Abstract
The disaccharide trehalose (TRE) represents a natural energy supply for distinct non-mammalian species. Evidence has shown that TRE impacts on various properties including the stabilization of protein structure and cell membranes, which are important neuroprotective features against neurodegeneration. In this study, we tested the specific effect of TRE on cell proliferation and mobilization using an established experimental paradigm of adult neural progenitor cells (NPCs) derived from murine hippocampus. NPC proliferation, both measured by growth curve analysis over 25 days and by bromodeoxyuridine (BrdU) incorporation, was not altered by adding TRE instead of GLC to the culture media. Using Boyden chamber experiments, the mobility in regular glucose-containing media did not differ from glucose-free TRE-supplemented media. Our observation suggests that TRE has the capacity to replace glucose (GLC) as energy source in neural cells in our experimental paradigm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trehalose (TRE) is an uncommon, but somehow well-described biological compound. This non-reducing disaccharide contains two d-glucose residues, α-1,1-linked (Venables et al. 2008). TRE serves as energy supply in fungus, bacteria and invertebrate animals (Jain and Roy 2008) and was found to allow for cellular survival for longer periods without water (Iturriaga et al. 2009). Trehalose gained particular interest from the general public because of the water bear tardigrade, a micro non-mammalian animal, which can reach a body length of 1.5 mm maximum. Found from the deep sea to volcanos, the tardigrades use trehalose to survive under extreme temperatures (Jonsson 2007), stressing their role in stabilizing membranes and other particular molecular assemblies under exceptional environmental conditions. As a result of this, the food industry takes advantage of their protective qualities against dryness, freezing and osmotic pressure stresses, after TRE has been manufactured artificially from starch.
In humans and most mammalian species, after absorption in the small intestine TRE is processed by trehalase into two glucose molecules; as such only minimal amounts of TRE reach the general blood circulation (Elbein 1974). Recent evidence has shown that TRE can be cleaved by trehalase in the mammalian brain including the hippocampus (Halbe and Rami 2019), where the NPCs used in our study were derived from. Besides, at least for cell culture experiments, it has been reported that TRE entered cells by endocytosis (Wolkers et al. 2001). Regarding TRE’s potential to replace GLC as energy supply, it should be mentioned that TRE does not induce rapid increases in blood glucose and consecutive insulin levels (Yoshizane et al. 2017). These non-glycemic properties render TRE an interesting candidate as glucose surrogate taking into account the numerous deleterious effects of GLC on brain atherosclerosis as a result of hyperglycemia for instance (Riederer et al. 2017).
As a first step, we wanted to analyze the impact of TRE on neural progenitor cells derived from the hippocampus of adult mice using our established in vitro paradigm (Benninghoff et al. 2010). The subgranular zone of the hippocampus is one of two known germinal zones, which continue to generate new neurons after birth—the other region is the subventricular zone (SVZ) lining the lateral ventricles (Snyder 2019). These regions contain stem or progenitor cells that can be isolated and grown in vitro as neurospheres, maintaining self-renewal capacity and multipotency over time (Ferrari et al. 2010). Therefore, adult neural stem or progenitor cells can be used as a reliable paradigm to study the impact of a given compound on neural proliferation and mobilization, providing empirical support for their potential to fight against neuropsychiatric disorders (Kempermann and Kronenberg 2003).
Given the reports on various beneficial molecular effects of TRE such as the induction of autophagy (DeBosch et al. 2016) in a general sense and—a more specific example—the suppression of osteoporosis (Arai et al. 2001), it is also noteworthy to reckon the mitigating effects of TRE on neuropsychiatric diseases such as Huntington’s (Tanaka et al. 2004), Alzheimer’s and even in an animal model of depression (Kara et al. 2013). Especially in the case of Alzheimer’s disease, our group has been looking into alternative therapy strategies on a molecular level. As a result, we focused on methylene blue (MB) using our stem cell paradigm (van der Ven et al. 2017), taking into account possible molecular explanations how MB might impact as a therapeutic agent especially in mitochondria, repairing impaired energy metabolism and respiratory stress (Beal 2005). Furthermore, parallel mechanisms seem to hold true for TRE in alleviating pathologic beta-amyloid load (Butterfield 2002).
