Abstract
Despite intensive effort, biomarker research for the detection of prodromal stage, diagnosis and progression of Parkinson’s disease (PD) falls short of expectations. This article reviews the attempts in the last 20 years to find a biomarker, addresses challenges along the biomarker search and suggests the steps that should be taken to overcome these challenges. Although several biomarkers are currently available, none of them is specific enough for diagnosis, prediction of future PD or disease progression. The main reason for the failure finding a strong biomarker seems to be drastic heterogeneity of PD, which exhibits itself in all domains; from the clinic to pathophysiology or genetics. The diversity in patient selection, assessment methods or outcomes in biomarker studies also limit the interpretation and generalizability of the data. In search of a reliable biomarker, consideration of novel approaches encompassing individual demographic, clinical, genetic, epigenetic and environmental differences, employment of strategies enabling marker combinations, designing multicenter studies with compatible assessment methods, integration of data from preclinical domains and utilization of novel technology-based assessments are necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term “biomarker” is the short form of “biological marker” and was defined by the National Institutes of Health Biomarkers Definitions Working Group as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” (Biomarkers Definitions Working Group 2001). A biomarker can be a clinical sign, biological sample or a measurement in imaging; however, should be objective and reproducible independent from the subjective “signs” perceived by the patients (Strimbu and Tavel 2010). With regard to PD, intensive effort has been expended in the last decades in various areas such as biochemistry, genetic, epigenetic, -omics, clinic or imaging to define a reliable biomarker for the prediction, diagnosis and progression of PD (Table 1). In this article, we attempt to review the progress that has been achieved in the last 20 years and discuss further challenges.
Although Dr. James Parkinson already defined the neurodegenerative disorder which was later given his name as Parkinson’s disease as a motor syndrome with non-motor aspects in his “Assay on the Shaking Palsy” (Parkinson 1817), this multisystemic character has taken a back seat for many decades. In fact, Parkinson’s disease (PD) has for many years been regarded as a disorder of the motor system which manifests itself primarily through bradykinesia, tremor and/ or rigidity. However, thanks to the vigorous research in the last decades not only non-motor but also epidemiologic, environmental, genetic and molecular aspects of the disease could be highlighted which changed our view of PD substantially. Still, in spite of all advance in many fields, PD is clinically defined (Lewis et al. 2005).
Importantly, motor symptoms form the key criteria for diagnosis and differential diagnosis of PD, which may deem remarkable as non-motor symptoms antecede the classical motor symptoms in most individuals later diagnosed as PD. For a long time the diagnosis of PD was based on the UK Queen Square Brain Bank criteria which suggested a stepwise method by introducing the core symptoms followed by supportive and exclusion criteria (Gibb and Lees 1988). The main symptom was bradykinesia which must be accompanied by one or more of the following: rest tremor, rigidity or postural instability. Recently, however, the “International Parkinson and Movement Disorders Society Task Force for the Definition of PD” (MDS-TF-PD) proposed a revised set of criteria (Postuma et al. 2015). They refined the core criteria by excluding postural instability and incorporated other supportive features such as imaging and red flags such as absence of any non-motor symptoms (which were absent in the former criteria) suggesting a more comprehensive approach based on the advances in the field of PD. Similar to the former, the latter criteria recognized motor symptoms as fundamental for diagnosis, but acknowledged movement disorders expert examination as reference to codify the diagnostic process and make it reproducible and applicable also by (relative-) non experts. Application of the MDS-TF-PD criteria in reference to an expert diagnosis showed a higher diagnostic accuracy (92.6%) compared to the UK Queen Square Brain Bank criteria (86.4%) in a recent multicenter study (Postuma et al. 2018).
Although diagnostic accuracy in the clinical phase has improved, it is not 100%, yet. Moreover, progression markers in the clinical phase, which are urgently needed to better understand the individual course of the disease, the effect of different treatment strategies, and as endpoints for clinical studies are still lacking. This may on the one hand be due to the heterogeneity of PD, on the other hand on individual compensatory mechanisms etc. Recently, a task force on PD subtypes has been assembled by the MDS (https://www.movementdisorders.org/MDS/About/Committees--Other-Groups/Task-Force-on-PD-Subtypes.htm), which is hoped to lay a strong basis for progression marker research by giving a clear definition on subtypes of the disease.
Given the fact, that PD is still a clinical diagnosis and that discovery of progression markers in the clinical phase may need to be set aside until subgroups are defined the question remains: What has biomarker research in the last decades accomplished?
The premotor stages of Parkinson’s disease
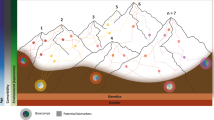
Parkinson’s disease occurs as a result of a long ongoing and so far relentlessly progressive degeneration which is reported to begin outside the substantia nigra, even outside the brain—with possible starting points in the gut or the olfactory bulb (Braak et al.). For the definition of the stages before the classical motor symptoms allow clinical diagnosis, i.e. the yet prediagnostic stage, the MDS-TF-PD suggests two terms, namely the “preclinical” and “prodromal” stages. The preclinical stage encompasses the presence of an ongoing neurodegenerative process without any detectable clinical symptom—neither motor, nor non-motor, whereas the prodromal stage was defined as the time when clinical symptoms or signs that may be related to the neurodegenerative process occur, but key motor criteria are still missing (Fig. 1) (Berg et al. 2014). This segregation of PD into stages provides a broader view and understanding of the relation between the clinical portrait and the neurodegenerative process, which has facilitated biomarker research for prediction of biomarkers for earlier diagnosis and progression in the prodromal phase.
Stages of Parkinson’s disease (PD) and the biomarkers of interest. The clinical stage begins with the diagnosis which is preceded by risk, preclinical (beginning of neurodegeneration without any symptom) and prodromal (presence of PD related symptoms without fulfilling the diagnostic criteria) stages. Currently, no biomarker exists that can specifically detect the prediagnostic stages, assist the diagnosis or predict disease progression. Domains of ongoing biomarker research are shown in relation to the disease stage
Thus, the prodromal stage has been the subject of several longitudinal population-based and enriched risk cohort studies since this long time frame provides an opportunity to investigate promising factors for the prediction of future PD.
