EH Jr, Geschwind D, Roeder K, Devlin B, State MW (2011) Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron 70(5):863–885. https://doi.org/10.1016/j.neuron.2011.05.002
Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A (2014) The familial risk of autism. JAMA 311(17):1770–1777. https://doi.org/10.1001/jama.2014.4144
Schaaf CP, Sabo A, Sakai Y, Crosby J, Muzny D, Hawes A, Lewis L, Akbar H, Varghese R, Boerwinkle E, Gibbs RA, Zoghbi HY (2011) Oligogenic heterozygosity in individuals with high-functioning autism spectrum disorders. Hum Mol Genet 20(17):3366–3375. https://doi.org/10.1093/hmg/ddr243
Shishido E, Aleksic B, Ozaki N (2014) Copy-number variation in the pathogenesis of autism spectrum disorder. Psychiatry Clin Neurosci 68(2):85–95
Slavotinek A, Maher E, Gregory P, Rowlandson P, Huson SM (1997) The phenotypic effects of chromosome rearrangement involving bands 7q21.3 and 22q13.3. J Med Genet 34(10):857–861. https://doi.org/10.1136/jmg.34.10.857
Smith M, Escamilla JR, Filipek P, Bocian ME, Modahl C, Flodman P, Spence MA (2001) Molecular genetic delineation of 2q37.3 deletion in autism and osteodystrophy: report of a case and of new markers for deletion screening by PCR. Cytogenet Genome Res 94(1–2):15–22
Somerville MJ, Mervis CB, Young EJ, Seo EJ, del Campo M, Bamforth S, Peregrine E, Loo W, Lilley M, Perez-Jurado LA, Morris CA, Scherer SW, Osborne LR (2005) Severe expressive-language delay related to duplication of the Williams-Beuren locus. N Engl J Med 353(16):1694–1701. https://doi.org/10.1056/NEJMoa051962
Soorya L, Kolevzon A, Zweifach J, Lim T, Dobry Y, Schwartz L, Frank Y, Wang AT, Cai G, Parkhomenko E, Halpern D, Grodberg D, Angarita B, Willner JP, Yang A, Canitano R, Chaplin W, Betancur C, Buxbaum JD (2013) Prospective investigation of autism and genotype-phenotype correlations in 22q13 deletion syndrome and SHANK3 deficiency. Mol Autism 4(1):18. https://doi.org/10.1186/2040-2392-4-18
Tellegen P, Winkel M, Laros J (2003) Snijders oomen non-verbal intelligence test revised (SON-R). Hofgrefe-Verlag, Göttingen, Germany
Tick B, Bolton P, Happe F, Rutter M, Rijsdijk F (2016) Heritability of autism spectrum disorders: a meta-analysis of twin studies. J Child Psychol Psychiatry 57(5):585–595. https://doi.org/10.1111/jcpp.12499
Torniero C, dalla Bernardina B, Novara F, Vetro A, Ricca I, Darra F, Pramparo T, Guerrini R, Zuffardi O (2007) Cortical dysplasia of the left temporal lobe might explain severe expressive-language delay in patients with duplication of the Williams-Beuren locus. Eur J Hum Genet 15(1):62–67. https://doi.org/10.1038/sj.ejhg.5201730
Tsang KM, Croen LA, Torres AR, Kharrazi M, Delorenze GN, Windham GC, Yoshida CK, Zerbo O, Weiss LA (2013) A genome-wide survey of transgenerational genetic effects in autism. PLoS One 8(10):e76978
Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Ponten F (2015) Proteomics. Tissue-based map of the human proteome. Science 347(6220):1260419. https://doi.org/10.1126/science.1260419
Uzunova G, Pallanti S, Hollander E (2016) Excitatory/inhibitory imbalance in autism spectrum disorders: implications for interventions and therapeutics. World J Biol Psychiatry 17(3):174–186. https://doi.org/10.3109/15622975.2015.1085597