Here, we present the first preliminary experiments on the impact of TRE on stem cell proliferation and mobilization. We evaluated the growth rates of NPCs over the period of 25 days and analyzed the actual proliferation using BrdU incorporation. We further in vitro analyzed the ability of TRE to induce cell mobility, simulating the in vivo setting of newly generated cells migrating a distinct path form their neurogenic niches to other areas of the brain.
Materials and methods
Establishment of primary NPC cultures
According to NIH (National Institutes of Health Guide for the Care and Use of Laboratory Animals)-equivalent animal care rules, adult wild-type mice (Charles River, 14 weeks old) were anesthetized by intraperitoneal injection of pentobarbital (120 mg/kg) and killed by cervical dislocation. The prepared brains were placed in chilled PBS (phosphate-buffered saline). The dentate gyrus of the hippocampus was carefully removed and put in a digestion solution [EBSS (Earle’s balanced salt solution) containing 0.94 mg/ml papain (Worthington Biochemicals), 0.2 mg/ml cysteine and EDTA (ethylenediaminetetraacetic acid)—both from Sigma-Aldrich)] for 50 min at 37 °C under gentle rocking. After digestion, tissues were washed twice in DMEM (Dulbecco’s modified Eagle medium from Gibco Life), mechanically dissociated using a fire-polished Pasteur pipette and finally placed in serum-free DMEM/F12 (1:1 v/v; Gibco Life) growth media containing 20 ng/ml EGF (epidermal growth factor) and 10 ng/ml FGF-2 (fibroblast growth factor—both human recombinant from Peprotech), 2 mM l-glutamine, 3.3 mM glucose, 9.6 µg/ml putrescine, 6.3 µg/ml progesterone, 5.2 ng/ml sodium selenite, 0.025 mg/ml insulin, 0.1 mg/ml transferrin and 0.2 µg/ml heparin (all Sigma) at a density of 20,000 cells/ml onto sterile, non-coated Petri dishes (Corning).
Cell culturing and propagation
Hippocampal stem or progenitor cells from the dentate gyrus were serially subcultured by mechanical dissociation every 4–7 days. Cells were collected as neurospheres and the total number of viable cells was assessed each passage by trypan blue exclusion (Sigma). Primary neurospheres consistently gave rise to continuously expanding cultures, which were used for long-term proliferation assessment, i.e., 25 days in total. Following evaluation of NPC counts, cells were always replated at the density of 200,000 cells. By taking into account the previous cell number and the amount of plated cells over a period of 25 days, we were subsequently able to establish growth curves and compare the proliferation rates of NPCs in different culture media following the formula: actual cell number N = (actual cell count × cell count of the previous passage) ÷ number of plated cells. The control with 3.3 mM glucose was compared to a group where glucose was replaced by 1.6 mM TRE For growth curves, each experiment was performed at least three times per passage. The cell lines tested were randomly assigned to the TRE or control (GLC) group. Data obtained from each experiment represent triplicates. For further experiments regarding migration and short-term proliferation, NPCs were collected 5 days after the last subculturing passage.
Cell culture treatments with trehalose
NPCs were treated with trehalose dihydrate (α-d-glucopyranosyl-α-d-glucopyranoside, C12H22O11·2H2O, CAS 6138-23-4). In preliminary experiments, the appropriate concentration of TRE was determined by toxicity assays using Alamar blue (Invitrogen) to exclude the cytotoxic effects, which could be mistaken as the result of survival or proliferation. For assessing the impact of TRE at the cellular level, 1.6 mM trehalose dihydrate was used (Sigma). TRE was soluble in culture media and was freshly prepared for each experiment. There are no reports about the toxic effects of TRE (Richards et al. 2002).