Biomarkers in the prodromal stage
The symptoms or traits associated with PD are numerous. These markers may be stable indicating a (decreased or increased) risk of PD and occurring independent of the neurodegenerative process—here the term “risk markers” is appropriate. Or they may be clinical manifestations of the ongoing neurodegenerative process—here the term “prodromal markers” is applied. Gathering the literature of population-based studies and examining the existing evidence for each marker, the MDS-TF-PD published a list of the currently known risk and the prodromal markers (Berg et al. 2015). In this classification, risk markers include demographic features such as gender, history of smoking, pesticide exposure or family history as well as increased nigral echogenicity detected by transcranial ultrasound. Prodromal markers comprise among others autonomic dysfunction, rapid eye movement sleep behavior disorder (RBD), slight motor impairment, olfaction, depression or detection of reduced nigrostriatal radioligand uptake in SPECT (Berg et al. 2015).
Other than SPECT of the dopaminergic terminals, imaging markers have not been included in the criteria for prodromal PD, even though there are several potential candidates for prodromal state markers which may indicate, how advanced the prodromal state of the disease has progressed.
First and foremost, absence of the dorsal nigral hyperintensity (DNH) using susceptibility-weighted Imaging (SWI) of the substantia nigra in 3T MRI has shown promise as a prodromal disease marker indicating an already advanced prodromal stage. De Marzi and colleagues showed that DNH was absent in 92% of PD patients, 77% of RBD cases and only in 3% of healthy controls (De Marzi et al. 2016). It is yet unknown, how well this marker may serve as a progression marker longitudinally, but a recent study showing good cross-sectional correlation of the DNH with SPECT of the dopaminergic terminals indicates relevant potential (Frosini et al. 2017).
Another recently emerged imaging marker with potential as a state marker in the prodromal stage is loss of the neuromelanin-dependent signal in a modified T1 sequence in 3T MRI of the substantia nigra and locus coeruleus. Volume and signal strength of the substantia nigra in neuromelanin MRI accurately distinguished RBD patients from controls in 88% of the cases. If combined with fractional anisotropy of the same area, accuracy increased to 92% (Pyatigorskaya et al. 2017). Interestingly, neuromelanin loss in the locus coeruleus seems to be dependent of the presence of RBD as a prodromal PD phenotype (García-Lorenzo et al. 2013; Knudsen et al. 2018).
Characteristic functional network aberrations may also be present in the prodromal phase, even though some of the changes observed may also stem from preclinical functional compensation (van Nuenen et al. 2009). Using resting-state fMRI, Rolinski and colleagues have shown reduction in basal ganglia connectivity in RBD of a similar magnitude to that in PD cases, even though striatal denervation as evidenced in DAT SPECT was less advanced (Rolinski et al. 2016). Moreover, RBD patients seem to express specific metabolic patterns in FDG PET which partially overlap with the well-described PD-related metabolic patterns (Meles et al. 2018).
These studies propose highly promising surrogate imaging markers for detection of individuals at the prodromal stage and/or for disease progression. However, with regard to prodromal PD more data should be accumulated to understand the strength of the candidate markers, their association with other markers as well as their contribution to the conceptualization of prodromal PD.
Another area of great expectation is biomarker research from body fluids or histological samples. Different biomarkers that are able to identify Alzheimer’s disease (AD) are available for a considerable period of time. Amyloid-β plaques and tau tangles, both pathological hallmarks of Alzheimer’s disease provide reliable information on whether the disease is present or not also in the very early stage of disease (Hansson et al. 2014; Zetterberg and Schott 2019). Using Elisa assay, beta-amyloid (1–40), tau protein and phospho-tau protein may separate AD from new diagnosed PD and allows an estimate of the progress of dementia in PD (Andreasen et al. 1998; Hansson et al. 2006; Siderowf et al. 2010).
The field of objective biomarkers for PD has made great progress; however, there is currently no reliable fluid biomarker. On the protein level, there are indications that (1) levels of α-synuclein oligomers in cerebrospinal fluid (CSF) and the oligomers/total-α-synuclein ratio can be useful for diagnosis and early detection of PD (Tokuda et al. 2010), (2) that metabolites and peptide levels in plasma and CSF may differentiate healthy controls from patients with newly diagnosed PD (Trupp et al. 2014), (3) that changes in endolysosomal enzyme activities in CSF may indicate disease status (van Dijk et al. 2013), and (4) that CSF levels of α-synuclein and UCH-L1 show distinct patterns in parkinsonian syndromes (Mondello et al. 2014).
Further studies evince alterations in neurotransmitters (decreases in 3,4-dihydroxyphenylacetic acid, l-3,4-dihydroxyphenylalanin, norepinephrine and 3,4-dihydroxyphenylglycolaldehyde as potential markers) (Goldstein et al. 2008, 2012; LeWitt et al. 2011). However, individual findings have never been replicated in large collectives. The involvement of excitotoxicity and oxidative stress and its impact as biomarker in PD has also been examined in several studies but found inconsistent results (Hong et al. 2010; Lewitt et al. 2013; Willkommen et al. 2018). Recent evidence also suggests contribution of the adaptive immune system in PD. Thus, immunological biomarkers (interleukins, tumor necrosis factors, major histocompatibility complex) are currently also of interest as potential biomarker in PD (Nilsonne and Lekander 2017; Kim et al. 2018). Moreover, growth factors have also become the focus of interest in PD biomarker research in the last years (Saal et al. 2017; Rahmani et al. 2019). Again, the results are partially contradictory and there are no blinded studies in large groups of patients. Furthermore, there is evidence for CSF plasma neurofilament light (NFL) as sensitive and specific biomarker that may help to rule out neurodegenerative others than idiopathic PD (multiple system atrophy, supranuclear palsy, corticobasal syndrome, frontotemporal dementia) (Herbert et al. 2015). Axonal damage releases NFLs into CSF and eventually into blood (Khalil et al. 2018). Considering age-dependent normal ranges, plasma NFL may represent a biomarker of cognitive decline in AD and PD, with more specificity for AD (Lin et al. 2018).
