Abstract
Purpose of Review
Patients with Parkinson’s disease (PD) often display gastrointestinal and genitourinary autonomic symptoms years or even decades prior to diagnosis. These symptoms are thought to be caused in part by pathological α-synuclein inclusions in the peripheral autonomic and enteric nervous systems. It has been proposed that the initial α-synuclein aggregation may in some PD patients originate in peripheral nerve terminals and then spread centripetally to the spinal cord and brainstem. In vivo imaging methods can directly quantify the degeneration of the autonomic nervous system as well as the functional consequences such as perturbed motility. Here, we review the methodological principles of these imaging techniques and the major findings in patients with PD and atypical parkinsonism.
Recent Findings
Loss of sympathetic and parasympathetic nerve terminals in PD can be visualized using radiotracer imaging, including 123I-MIBG scintigraphy, and 18F-dopamine and 11C-donepezil PET. Recently, ultrasonographical studies disclosed reduced diameter of the vagal nerves in PD patients. Radiological and radioisotope techniques have demonstrated dysmotility and prolonged transit time throughout all subdivisions of the gastrointestinal tract in PD. The prevalence of objective dysfunction as measured with these imaging methods is often considerably higher compared to the prevalence of subjective symptoms experienced by the patients.
Summary
Degeneration of the autonomic nervous system may play a key role in the pathogenesis of PD. In vivo imaging techniques provide powerful and noninvasive tools to quantify the degree and extent of this degeneration and its functional consequences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a multisystem neurodegenerative disorder with early and widespread involvement of the autonomic and enteric nervous systems [1••]. A substantial fraction of PD patients experience genitourinary and gastrointestinal symptoms years or even decades prior to diagnosis [2,3,4]. Inclusions, consisting of misfolded and aggregated α-synuclein, have been detected in sympathetic and parasympathetic nerve fibers and throughout the gastrointestinal canal in manifest and premotor PD patients [5,6,7,8,9]. It has been suggested that α-synuclein aggregation initially starts in nonmyelinated, hyperbranched axons and terminals of the autonomic nerves, and then spreads by axonal transport to the central nervous system. This hypothesis offers an attractive explanation for the abundance of α-synuclein pathology observed in both sympathetic and parasympathetic neurons [10, 11].
Functional imaging presents powerful and noninvasive opportunities for exploring the consequences of autonomic denervation in movement disorders. Direct measures of sympathetic and parasympathetic nerve terminal degeneration can be obtained by positron emission tomography (PET) and gamma camera methodology. Other imaging techniques can quantify the functional consequences of autonomic degeneration. Delayed gastric emptying, esophageal and intestinal dysmotility, and anorectal dysfunction are all amenable to quantification by radioisotope and radiological imaging methods. Here, we review the methods for direct imaging of autonomic nervous system degeneration, as well as the functional consequences in the context of PD and atypical movement disorders.
The Autonomic Nervous System
The autonomic nervous system comprises three major divisions: the sympathetic, parasympathetic, and enteric nervous systems [1••]. The efferent outflow of the sympathetic and parasympathetic system is organized in serial two-neuron arrangements. The preganglionic neurons of the sympathetic system are located in four major nuclei of the intermediate zone of the T1 to L2 spinal cord levels, with the intermediolateral cell column (IML) being the most important contributor of sympathetic efferent outflow [12]. The preganglionic sympathetic fibers project to the cell bodies of postganglionic neurons residing in the prevertebral and paravertebral ganglia. The preganglionic neurons thereby provide the only connecting link between the central and peripheral sympathetic systems [1••]. The often very long axons of the postganglionic neurons then project to end effector organs throughout the body. All postganglionic sympathetic neurons release noradrenaline at their synapses, except for the well-known exceptions of sweat glands, adrenal medulla, and the kidney.
The preganglionic neurons of the parasympathetic nervous system are located in several brainstem nuclei and the sacral parasympathetic nucleus of sacral spinal segments S2 to S4. The dorsal motor nucleus of the vagus (DMV), located in the medulla oblongata, contributes the most significant part of visceral motor efferents and innervates the thoracic and most abdominal organs, including the gastrointestinal tract to the level of the proximal transverse colon. The sacral parasympathetic nucleus innervates pelvis organs and the distal colon and rectum. Postganglionic parasympathetic ganglia are mostly embedded within target organs giving rise to short and localized postganglionic fiber end fields. Both pre- and postganglionic parasympathetic neurons are cholinergic.