BrdU incorporation/short-term proliferation assay
In short-term proliferation experiments, 35,000 undifferentiated NPCs in growth medium containing 1.6 mM TRE or regular growth medium (control) 3.3 mM GLC were plated onto Matrigel-coated glass coverslips (Menzel Deckgläser). Cells were incubated at 37 °C, 5% CO2 for 24 h. After incubation, the cell suspension was largely removed and replaced with fresh prewarmed media containing 0.1% labeling reagent (bromodeoxyuridine, BrdU- and 5-flouro-2′deoxyuridine, Amersham Cell Proliferation Biotrak ELISA System). After 60 min of incubation at 37 °C in 5% CO2, the media were entirely removed and glass coverslips were washed with phosphate-buffered saline (PBS, Gibco). The remaining cells were fixed (4% paraformaldehyde in PBS, pH 7.4, for 20 min). Then freeze-dried nuclease (Amersham Cell Proliferation Biotrak ELISA System) and Anti-BrdU were added. DNA digestion and binding of anti-BrdU were allowed within the following 60 min. After washing with PBS, Cy3-labeled anti-BrdU-antibody (Cy3-goat-anti-mouse, whole IgG H + L, Dianova) was added (1:1000, 30 min at room temperature) for selective visualization of proliferating cells. To assess the total number of cells independently of their proliferation status, all cell nuclei were counterstained with DAPI (4,6-diamidino-2-phenylindole, 1:1000 dilution of stock solution, 10 min at room temperature). Samples were examined (and photographed) at 200-fold magnification using a fluorescence microscope. Immunoreactive cells were counted in at least five non-overlapping fields in each of five samples and expressed as a percentage of the total number of nuclei.
Mobility assay
For mobility experiments, Boyden chambers were used. PVP (polyvinylpyrrolidone)-free polycarbonate filters with 8 μm pores (Micron) were coated with Matrigel (BD Biosciences). DMEM media with either GLC or TRE but without any growth factors was tested. As an internal standard culture, media containing EGF and FGF2 were used. Growth factor-free culture media were placed in the lower chambers. Undifferentiated NPCs with a regular cell diameter of ~ 16 μm were used for the assays 24 h following the last subculturing passage. 50,000 cells were resuspended in 200 μl DMEM, placed in the upper chambers and incubated for 6 h at 37 °C in 5% CO2. NPCs remaining on the upper surface of the filters were mechanically removed, while those that had migrated to the lower surface were fixed with 99.8% ethanol (Carl-Roth), Giemsa (Sigma-Aldrich) stained and counted at 400-fold magnification in five random fields per filter.
Statistical analysis
Experimental data are reported as mean ± standard deviation (SD) given the Gaussian distribution of the data if not declared otherwise. Differences between means of TRE and control groups were generally determined by unpaired Student’s t test, with p < 0.05 being considered significant.
Results
We examined the effect of replacing glucose by TRE in proliferation and mobilization of adult neural progenitor cells derived from mouse hippocampus. In our in vitro model, cells proliferated as indicated by BrdU incorporation and survived long term for 25 days, indicating that TRE sufficiently replaced glucose in cell culture. Further, TRE as chemoattractant in mobility assays did not enhance NPC mobility compared to glucose.
Proliferation
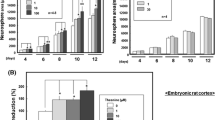
For long-term proliferation, fresh NPCs were inoculated with either 3.3 mM GLC as control or 1.6 mM TRE. There was no reduction of cellular proliferation in the TRE-incubated compared to the glucose-incubated cells over 25 days. The growth rate of glucose- (y = 0.1432e0.571x) and the TRE-treated cells (y = 0.1348e0.5504x) did not differ significantly (p = 0.093) (Fig. 1a).
a NPC proliferation kinetics: no effect on NPC proliferation during the replacement of 3.3 mM GLC by 1.6 mM TRE over 25 days. The growth rate of glucose (y = 0.1432e0.571x) and the TRE-treated cells (y = 0.1348e0.5504x) did not differ significantly (p = 0.093). b The percentage of proliferating undifferentiated NPC demonstrated by BrdU incorporation showed no significant difference (t test p = 0.72) between glucose (mean 0.275, SD 0.088) and TRE (mean 0.28, SD 0.095)
BrdU incorporation
To exclude the clonal effects, which could potentially have caused the above seen parallel regression curves of the tested compounds, we analyzed data from short-term proliferation assays by BrdU incorporation. In short, only proliferating cells incorporate BrdU, a thymidine analog. Therefore, the BrdU incorporating cells account for the amount of proliferating cells at a given time. Showing the same outcome as long-term proliferation, we concluded that in TRE-supplemented media undifferentiated NPCs had a similar proliferation as the glucose-treated NPCs without any statistically significant difference (p = 0.72) (Fig. 1b).