Current basic research studies integrate the findings of proteomics (investigation of the entire set of proteins that is expressed by a genome), microbiome (community of commensal, symbiotic and pathogenic microorganisms), epigenetics (study of heritable phenotype changes that do not involve alterations in the DNA sequence such as DNA methylation and histone modification and RNA transcripts) with other omics data to characterize and quantify biological molecules that translate into the structure, function, and dynamics in the organism affected from PD (Halbgebauer et al. 2016; Hopfner et al. 2017; Ping et al. 2018; Navarro-Sánchez et al. 2018; Scheperjans et al. 2018; Smith et al. 2019). The validation of these potential biomarkers in large-scale clinical studies is necessary to evaluate the diagnostic potential in future.
In the last years great attention has been paid to the studies histologically examining phosphorylated α-synuclein deposits in different tissues of PD cases. Skin biopsies might be a promising tool for pre-mortem histopathological diagnosis of idiopathic PD and early diagnosis in preliminary stages of PD (such as RBD) (Doppler et al. 2016; Vilas et al. 2016). The results from this histological biomarker are promising, but the sensitivity and the analysis protocols are still not high enough for clinical routine despite easily accessible tissues for biopsies (skin, salivary glands) (Doppler et al. 2016).
Calculation of probability for prodromal PD
In the absence of valid imaging and biosample-derived biomarkers and considering the fact that there is no single marker that allows the diagnosis of prodromal PD the MDS-TF-PD established a model to calculate the risk for an individual to be in the prodromal phase. Thus, for the very first time a mathematical model is used to form research criteria for a prodromal stage of a disease—in this case: PD. As a first step the strength of each marker for the association with PD was defined by its likelihood ratio (LR) which was calculated according to the marker’s sensitivity and specificity for being associated with the later diagnosis of PD. Each marker has two LR values, one positive (LR+) and one negative (LR−) indicating a positive or a negative test result (presence or absence of the marker meaning increased or decreased likelihood for PD) for the assessed individual. In a second step the method uses a Bayesian naïve classifier in which the a priori (pre-test) risk/probability for PD is defined according to the age of the individual. Then the pre-test probability value is multiplied with the LRs of the assessments. Each added LR+ increases, whereas each LR− decreases the overall probability of the assessed individual to be in the prodromal phase. For instance, a 72 years-old has a pre-test probability of 2.5% (pre-test odds = 0.025) for PD. Being male leads to a slight increase of likelihood of 1.2, whereas the history of smoking decrease the likelihood by multiplication with 0.8. Assuming that he has a first-degree relative with PD (LR+ = 2.5), an increased substantia echogenicity on transcranial ultrasound (LR+ = 4.7), RBD detected by the RBD screening questionnaire (LR+ = 2.3), hyposmia (LR+ = 4.0), subthreshold parkinsonism (LR+ = 10) and a severe erectile dysfunction (LR+ = 2.0), but no constipation (LR− = 0.8), depression (LR− = 0.85) or orthostatic hypotension (LR− = 0.87) a multitude of markers is at hand to calculate the individual risk. Multiplying all these LRs gives the value of 1228 (total LR) which is further multiplied by the pre-test odds (in this case 0.025) resulting in the post-test odds of 30.7 which corresponds to a post-test probability of 97% according to the universal formula of probability (probability = odds/(1 + odds)) suggesting that this individual has a 97% probability of being in the prodromal stage of PD.
The MDS-TF-PD determined a cut-off value of 80% for the definition of probable prodromal PD. Later the value of 50% was also suggested for possible prodromal PD (Mahlknecht et al. 2016). The performance of the method has been investigated in several longitudinal studies and proven to be capable of detecting individuals who are in the prodromal stage of PD (Mahlknecht et al. 2016; Pilotto et al. 2017; Fereshtehnejad et al. 2017).
Challenges for the detection of prodromal PD
By suggesting this calculation method the MDS-TF-PD also aimed to introduce a scheme for participant selection for neuroprotection trials. However, for predicting prodromal PD with this method, one needs to take some factors into account that influence the assessment process. Surely, the outcome of the calculation depends on several factors including (1) the number and (2) the predictive value of the detected markers as well as (3) the diagnostic strength of the performed test for the respective marker. More assessments mean more LRs to multiply indicating a more comprehensive assessment and a more reliable conclusion. Additionally the reliability of detecting prodromal PD increases with more specific markers. For instance, prevalence of constipation in the elderly is at least 20% (Vazquez Roque and Bouras 2015) which indicates a low predictive value for future PD. On the other hand, RBD has the highest specificity of all known markers (Postuma and Berg 2016). It is known that within 10 years, more than 80% of patients with RBD proceed to an α-synucleinopathy (Postuma and Berg 2016). However, the way of assessing is crucial. For instance, a positive polysomnography (PSG) for the detection of RBD has a very high LR (LR+ = 130) in comparison to the RBD screening questionnaire (LR+ = 2.3) (Berg et al. 2015).
Another factor that influences the prediction accuracy is (4) the “lead time” of the marker which indicates the period between emerging of the marker and the clinical diagnosis (Postuma and Berg 2016). For many markers time of occurrence in the neurodegenerative process is not clear, neither is time of progression until motor symptoms allow diagnosis. In some (but not all) individuals the number of detectable symptoms increases over time. This suggests that the efficiency of the method is dependent on the lead time of the prodromal markers and the sensitivity of the criteria may increase over the course of the prodromal stage. However, for studies on strategies with presumed neuroprotective properties lead time is essential. A very short lead time would provide little window for any neuroprotective intervention to get effective, especially as the neurodegeneration would have proceeded too far at that stage. On the other hand it is hardly possible to conduct a trial over many years when there are no progression markers other than conversion to PD that may be used as endpoint.