Van der Aa N, Rooms L, Vandeweyer G, van den Ende J, Reyniers E, Fichera M, Romano C, Delle Chiaie B, Mortier G, Menten B, Destree A, Maystadt I, Mannik K, Kurg A, Reimand T, McMullan D, Oley C, Brueton L, Bongers EM, van Bon BW, Pfund R, Jacquemont S, Ferrarini A, Martinet D, Schrander-Stumpel C, Stegmann AP, Frints SG, de Vries BB, Ceulemans B, Kooy RF (2009) Fourteen new cases contribute to the characterization of the 7q11.23 microduplication syndrome. Eur J Med Genet 52(2–3):94–100. https://doi.org/10.1016/j.ejmg.2009.02.006
van der Zwaag B, Franke L, Poot M, Hochstenbach R, Spierenburg HA, Vorstman JA, van Daalen E, de Jonge MV, Verbeek NE, Brilstra EH, van ‘t Slot R, Ophoff RA, van Es MA, Blauw HM, Veldink JH, Buizer-Voskamp JE, Beemer FA, van den Berg LH, Wijmenga C, van Amstel HK, van Engeland H, Burbach JP, Staal WG (2009) Gene-network analysis identifies susceptibility genes related to glycobiology in autism. PLoS One 4(5):e5324. https://doi.org/10.1371/journal.pone.0005324
Verheij JB, de Munnik SA, Dijkhuizen T, de Leeuw N, Olde Weghuis D, van den Hoek GJ, Rijlaarsdam RS, Thomasse YE, Dikkers FG, Marcelis CL, van Ravenswaaij-Arts CM (2009) An 8.35 Mb overlapping interstitial deletion of 8q24 in two patients with coloboma, congenital heart defect, limb abnormalities, psychomotor retardation and convulsions. Eur J Med Genet 52(5):353–357. https://doi.org/10.1016/j.ejmg.2009.05.006
Vissers ME, Cohen MX, Geurts HM (2012) Brain connectivity and high functioning autism: a promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neurosci Biobehav Rev 36(1):604–625. https://doi.org/10.1016/j.neubiorev.2011.09.003
Vorstman JAS, Staal WG, van Daalen E, van Engeland H, Hochstenbach PFR, Franke L (2005) Identification of novel autism candidate regions through analysis of reported cytogenetic abnormalities associated with autism. Mol Psychiatry 11 (1):18–28. doi:http://www.nature.com/mp/journal/v11/n1/suppinfo/4001757s1.html
Wang K, Zhang H, Ma D, Bucan M, Glessner JT, Abrahams BS, Salyakina D, Imielinski M, Bradfield JP, Sleiman PM, Kim CE, Hou C, Frackelton E, Chiavacci R, Takahashi N, Sakurai T, Rappaport E, Lajonchere CM, Munson J, Estes A, Korvatska O, Piven J, Sonnenblick LI, Alvarez Retuerto AI, Herman EI, Dong H, Hutman T, Sigman M, Ozonoff S, Klin A, Owley T, Sweeney JA, Brune CW, Cantor RM, Bernier R, Gilbert JR, Cuccaro ML, McMahon WM, Miller J, State MW, Wassink TH, Coon H, Levy SE, Schultz RT, Nurnberger JI, Haines JL, Sutcliffe JS, Cook EH, Minshew NJ, Buxbaum JD, Dawson G, Grant SF, Geschwind DH, Pericak-Vance MA, Schellenberg GD, Hakonarson H (2009) Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature 459(7246):528–533. https://doi.org/10.1038/nature07999
Wassink TH, Vieland VJ, Sheffield VC, Bartlett CW, Goedken R, Childress D, Piven J (2008) Posterior probability of linkage analysis of autism dataset identifies linkage to chromosome 16. Psychiatr Genet 18(2):85–91. https://doi.org/10.1097/YPG.0b013e3282f9b48e
Wechsler D (1981) WAIS-R manual: Wechsler adult intelligence scale-revised. Psychological Corporation
Weiss R (2006) CFT 20-R: Grundintelligenztest Skala 2—Revision. Hofgrefe-Verlag, Göttingen
Werling AM, Bobrowski E, Taurines R, Gundelfinger R, Romanos M, Grünblatt E, Walitza S (2015) CNTNAP2 gene in high functioning autism: no association according to family and meta-analysis approaches. J Neural Transm (Vienna). https://doi.org/10.1007/s00702-015-1458-5
Wheeler PG, Huang D, Dai Z (2014) Haploinsufficiency of HDAC4 does not cause intellectual disability in all affected individuals. Am J Med Genet A 164(7):1826–1829
Yingjun X, Haiming Y, Mingbang W, Liangying Z, Jiaxiu Z, Bing S, Qibin Y, Xiaofang S (2017) Copy number variations independently induce autism spectrum disorder. Biosci Rep. https://doi.org/10.1042/bsr20160570