Mobility assay
Next, we look into the mobilization activity of NPCs. Compared to the glucose control, incubation using TRE did not affect the number of migrated cells over 6 h (p = 0.06). As internal standard, we exposed NPCs to Completo media containing EGF/FGF-2 as an internal standard and positive control (data not shown in the figure), since EGF and FGF-2 are strong chemoattractants and NPC cells migrated two times more compared to glucose or TRE (Fig. 2).
NPC migration toward either glucose or TRE per optical field (400 fold magnification): analysis of the mobility of single NPC (mean diameter 16 µm) in Boyden chambers from one surface side to the other surface by migration through micropores (diameter 8 µm) revealed no significant difference (p = 0.06) between glucose (mean number of migrated cells 28.99) and TRE (mean 23.61). Inside the box, all values min to max are included; lines inside represent median values (GLC 26, TRE 19)
Discussion
We investigated the substitution of glucose by TRE in NPC culture. Our data demonstrated that the growth rates of both compounds over a period of 25 days in vitro did not differ in a significant way. In long-term proliferation experiments, NPC survived and proliferated with TRE as the only sugary source in GLC-free culture media containing growth factors FGF-2 and EGF.
Further, TRE neither enhances nor depresses the mobilization of NPC compared to glucose. Although other groups have been using higher concentrations of TRE up to 100 mM (Sarkar et al. 2007; Kruger et al. 2012), we believe that using TRE at a concentration close to that of glucose underlines the notion that TRE might replace glucose as a nutritive factor. Taking into account that the relative sweetness of TRE is 45% of the amount of GLC, TRE with its high thermostability and wide pH stability range represents a potential nutritional alternative to GLC. However, the wide range use of TRE as a nutritive factor may have some drawbacks, since dietary TRE has been shown to enhance the virulence of epidemic and hypervirulent strains of C. difficile (Collins et al. 2018).
Pathologic GLC utilization is linked to disturbed insulin-adherent mechanisms. In the diabetic brain this can lead to insulin resistance, which constitutes a risk factor for Alzheimer’s (AD) and vascular dementia (Riederer et al. 2017). Therefore, therapeutic alternatives to GLC such as TRE may have an impact in the prevention of dementia. Although the precise mechanism has not been elucidated yet, there is good evidence for a connection between cognitive impairment and diabetes mellitus (DM) (Feinkohl et al. 2015). For instance, insulin resistance not only predicted mild cognitive impairment (MCI) conversion to AD (Willette et al. 2015b), but also it projected the beta-amyloid deposition rate, one of the main biomarkers for AD, in late middle-aged adults (Willette et al. 2015a).
Furthermore, it is an intriguing finding that hyperglycemia caused decreased neurogenesis in a rodent insulin-depletion model (Alvarez et al. 2009). Accordingly, another neuroplastic effect was impaired in hyperglycemia, i.e., synaptic integrity (Jacobson et al. 2007). In addition to this, our group has recently stressed the role of synaptophysin as an indicator of protective effects after methylene blue treatment using the given NPC model (van der Ven et al. 2017). This is of particular interest when it comes to stem cell mobility and migratory capacity, because migrating into neurogenic niches is putatively followed by stem cell differentiation into neurons and synaptic sprouting in vivo. The maintenance and proliferation of adult neural progenitor cells has also been shown to be influenced by the monoamine serotonin (Benninghoff et al. 2010). Serotonin (5-HT) also is a target for antidepressant effects, which makes sense taking into account the role of depleted neurogenesis in depression or rather animal models of depression (Samuels et al. 2016). Providing that neurogenesis plays a role in the complex interrelation of depression, it is also an intriguing finding that TRE induced antidepressant effects in a mouse model (Kara et al. 2013).