Additional factors that influence the performance of the proposed criteria are (5) the cost and the practicality of the assessment. The LRs+ of PSG proven RBD and nigrostriatal dysfunction detected by DATScan are high, assuring a more accurate conclusion. However, these assessments are rather expensive, elaborate, are available in selected centers only or entail radiation (SPECT). Thus, they are not practically well suited as screening instruments outside of clinical trials.
The classification of prodromal markers with respect to disease progression is also an important issue. It may be assumed that some of them contribute not only to disease prediction but may also serve as markers for measuring progression (being indirect indicators of the underlying neurodegenerative process) since they are de facto disease related symptoms which are expected to worsen in the course of the disease. However, results from different studies show that this is not always the case. Some, like hyposmia may even improve in the course of the disease (Fullard et al. 2017). Other markers such as autonomic dysfunction or depression may fluctuate, at least do not show a steady worsening.
Still, there is some hope from neuroimaging studies. Follow-up of the PARS study shows that hyposmic individuals with a DATScan deficit ≤ 65% of normal show further decline of tracer uptake of 20% (SD 15%) over the next 4 years (Jennings et al. 2017). Moreover, imaging of the Braak stages with peripheral markers as proposed by the group of P. Borghammer seems promising (Knudsen and Borghammer 2018), although results need to be confirmed and it needs to be acknowledged that this comprehensive neuroimaging assessment battery cannot be performed in larger groups of individuals.
New insights into the understanding of progression of neurodegeneration may also be expected from quantitative motor assessments. Several groups are currently measuring movements in various conditions longitudinally in individuals supposed to be in the prodromal phase of PD (see http://www.trend-study.de as one example). Although algorithms for a better understanding of slight changes in movements are still in the process of being developed first promising results indicate that quantitative movement assessment may predict PD 4–6 years prior to clinical diagnosis and that worsening of the parameters over time can be seen (Del Din et al. 2019, submitted).
Overall, validation studies showed that the suggested criteria can identify prodromal PD when suspected individuals are investigated thoroughly, preferably with high quality tests. As none of these biomarkers can predict PD with 100% accuracy in a clearly defined time span (neither alone nor in combination) support from other research areas is urgently needed.
Challenges in disease approach
One of the most important reasons for the failure of attempts to find a reliable biomarker for PD is its striking heterogeneity. Clinically, vast diversity is seen regarding motor or non-motor symptoms. Furthermore, the reasons for differences in treatment response and symptom progression are also still unsolved, challenging clinicians. Thus, subtyping is necessary to better categorize individual cases and—importantly to better understand the underlying pathophysiology and to design causative, disease modulating trials.
So far, PD has been divided into three main subtypes with regard to motor manifestations as tremor dominant, postural instability gait disorder or akinetic/rigid form (Obeso et al. 2017). It has been reported that these subtypes may differ in terms of disease severity and progression (Marras et al. 2002; Obeso et al. 2017). However, little can be explained with the help of this broad classification. Despite further efforts for a more comprehensive subtyping (Marras and Lang 2013), no sufficient grouping regarding the diversity in phenotype has been provided, yet (Berg et al. 2014). One reason may be that most of the suggested subtyping systems applied cluster analyses using only clinical data. However, heterogeneity of PD is not only found in clinical symptoms (motor and non-motor manifestations) and clinical disease progression, but also in genetics, pathways involved in the pathophysiology and even in the histopathology of the disease. For instance, disease phenotype and progression are different between patients with LRRK2 or GBA mutations in comparison to idiopathic PD (Davis et al. 2016; Saunders-Pullman et al. 2018). Likewise occurrence and distribution of pathological substrates may vary on the one hand between individual patients possibly accounting for different phenotypes, on the other hand between different forms—postmortem investigation of brains of PD patients due to Parkin mutation for example lack in general Lewy-bodies. Moreover, additional pathology (vascular, abeta-pathology etc.) may contribute to the clinical phenotype (Obeso et al. 2017).
Thus, PD needs to be considered as a multifaceted syndrome with clinical, epidemiological and genetic subtypes (Espay et al. 2017) and has to be conceptualized within the notion of precision medicine (Funke et al. 2013; Espay et al. 2017), which is a systems biology approach recognizing the disease as a whole, embracing infrequent manifestations and outliers rather than a reductionist approach which focuses on the common feature of all different PD phenotypes (Espay et al. 2017). Past unsuccessful attempts to find neuroprotective or disease modifying therapeutic strategies may at least in part be explained by the reductionist approach since little attention has been paid to disease variability in the trials conducted that far.
The importance of decent subtyping, e.g. integration of molecular and genetic portraits of patients in study designs to meet a refined trial outcome (Chen-Plotkin and Zetterberg 2018) can be exemplified in trials conducted thus far with co-enzyme Q10, which is supposed to support metabolism of the mitochondria. Several studies have been conducted thus far with either non-significant outcomes between the verum and placebo groups or diverse results (Zhu et al. 2017). Importantly, it needs to be considered that with regard to the genotype unstratified populations had been included in these trials. Based on the knowledge that specific mutations (mutations in the Parkin, PINK1 or DJ1 gene) are associated with severe changes in mitochondrial metabolism whereas these metabolic abnormalities play only a minor role in other forms of PD, a study on the effect of co-enzyme Q10 and vitamin K2 which acts as an electron carrier in the mitochondria and promotes ATP production in genetically stratified patients has just been initiated (Vos et al. 2012). Similarly a clinical study investigating the efficacy of GZ/SAR402671, which is supposed to lower the increased glucosylceramide levels inducing α-synuclein formation in selected patients with a glucocerebrosidase (GBA) mutation is ongoing (ClinicalTrials.gov identifier: NCT02906020). These studies in specific genetically stratified PD subtypes obviously include a more homogenous group, which is of great importance when pathomechanism-related strategies are applied.