Zoghbi HY (2003) Postnatal neurodevelopmental disorders: meeting at the synapse? Science 302(5646):826–830. https://doi.org/10.1126/science.1089071
Acknowledgments
The authors thank the families, patients, and control volunteers who participated in this research. The authors would like to acknowledge Miryame Hofmann of the Translational Molecular Psychiatry Laboratory, Department of Child and Adolescent Psychiatry and Psychotherapy Zurich, for her technical support.
Funding
The current study was funded by the University of Zurich. The funding body was not involved in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
AW and EG drafted the manuscript, performed literature search and worked on the interpretation of the data. EG participated in the study design and performed the statistical analysis. BO planned and carried out the genetic studies, worked on the interpretation of the data, and revised the manuscript. EB was involved in the clinical design, recruitment and acquisition of clinical data in Zurich. RG was involved in the supervision of the acquisition of the clinical data in Zurich and was involved in the interpretation of data. RT was involved in the clinical acquisition of data in Würzburg and interpretation of data and revised the manuscript. MR was responsible in the clinical acquisition of data in Würzburg. AR was responsible for the CNV study design, the interpretation of the data and revised the manuscript. SW was responsible for the underlying clinical study design, initiated and created together with AR the CNV study; she was involved in the interpretation of data and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
SW has received in the last 5 years royalities from Thieme Hogrefe, Kohlhammer, Springer, Beltz. SW has received lecture honoraria from Opopharma in the last 5 years. Her work was supported in the last 5 years by the Swiss National Science Foundation (SNF), diff. EU FP7s, HSM Hochspezialisierte Medizin of the Kanton Zurich, Switzerland, Bfarm Germany, ZInEP, Hartmann Müller Stiftung, Olga Mayenfisch, and Gertrud Thalmann Fonds. Outside professional activities and interests are declared under the link of the University of Zurich www.uzh.ch/prof/ssl-dir/interessenbindungen/client/web/. AR was supported by the Swiss National Science Foundation, E-rare, and Von Sick foundation. Outside professional activities and interests are declared under the link of the University of Zurich https://www.uzh.ch/prof/ssl-dir/interessenbindungen/client/web/R. The other authors declare no conflict of interest.
Ethical approval and consent to participate
All procedures were performed with the written informed consent of the parents of all participants and the study was approved by the local ethics committees of the Canton of Zurich (Switzerland, E-36/2009), and of Würzburg (Germany, study numbers 8/06 and 227/09), respectively.
Consent for publication
The aforementioned consent form includes publication consent as well.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Werling, A.M., Grünblatt, E., Oneda, B. et al. High-resolution chromosomal microarray analysis for copy-number variations in high-functioning autism reveals large aberration typical for intellectual disability. J Neural Transm 127, 81–94 (2020). https://doi.org/10.1007/s00702-019-02114-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-019-02114-9