Therefore, future research should focus on the interplay of TRE and elements of the serotonergic machinery such as 5-HT receptors and the effect of tryptophan hydroxylases (TPH), the rate limiting enzymes in the 5-HT biosynthesis. The aspect of TRE degradation by trehalase should also be considered, since trehalase, the TRE-degrading enzyme, was found in the hippocampus, after it has already been detected in the gastrointestinal tract and in the kidneys (Oesterreicher et al. 2001; Sacktor and Berger 1969). Trehalase induces autophagy by inhibiting the transportation of GLC by SLC2A (GLUT) transporters into the cell to create a starvation state (Mardones et al. 2016). This is interesting because autophagy may also play a role in the antidepressant effect of TRE (Kara et al. 2013): In a mouse model, TRE reduced immobility in the forced swim test, a standard tool for antidepressant-like effects.
Neurodegenerative diseases caused by prions, misfolded proteins or aggregation complexes might benefit from enhancing autophagy or stabilizing the protein structure by TRE (Sarkar et al. 2007; Beranger et al. 2008; Aguib et al. 2009). In Huntington’s chorea, this mode of action, i.e., reduction of protein aggregation, was enhanced in a mouse model both in vitro and in vivo (Tanaka et al. 2004) and thus prolonging the murine life span by 20%. The mechanism was detected by Sarkar et al. (2007). He showed that TRE works as an m-tor-independent autophagocytosis activator. In addition, he showed that TRE protects against apoptosis. In the case of prion disease, Beranger et al. (2008) showed that TRE reduced the rate of prion protein and protected infected cells from oxidative stress, but in a different study TRE was not able to prolong the life span in a mouse model of prion disease (Aguib et al. 2009).
Intracerebral transplantation of neural stem cells in combination with TRE feeding palliated the pathology of Huntington’s disease in mouse. Not only was the life span extended, but also the motor function improved, indicating a positive effect of TRE and its availability after feeding (Yang and Yu 2009). Likewise, recent evidence has shown that TRE influences the morphology of neurons, because it augmens the dendritic arborization during the process of neural maturation (Martano et al. 2017), which might be one possible mechanism how a pathologic process is repaired. In parallel, this mode of action could also be useful in treating early stages of Alzheimer’s. In Alzheimer’s, the in vitro aggregation of beta-amyloid 1–40 was reduced by TRE, whereas the amount of the more toxic variant 1–42 was not (Liu et al. 2005). In general, it seems that TRE stabilizes protein structures, especially lipid bilayers (Pereira et al. 2004). In a mouse model of tautopathy (Rodriguez-Navarro et al. 2010), the overexpressing of human mutated Tau protein led to a Parkinson-like behavior and after treatment with 1% TRE in drinking water, the levels of phosphorylated tau and neuritic plaques decreased (Fig. 3).
Possible modes of action how TRE might prevent protein aggregation and misfolding: TRE may form a chaperone conferring cellular resistance against oxidative stress, dehydration or heat (1) around the protein structures. As an alternative, it integrates into hydrogen bonds to repair its structure (2)
All these findings have not been put into the perspective of neurogenesis and neural progenitor cells in particular. Neurogenesis seems to be important as a pivotal mechanism to replace damaged or undergone neural cells. Adult neural stem cells (NPC) de novo generate all cells of the neural lineage such as neurons, astrocytes and oligodendrocytes (Galli et al. 2008). Although human neurogenesis is limited compared to rodents’, e.g., (Jessberger and Gage 2014), the alteration in adult neurogenesis is now in the focus of explaining cognitive decline that takes place during aging and as a potential contributor to neuropsychiatric illnesses (Ming and Song 2005; Lazarov and Marr 2010).
It may seem far fetched to draw conclusions from in vitro experiments with regard to the diabetic brain and cognition. Of course, we have not tested our approach in vivo. Still, there are data on glycemia and insulin production in humans (Yoshizane et al. 2017). These data showed that TRE had a benefit in terms of insulin secretion compared to glucose. This might open the avenue to reduce the deleterious sequelae of high glucose consumption as a risk factor for neurological disorders in humans by taking TRE instead and still protect neurogenesis in vivo.