In the precision medicine approach, integration of information from different fields such as epidemiology, pathology, molecular/genetics or -omics may contribute to a better understanding of subtypes regarding clinical presentation and progression. For example transcriptome studies reported that a subset of RNA biomarkers in blood may predict disease characteristics such as motor progression or cognitive impairment (Santiago et al. 2018). Another attempt to integrate the pathophysiology in subtyping is the assessment of inflammatory markers. It has been proposed that serum inflammatory markers correlate with disease progression and cognitive impairment (Williams-Gray et al. 2016). Although the findings in these fields need to be confirmed in further studies, easily obtained inflammatory or -omics based biomarkers from blood or CSF may be promising for subtyping and explaining the heterogeneity in disease progression.
Directions for future biomarker research
The attempt to detect reliable biomarkers for PD has been ongoing on different levels in clinical and basic science and more effort is needed. Yet, much has been learned from the accumulating literature in terms of what sort of research approaches can be applied or what types of biomarkers can be developed (Chahine and Stern 2017). The detection of a candidate biomarker is mostly based on the existing conjecture or biological coherence. This type of research is in itself biased since it originates from a deduction. The second approach is using an unbiased technique such as detection of peptides using mass spectroscopy, whole genome-wide genetic or epigenetic approaches without any a priori hypothesis in the fields of proteomics or metabolomics. Both approaches should be accepted as valid and have their own advantages and disadvantages for finding a biomarker. In any case, the ultimate aim should be reaching optimal biomarkers which are easy to access, inexpensive, valid and reproducible. These biomarkers must then also be easily integrated with other markers to increase the strength of the prediction.
The potential sites for such biomarkers are tissues, biofluids and the fields of imaging, genetic or molecular biology (Chahine and Stern 2017). Another emerging area is technology-based motor assessments which yield more objective information than the limited routine clinical examination. Using algorithms, detailed movement maps of patients can be created by application of small accelerometric body-sensors that can be worn day and night even at home and monitor everyday activities of patients (Hansen et al. 2018). These home-based assessment systems are being used increasingly since they are unbiased in nature and provide reliable information for the difficulties in daily life that can hardly be detected in the clinical setting. Utilization of such technology including advanced imaging techniques, bioinformatics and other computer-based platforms may provide enormous assistance for disease diagnosis and prediction (Chahine and Stern 2017).
A further essential strategy to accelerate research and improve data quality is combining the effort of different centers. A review on longitudinal cohort studies in PD revealed that in 2017, 44 cohort studies were being followed the majority of which had a sample size smaller than 100 participants (Heinzel et al. 2017). Only less than half of the cohorts had a sample size larger than 200 indicating a possibly limited statistical power considering the outliers and drop-outs. Unfortunately, comparison of results between these studies also appears to be extremely difficult, as a huge variety of scales, assessments tools and techniques had been applied. Thus, harmonizing of assessment is seen as a major goal for deriving the important information from ongoing studies. Once assessments are harmonized, combination of the results of these studies would create a sample size of thousands boosting the statistical power extremely and granting valuable information for rarely studied variables. Moreover, comparison of data would be facilitated. An example of center overarching longitudinal data collection is the “Parkinson’s Progression Marker Initiative” (PPMI) study, which includes more than 30 centers across the world with detailed and standardized assessments to find biological, imaging or clinical biomarkers for the progression of PD (Marek et al. 2011). Fortunately, more multicenter center studies are being planned to accelerate research progress and increase data quality and harmonization between centers. The “Systemic Synuclein Sampling Study” is such an example which set out to measure α-synuclein from four different tissue samples in six different centers to define a reproducible way of detecting a biomarker for the diagnosis and progression of PD (Visanji et al. 2017).
Still, additional genetic, environmental and life style factors should also be taken into account. For instance, individuals with a traumatic brain injury (TBI) have shown to have a 44% increased risk for PD in 5–7 years following the TBI (Gardner et al. 2015). This risk increases up to 83% in patients with a severe TBI in 12 years (Gardner et al. 2018). However, not in all. There seems to be a genetic vulnerability which increases the risk to develop PD after TBI. Individuals with an expanded SNCA Rep1 genotype were found to have an increased risk, while short Rep1 is associated with a decreased risk (Goldman et al. 2012). This finding is a perfect example for gene-environment interaction.
Moreover, possible environmental factors can account for the diverse penetrance even in monogenic PD forms. It has been reported that the penetrance of Gly2019Ser pathogenic variant of LRRK2 mutation is more frequent in Tunisian or Ashkenazi Jewish in comparison to Norwegian PD patients pointing out an effect of outer influences (Hentati et al. 2014). Probably, environmental factors operate their effects through epigenetic mechanisms which affect gene expression without changing the DNA itself. It is known that DNA transcription is inhibited with methylation of the DNA (Wüllner et al. 2016). In sporadic PD, for instance, methylation of the PGC-1 gene (stimulated by the pro-inflammatory fatty acid palmitate) has been shown to contribute to the diagnosis of PD (Su et al. 2015). The effects of the methylation pattern of the DNA on other PD features such as the age of onset, male susceptibility or drug response have also been demonstrated, highlighting the pivotal role of epigenetics on PD heterogeneity.
Conclusion
Despite considerable advance in the last decades, biomarker research in PD has yielded only limited success. One major reason for the difficulty in biomarker detection seems to be the heterogeneity of PD which can be observed in every aspect of the disease, from pathology to clinical phenotype including disease progression. Inconsistencies between biomarker studies in patient selection or assessment methods can also partly explain the lack of success for developing a biomarker. Nevertheless, a lot has been learned from past failures. For the detection of a convenient and strong biomarker we need unbiased biomarker discovery programs in large (multicenter) cohorts, transparent reporting and harmonization of assessment methods across centers, public data sharing, utilization of complex analysis strategies and automated standardized assessment methods. This will set the basis for replication and reproduction (validation) of findings, creating models to integrate different biomarkers in multimodal platforms and employing a precision medicine approach, which takes additional and individual factors such as life style or comorbidities into account. Future research integrating these aspects should help setting criteria for PD subtyping by clarifying disease heterogeneity, allowing individualized counseling by predicting disease progression and enabling the development of neuroprotective interventions by predicting the prodromal stage.
References
Andreasen N, Vanmechelen E, Van de Voorde A et al (1998) Cerebrospinal fluid tau protein as a biochemical marker for Alzheimer’s disease: a community based follow up study. J Neurol Neurosurg Psychiatry 64:298–305
Berg D, Postuma RB, Bloem B et al (2014) Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson’s disease. Mov Disord 29:454–462. https://doi.org/10.1002/mds.25844
Berg D, Postuma RB, Adler CH et al (2015) MDS research criteria for prodromal Parkinson’s disease. Mov Disord 30:1600–1611. https://doi.org/10.1002/mds.26431
Biomarkers Definitions Working Group (2001) Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 69:89–95. https://doi.org/10.1067/mcp.2001.113989
Braak H, Del Tredici K, Rüb U et al Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Chahine LM, Stern MB (2017) Parkinson’s disease biomarkers: where are we and where do we go next? Mov Disord Clin Pract 4:796–805. https://doi.org/10.1002/mdc3.12545
Chen-Plotkin AS, Zetterberg H (2018) Updating our definitions of Parkinson’s disease for a molecular age. J Parkinson’s Dis 8:S53–S57. https://doi.org/10.3233/JPD-181487
Davis MY, Johnson CO, Leverenz JB et al (2016) Association of GBA mutations and the E326K polymorphism with motor and cognitive progression in Parkinson disease. JAMA Neurol 73:1217–1224. https://doi.org/10.1001/jamaneurol.2016.2245
De Marzi R, Seppi K, Högl B et al (2016) Loss of dorsolateral nigral hyperintensity on 3.0 T susceptibility-weighted imaging in idiopathic rapid eye movement sleep behavior disorder. Ann Neurol 79:1026–1030. https://doi.org/10.1002/ana.24646
Doppler K, Volkmann J, Sommer C (2016) Skin biopsies in the differential diagnosis of parkinsonism: are we ready for simplified protocols? Brain 139:e5. https://doi.org/10.1093/brain/awv251
Espay AJ, Brundin P, Lang AE (2017) Precision medicine for disease modification in Parkinson disease. Nat Rev Neurol 13:119–126. https://doi.org/10.1038/nrneurol.2016.196
Fereshtehnejad S-M, Montplaisir JY, Pelletier A et al (2017) Validation of the MDS research criteria for prodromal Parkinson’s disease: longitudinal assessment in a REM sleep behavior disorder (RBD) cohort. Mov Disord 32:865–873. https://doi.org/10.1002/mds.26989
Frosini D, Cosottini M, Donatelli G et al (2017) Seven tesla MRI of the substantia nigra in patients with rapid eye movement sleep behavior disorder. Parkinsonism Relat Disord 43:105–109. https://doi.org/10.1016/j.parkreldis.2017.08.002
Fullard ME, Morley JF, Duda JE (2017) Olfactory dysfunction as an early biomarker in Parkinson’s disease. Neurosci Bull 33:515–525. https://doi.org/10.1007/s12264-017-0170-x
Funke C, Schneider SA, Berg D, Kell DB (2013) Genetics and iron in the systems biology of Parkinson’s disease and some related disorders. Neurochem Int 62:637–652. https://doi.org/10.1016/j.neuint.2012.11.015
García-Lorenzo D, Longo-Dos Santos C, Ewenczyk C et al (2013) The coeruleus/subcoeruleus complex in rapid eye movement sleep behaviour disorders in Parkinson’s disease. Brain 136:2120–2129. https://doi.org/10.1093/brain/awt152
Gardner RC, Burke JF, Nettiksimmons J et al (2015) Traumatic brain injury in later life increases risk for Parkinson disease. Ann Neurol 77:987–995. https://doi.org/10.1002/ana.24396
Gardner RC, Byers AL, Barnes DE et al (2018) Mild TBI and risk of Parkinson disease. Neurology 90:e1771–e1779. https://doi.org/10.1212/WNL.0000000000005522
Gibb WR, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 51:745–752
Goldman SM, Kamel F, Ross GW et al (2012) Head injury, alpha-synuclein Rep1, and Parkinson’s disease. Ann Neurol 71:40–48. https://doi.org/10.1002/ana.22499
Goldstein DS, Holmes C, Bentho O et al (2008) Biomarkers to detect central dopamine deficiency and distinguish Parkinson disease from multiple system atrophy. Parkinsonism Relat Disord 14:600–607. https://doi.org/10.1016/j.parkreldis.2008.01.010
Goldstein DS, Holmes C, Sharabi Y (2012) Cerebrospinal fluid biomarkers of central catecholamine deficiency in Parkinson’s disease and other synucleinopathies. Brain 135:1900–1913. https://doi.org/10.1093/brain/aws055
Halbgebauer S, Öckl P, Wirth K et al (2016) Protein biomarkers in Parkinson’s disease: focus on cerebrospinal fluid markers and synaptic proteins. Mov Disord 31:848–860. https://doi.org/10.1002/mds.26635
Hansen C, Sanchez-Ferro A, Maetzler W (2018) How mobile health technology and electronic health records will change care of patients with Parkinson’s disease. J Parkinson’s Dis 8:S41–S45. https://doi.org/10.3233/JPD-181498
Hansson O, Zetterberg H, Buchhave P et al (2006) Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol 5:228–234. https://doi.org/10.1016/S1474-4422(06)70355-6
Hansson O, Hall S, Ohrfelt A et al (2014) Levels of cerebrospinal fluid α-synuclein oligomers are increased in Parkinson’s disease with dementia and dementia with Lewy bodies compared to Alzheimer’s disease. Alzheimers Res Ther 6:25. https://doi.org/10.1186/alzrt255
Heinzel S, Lerche S, Maetzler W, Berg D (2017) Global, yet incomplete overview of cohort studies in Parkinson’s disease. J Parkinson’s Dis 7:423–432. https://doi.org/10.3233/JPD-171100