References
Aguib Y, Heiseke A, Gilch S, Riemer C, Baier M, Schatzl HM, Ertmer A (2009) Autophagy induction by trehalose counteracts cellular prion infection. Autophagy 5(3):361–369
Alvarez EO, Beauquis J, Revsin Y, Banzan AM, Roig P, De Nicola AF, Saravia F (2009) Cognitive dysfunction and hippocampal changes in experimental type 1 diabetes. Behav Brain Res 198(1):224–230. https://doi.org/10.1016/j.bbr.2008.11.001
Arai C, Kohguchi M, Akamatsu S, Arai N, Yoshizane C, Hasegawa N, Hanaya T, Arai S, Ikeda M, Kurimoto M (2001) Trehalose suppresses lipopolysaccharide-induced osteoclastogenesis bone marrow in mice. Nutr Res 21(7):993–999
Beal MF (2005) Oxidative damage as an early marker of Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 26(5):585–586. https://doi.org/10.1016/j.neurobiolaging.2004.09.022
Benninghoff J, Gritti A, Rizzi M, Lamorte G, Schloesser RJ, Schmitt A, Robel S, Genius J, Moessner R, Riederer P, Manji HK, Grunze H, Rujescu D, Moeller HJ, Lesch KP, Vescovi AL (2010) Serotonin depletion hampers survival and proliferation in neurospheres derived from adult neural stem cells. Neuropsychopharmacology 35(4):893–903. https://doi.org/10.1038/npp.2009.181
Beranger F, Crozet C, Goldsborough A, Lehmann S (2008) Trehalose impairs aggregation of PrPSc molecules and protects prion-infected cells against oxidative damage. Biochem Biophys Res Commun 374(1):44–48. https://doi.org/10.1016/j.bbrc.2008.06.094
Butterfield DA (2002) Amyloid beta-peptide (1–42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic Res 36(12):1307–1313
Collins J, Robinson C, Danhof H, Knetsch CW, van Leeuwen HC, Lawley TD, Auchtung JM, Britton RA (2018) Dietary trehalose enhances virulence of epidemic Clostridium difficile. Nature 553(7688):291–294. https://doi.org/10.1038/nature25178
DeBosch BJ, Heitmeier MR, Mayer AL, Higgins CB, Crowley JR, Kraft TE, Chi M, Newberry EP, Chen Z, Finck BN, Davidson NO, Yarasheski KE, Hruz PW, Moley KH (2016) Trehalose inhibits solute carrier 2A (SLC2A) proteins to induce autophagy and prevent hepatic steatosis. Sci Signal 9(416):ra21. https://doi.org/10.1126/scisignal.aac5472
Elbein AD (1974) The metabolism of alpha, alpha-trehalose. Adv Carbohydr Chem Biochem 30:227–256
Feinkohl I, Price JF, Strachan MW, Frier BM (2015) The impact of diabetes on cognitive decline: potential vascular, metabolic, and psychosocial risk factors. Alzheimers Res Ther 7(1):46. https://doi.org/10.1186/s13195-015-0130-5
Ferrari D, Binda E, De Filippis L, Vescovi AL (2010) Isolation of neural stem cells from neural tissues using the neurosphere technique. Curr Protoc Stem Cell Biol Chapter 2(Unit2D):6. https://doi.org/10.1002/9780470151808.sc02d06s15
Galli R, Gritti A, Vescovi AL (2008) Adult neural stem cells. Methods Mol Biol 438:67–84. https://doi.org/10.1007/978-1-59745-133-8_7
Halbe L, Rami A (2019) Trehalase localization in the cerebral cortex, hippocampus and cerebellum of mouse brains. J Adv Res 18:71–79. https://doi.org/10.1016/j.jare.2019.01.009
Iturriaga G, Suarez R, Nova-Franco B (2009) Trehalose metabolism: from osmoprotection to signaling. Int J Mol Sci 10(9):3793–3810. https://doi.org/10.3390/ijms10093793
Jacobson AM, Musen G, Ryan CM, Silvers N, Cleary P, Waberski B, Burwood A, Weinger K, Bayless M, Dahms W, Harth J (2007) Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med 356(18):1842–1852. https://doi.org/10.1056/NEJMoa066397
Jain NK, Roy I (2008) Role of trehalose in moisture-induced aggregation of bovine serum albumin. Eur J Pharm Biopharm 69(3):824–834. https://doi.org/10.1016/j.ejpb.2008.01.032
Jessberger S, Gage FH (2014) Adult neurogenesis: bridging the gap between mice and humans. Trends Cell Biol 24(10):558–563. https://doi.org/10.1016/j.tcb.2014.07.003
Jonsson KI (2007) Tardigrades as a potential model organism in space research. Astrobiology 7(5):757–766. https://doi.org/10.1089/ast.2006.0088
Kara NZ, Toker L, Agam G, Anderson GW, Belmaker RH, Einat H (2013) Trehalose induced antidepressant-like effects and autophagy enhancement in mice. Psychopharmacology 229(2):367–375. https://doi.org/10.1007/s00213-013-3119-4
Kempermann G, Kronenberg G (2003) Depressed new neurons–adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol Psychiatry 54(5):499–503
Kruger U, Wang Y, Kumar S, Mandelkow EM (2012) Autophagic degradation of tau in primary neurons and its enhancement by trehalose. Neurobiol Aging 33(10):2291–2305. https://doi.org/10.1016/j.neurobiolaging.2011.11.009
Lazarov O, Marr RA (2010) Neurogenesis and Alzheimer’s disease: at the crossroads. Exp Neurol 223(2):267–281. https://doi.org/10.1016/j.expneurol.2009.08.009
Liu R, Barkhordarian H, Emadi S, Park CB, Sierks MR (2005) Trehalose differentially inhibits aggregation and neurotoxicity of beta-amyloid 40 and 42. Neurobiol Dis 20(1):74–81. https://doi.org/10.1016/j.nbd.2005.02.003
Mardones P, Rubinsztein DC, Hetz C (2016) Mystery solved: trehalose kickstarts autophagy by blocking glucose transport. Sci Signal 9(416):fs2. https://doi.org/10.1126/scisignal.aaf1937
Martano G, Gerosa L, Prada I, Garrone G, Krogh V, Verderio C, Passafaro M (2017) Biosynthesis of astrocytic trehalose regulates neuronal arborization in hippocampal neurons. ACS Chem Neurosci 8(9):1865–1872. https://doi.org/10.1021/acschemneuro.7b00177
Ming GL, Song H (2005) Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 28:223–250. https://doi.org/10.1146/annurev.neuro.28.051804.101459
Oesterreicher TJ, Markesich DC, Henning SJ (2001) Cloning, characterization and mapping of the mouse trehalase (Treh) gene. Gene 270(1–2):211–220
Pereira CS, Lins RD, Chandrasekhar I, Freitas LC, Hunenberger PH (2004) Interaction of the disaccharide trehalose with a phospholipid bilayer: a molecular dynamics study. Biophys J 86(4):2273–2285. https://doi.org/10.1016/S0006-3495(04)74285-X
Richards AB, Krakowka S, Dexter LB, Schmid H, Wolterbeek AP, Waalkens-Berendsen DH, Shigoyuki A, Kurimoto M (2002) Trehalose: a review of properties, history of use and human tolerance, and results of multiple safety studies. Food Chem Toxicol 40(7):871–898
Riederer P, Korczyn AD, Ali SS, Bajenaru O, Choi MS, Chopp M, Dermanovic-Dobrota V, Grunblatt E, Jellinger KA, Kamal MA, Kamal W, Leszek J, Sheldrick-Michel TM, Mushtaq G, Meglic B, Natovich R, Pirtosek Z, Rakusa M, Salkovic-Petrisic M, Schmidt R, Schmitt A, Sridhar GR, Vecsei L, Wojszel ZB, Yaman H, Zhang ZG, Cukierman-Yaffe T (2017) The diabetic brain and cognition. J Neural Transm (Vienna) 124(11):1431–1454. https://doi.org/10.1007/s00702-017-1763-2
Rodriguez-Navarro JA, Rodriguez L, Casarejos MJ, Solano RM, Gomez A, Perucho J, Cuervo AM, Garcia de Yebenes J, Mena MA (2010) Trehalose ameliorates dopaminergic and tau pathology in parkin deleted/tau overexpressing mice through autophagy activation. Neurobiol Dis 39(3):423–438. https://doi.org/10.1016/j.nbd.2010.05.014