Hentati F, Trinh J, Thompson C et al (2014) LRRK2 parkinsonism in Tunisia and Norway: a comparative analysis of disease penetrance. Neurology 83:568–569. https://doi.org/10.1212/WNL.0000000000000675
Herbert MK, Aerts MB, Beenes M et al (2015) CSF neurofilament light chain but not FLT3 ligand discriminates parkinsonian disorders. Front Neurol 6:91. https://doi.org/10.3389/fneur.2015.00091
Hong Z, Shi M, Chung KA et al (2010) DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson’s disease. Brain 133:713–726. https://doi.org/10.1093/brain/awq008
Hopfner F, Künstner A, Müller SH et al (2017) Gut microbiota in Parkinson disease in a northern German cohort. Brain Res 1667:41–45. https://doi.org/10.1016/j.brainres.2017.04.019
Jennings D, Siderowf A, Stern M et al (2017) Conversion to Parkinson disease in the PARS hyposmic and dopamine transporter-deficit prodromal cohort. JAMA Neurol 74:933–940. https://doi.org/10.1001/jamaneurol.2017.0985
Khalil M, Teunissen CE, Otto M et al (2018) Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 14:577–589. https://doi.org/10.1038/s41582-018-0058-z
Kim R, Kim H-J, Kim A et al (2018) Peripheral blood inflammatory markers in early Parkinson’s disease. J Clin Neurosci 58:30–33. https://doi.org/10.1016/j.jocn.2018.10.079
Knudsen K, Borghammer P (2018) Imaging the Autonomic nervous system in parkinson’s disease. Curr Neurol Neurosci Rep 18:79. https://doi.org/10.1007/s11910-018-0889-4
Knudsen K, Fedorova TD, Hansen AK et al (2018) In-vivo staging of pathology in REM sleep behaviour disorder: a multimodality imaging case–control study. Lancet Neurol 17:618–628. https://doi.org/10.1016/S1474-4422(18)30162-5
Lewis SJG, Foltynie T, Blackwell AD et al (2005) Heterogeneity of Parkinson’s disease in the early clinical stages using a data driven approach. J Neurol Neurosurg Psychiatry 76:343–348. https://doi.org/10.1136/jnnp.2003.033530
LeWitt P, Schultz L, Auinger P et al (2011) CSF xanthine, homovanillic acid, and their ratio as biomarkers of Parkinson’s disease. Brain Res 1408:88–97. https://doi.org/10.1016/j.brainres.2011.06.057
Lewitt PA, Li J, Lu M et al (2013) 3-hydroxykynurenine and other Parkinson’s disease biomarkers discovered by metabolomic analysis. Mov Disord 28:1653–1660. https://doi.org/10.1002/mds.25555
Lin Y-S, Lee W-J, Wang S-J, Fuh J-L (2018) Levels of plasma neurofilament light chain and cognitive function in patients with Alzheimer or Parkinson disease. Sci Rep 8:17368. https://doi.org/10.1038/s41598-018-35766-w
Mahlknecht P, Gasperi A, Willeit P et al (2016) Prodromal Parkinson’s disease as defined per MDS research criteria in the general elderly community. Mov Disord 31:1405–1408. https://doi.org/10.1002/mds.26674
Marek K, Jennings D, Lasch S et al (2011) The Parkinson progression marker initiative (PPMI). Prog Neurobiol 95:629–635. https://doi.org/10.1016/j.pneurobio.2011.09.005
Marras C, Lang A (2013) Parkinson’s disease subtypes: lost in translation? J Neurol Neurosurg Psychiatry 84:409–415. https://doi.org/10.1136/jnnp-2012-303455
Marras C, Rochon P, Lang AE (2002) Predicting motor decline and disability in Parkinson disease. Arch Neurol 59:1724. https://doi.org/10.1001/archneur.59.11.1724
Meles SK, Renken RJ, Janzen A et al (2018) The metabolic pattern of idiopathic REM sleep behavior disorder reflects early-stage parkinson disease. J Nucl Med 59:1437–1444. https://doi.org/10.2967/jnumed.117.202242
Mondello S, Constantinescu R, Zetterberg H et al (2014) CSF α-synuclein and UCH-L1 levels in Parkinson’s disease and atypical parkinsonian disorders. Parkinsonism Relat Disord 20:382–387. https://doi.org/10.1016/j.parkreldis.2014.01.011
Navarro-Sánchez L, Águeda-Gómez B, Aparicio S, Pérez-Tur J (2018) Epigenetic study in Parkinson’s disease: a pilot analysis of DNA methylation in candidate genes in brain. Cells 7:150. https://doi.org/10.3390/cells7100150
Nilsonne G, Lekander M (2017) Circulating interleukin 6 in Parkinson disease. JAMA Neurol 74:607–608. https://doi.org/10.1001/jamaneurol.2017.0037
Obeso JA, Stamelou M, Goetz CG et al (2017) Past, present, and future of Parkinson’s disease: a special essay on the 200th Anniversary of the Shaking Palsy. Mov Disord 32:1264–1310. https://doi.org/10.1002/mds.27115
Parkinson J (1817) An essay on the shaking palsy. Whittingham and Rowland Sherwood, Neely and Jones, London
Pilotto A, Heinzel S, Suenkel U et al (2017) Application of the movement disorder society prodromal Parkinson’s disease research criteria in 2 independent prospective cohorts. Mov Disord. https://doi.org/10.1002/mds.27035
Ping L, Duong DM, Yin L et al (2018) Global quantitative analysis of the human brain proteome in Alzheimer’s and Parkinson’s disease. Sci data 5:180036. https://doi.org/10.1038/sdata.2018.36
Postuma RB, Berg D (2016) Advances in markers of prodromal Parkinson disease. Nat Rev Neurol 12:622–634. https://doi.org/10.1038/nrneurol.2016.152
Postuma RB, Berg D, Stern M et al (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30:1591–1601. https://doi.org/10.1002/mds.26424
Postuma RB, Poewe W, Litvan I et al (2018) Validation of the MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 33:1601–1608. https://doi.org/10.1002/mds.27362
Pyatigorskaya N, Gaurav R, Arnaldi D et al (2017) Magnetic resonance imaging biomarkers to assess substantia nigra damage in idiopathic rapid eye movement sleep behavior disorder. Sleep. https://doi.org/10.1093/sleep/zsx149
Rahmani F, Saghazadeh A, Rahmani M et al (2019) Plasma levels of brain-derived neurotrophic factor in patients with Parkinson disease: a systematic review and meta-analysis. Brain Res 1704:127–136. https://doi.org/10.1016/j.brainres.2018.10.006
Rolinski M, Griffanti L, Piccini P et al (2016) Basal ganglia dysfunction in idiopathic REM sleep behaviour disorder parallels that in early Parkinson’s disease. Brain 139:2224–2234. https://doi.org/10.1093/brain/aww124
Saal K-A, Galter D, Roeber S et al (2017) Altered expression of growth associated protein-43 and Rho kinase in human patients with Parkinson’s disease. Brain Pathol 27:13–25. https://doi.org/10.1111/bpa.12346
Santiago JA, Bottero V, Potashkin JA (2018) Evaluation of RNA blood biomarkers in the Parkinson’s disease biomarkers program. Front Aging Neurosci 10:157. https://doi.org/10.3389/fnagi.2018.00157
Saunders-Pullman R, Mirelman A, Alcalay RN et al (2018) Progression in the LRRK2-asssociated Parkinson disease population. JAMA Neurol 75:312–319. https://doi.org/10.1001/jamaneurol.2017.4019
Scheperjans F, Derkinderen P, Borghammer P (2018) The gut and Parkinson’s disease: hype or hope? J Parkinson’s Dis 8:S31–S39. https://doi.org/10.3233/JPD-181477
Siderowf A, Xie SX, Hurtig H et al (2010) CSF amyloid 1–42 predicts cognitive decline in Parkinson disease. Neurology 75:1055–1061. https://doi.org/10.1212/WNL.0b013e3181f39a78
Smith AR, Smith RG, Burrage J et al (2019) A cross-brain regions study of ANK1 DNA methylation in different neurodegenerative diseases. Neurobiol Aging 74:70–76. https://doi.org/10.1016/j.neurobiolaging.2018.09.024
Strimbu K, Tavel JA (2010) What are biomarkers? Curr Opin HIV AIDS 5:463–466. https://doi.org/10.1097/COH.0b013e32833ed177
Su X, Chu Y, Kordower JH et al (2015) PGC-1α promoter methylation in Parkinson’s disease. PLoS One 10:e0134087. https://doi.org/10.1371/journal.pone.0134087
Tokuda T, Qureshi MM, Ardah MT et al (2010) Detection of elevated levels of α-synuclein oligomers in CSF from patients with Parkinson disease. Neurology 75:1766–1772. https://doi.org/10.1212/WNL.0b013e3181fd613b
Trupp M, Jonsson P, Ohrfelt A et al (2014) Metabolite and peptide levels in plasma and CSF differentiating healthy controls from patients with newly diagnosed Parkinson’s disease. J Parkinson’s Dis 4:549–560. https://doi.org/10.3233/JPD-140389
van Nuenen BFL, van Eimeren T, van der Vegt JPM et al (2009) Mapping preclinical compensation in Parkinson’s disease: an imaging genomics approach. Mov Disord 24:S703–S710. https://doi.org/10.1002/mds.22635
van Dijk KD, Persichetti E, Chiasserini D et al (2013) Changes in endolysosomal enzyme activities in cerebrospinal fluid of patients with Parkinson’s disease. Mov Disord 28:747–754. https://doi.org/10.1002/mds.25495
Vazquez Roque M, Bouras EP (2015) Epidemiology and management of chronic constipation in elderly patients. Clin Interv Aging 10:919–930. https://doi.org/10.2147/CIA.S54304
Vilas D, Iranzo A, Tolosa E et al (2016) Assessment of α-synuclein in submandibular glands of patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case–control study. Lancet Neurol 15:708–718. https://doi.org/10.1016/S1474-4422(16)00080-6
Visanji NP, Mollenhauer B, Beach TG et al (2017) The systemic synuclein sampling study: toward a biomarker for Parkinson’s disease. Biomark Med 11:359–368. https://doi.org/10.2217/bmm-2016-0366
Vos M, Esposito G, Edirisinghe JN et al (2012) Vitamin K2 is a mitochondrial electron carrier that rescues Pink1 deficiency. Science 336:1306–1310. https://doi.org/10.1126/science.1218632
Williams-Gray CH, Wijeyekoon R, Yarnall AJ et al (2016) Serum immune markers and disease progression in an incident Parkinson’s disease cohort (ICICLE-PD). Mov Disord 31:995–1003. https://doi.org/10.1002/mds.26563
Willkommen D, Lucio M, Moritz F et al (2018) Metabolomic investigations in cerebrospinal fluid of Parkinson’s disease. PLoS One 13:e0208752. https://doi.org/10.1371/journal.pone.0208752
Wüllner U, Kaut O, deBoni L et al (2016) DNA methylation in Parkinson’s disease. J Neurochem 139:108–120. https://doi.org/10.1111/jnc.13646
Zetterberg H, Schott JM (2019) Biomarkers for Alzheimer’s disease beyond amyloid and tau. Nat Med 25:201–203. https://doi.org/10.1038/s41591-019-0348-z
Zhu Z-G, Sun M-X, Zhang W-L et al (2017) The efficacy and safety of coenzyme Q10 in Parkinson’s disease: a meta-analysis of randomized controlled trials. Neurol Sci 38:215–224. https://doi.org/10.1007/s10072-016-2757-9
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yilmaz, R., Hopfner, F., van Eimeren, T. et al. Biomarkers of Parkinson’s disease: 20 years later. J Neural Transm 126, 803–813 (2019). https://doi.org/10.1007/s00702-019-02001-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-019-02001-3