Sacktor B, Berger SJ (1969) Formation of trehalose from glucose in the renal cortex. Biochem Biophys Res Commun 35(6):796–800
Samuels BA, Mendez-David I, Faye C, David SA, Pierz KA, Gardier AM, Hen R, David DJ (2016) Serotonin 1A and serotonin 4 receptors: essential mediators of the neurogenic and behavioral actions of antidepressants. Neuroscientist 22(1):26–45. https://doi.org/10.1177/1073858414561303
Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC (2007) Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem 282(8):5641–5652. https://doi.org/10.1074/jbc.M609532200
Snyder JS (2019) Recalibrating the relevance of adult neurogenesis. Trends Neurosci 42(3):164–178. https://doi.org/10.1016/j.tins.2018.12.001
Tanaka M, Machida Y, Niu S, Ikeda T, Jana NR, Doi H, Kurosawa M, Nekooki M, Nukina N (2004) Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease. Nat Med 10(2):148–154. https://doi.org/10.1038/nm985
van der Ven AT, Pape JC, Hermann D, Schloesser R, Genius J, Fischer N, Mossner R, Scherbaum N, Wiltfang J, Rujescu D, Benninghoff J (2017) Methylene blue (tetramethylthionine chloride) influences the mobility of adult neural stem cells: a potentially novel therapeutic mechanism of a therapeutic approach in the treatment of Alzheimer’s disease. J Alzheimers Dis 57(2):531–540. https://doi.org/10.3233/JAD-160755
Venables MC, Brouns F, Jeukendrup AE (2008) Oxidation of maltose and trehalose during prolonged moderate-intensity exercise. Med Sci Sports Exerc 40(9):1653–1659. https://doi.org/10.1249/MSS.0b013e318175716c
Willette AA, Johnson SC, Birdsill AC, Sager MA, Christian B, Baker LD, Craft S, Oh J, Statz E, Hermann BP, Jonaitis EM, Koscik RL, La Rue A, Asthana S, Bendlin BB (2015a) Insulin resistance predicts brain amyloid deposition in late middle-aged adults. Alzheimers Dement 11(5):504–510e501. https://doi.org/10.1016/j.jalz.2014.03.011
Willette AA, Modanlo N, Kapogiannis D, Alzheimer’s Disease Neuroimaging I (2015b) Insulin resistance predicts medial temporal hypermetabolism in mild cognitive impairment conversion to Alzheimer disease. Diabetes 64(6):1933–1940. https://doi.org/10.2337/db14-1507
Wolkers WF, Walker NJ, Tablin F, Crowe JH (2001) Human platelets loaded with trehalose survive freeze-drying. Cryobiology 42(2):79–87. https://doi.org/10.1006/cryo.2001.2306
Yang CR, Yu RK (2009) Intracerebral transplantation of neural stem cells combined with trehalose ingestion alleviates pathology in a mouse model of Huntington’s disease. J Neurosci Res 87(1):26–33. https://doi.org/10.1002/jnr.21817
Yoshizane C, Mizote A, Yamada M, Arai N, Arai S, Maruta K, Mitsuzumi H, Ariyasu T, Ushio S, Fukuda S (2017) Glycemic, insulinemic and incretin responses after oral trehalose ingestion in healthy subjects. Nutr J 16(1):9. https://doi.org/10.1186/s12937-017-0233-x
Acknowledgements
The authors thank Caroline Strenkert for her excellent graphic work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All applicable international guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bertl, A., Brantl, V., Scherbaum, N. et al. Trehalose as glucose surrogate in proliferation and cellular mobility of adult neural progenitor cells derived from mouse hippocampus. J Neural Transm 126, 1485–1491 (2019). https://doi.org/10.1007/s00702-019-02070-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-019-02070-4