The enteric nervous system is a distributed system of intramural neuronal ganglia of the gastrointestinal tract. The enteric nervous system displays a high degree of autonomy but is also modulated to varying degrees by sympathetic and parasympathetic input [13, 14].
Imaging the Sympathetic Nervous System
The presence of pathological α-synuclein (α-syn) inclusions in the IML is an almost ubiquitous finding in PD patients [5, 15,16,17], and it may be among the earliest sites affected by the pathology [15, 18]. Several studies have reported that more severe pathology is often seen in the peripheral sympathetic ganglia as compared to the IML, suggestive of a peripheral-to-central propagation process [15, 19].
In multiple system atrophy (MSA), the IML very frequently shows marked degeneration and cell loss [20, 21], whereas the postganglionic system is mostly spared. Nevertheless, some authors did report the involvement of the postganglionic neurons [22, 23], and in six of 15 MSA patients, the number of tyrosine hydroxylase-immunoreactive cardiac nerve fibers was also slightly to moderately reduced [24].
Postmortem studies of cases with progressive supranuclear palsy (PSP) disclosed consistent involvement of the cervical and thoracic spinal cord, but no cell loss was seen in the IML [25, 26]. Also, no neurofibrillary tau tangles were seen in peripheral sympathetic ganglia of eight PSP cases [27], and tyrosine hydroxylase-immunoreactivity in cardiac nerve fibers was similar to healthy controls [28].
The sympathetic postganglionic nerve terminals are most commonly assessed in vivo using 123I-meta-iodobenzylguanidin (MIBG) cardiac scintigraphy. Less commonly used imaging methods include 18F-fluorodopamine and 11C-meta-hydroxyephedrine (HED) positron emission tomography (PET). In similarity to MIBG, these tracers are stored in vesicles of noradrenergic terminals [29, 30].
MIBG scintigraphy has been used for decades to image the loss of cardiac innervation in PD and has been the subject of several recent reviews [31,32,33]. Early (15 min) and late (3–4 h) MIBG static images are obtained using a gamma camera, and heart-to-mediastinum (H/M) ratios are calculated on these images. The H/M ratio difference between the early and late images allows estimation of tracer delivery and vesicular storage [34].
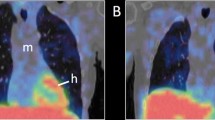
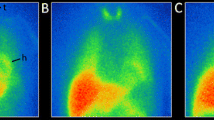
A remarkable loss of cardiac signal is evident in most PD patients (Fig. 1). Indeed, to our knowledge, no other neurotransmitter system displays such fulminant loss of innervation. In total, 80–90% of PD and DLB patients have pathological MIBG scintigraphies, but 40–50% of de novo Hoehn and Yahr stage I PD patients still have H/M ratios within normal limits [33, 35,36,37,38]. However, at Hoehn and Yahr stage III, nearly all PD patients show profound loss of MIBG signal [39, 40]. Using 18F-dopamine PET, Goldstein and colleagues have shown that the initial denervation occurs in the lateral wall of the left ventricle, subsequently followed by degeneration of the septum (Fig. 2) [41]. Increasing H/M ratios from the early to late time points are seen in healthy subjects. In contrast, most PD and DLB patients show declining H/M ratios, which signifies progressive dysfunction and loss of vesicular storage capacity in surviving neurons [42, 43]. Lately, it has been shown that 3D tomographical MIBG imaging improves the diagnostic accuracy, as it allows sensitive detection of the initial denervation in subregions of the myocardium [44,45,46].
18F-dopamine PET images of the heart. a Normal cardiac uptake in a healthy control subject. b Regionally decreased uptake in the lateral wall but conserved septal uptake of an early-stage PD patient. c Globally reduced uptake in a PD patient. [18F-dopamine images provided with courtesy of Professor David S. Goldstein, NIH]
Tremor-predominant PD patients generally show more conserved cardiac MIBG uptake compared to the akinetic-rigid phenotype [47]. In contrast, PD patients with clinical REM sleep behavior disorder (RBD) have significantly more pronounced loss of MIBG uptake than PD patients with subclinical RBD or without RBD [48, 49]. Nearly all patients with idiopathic RBD, most of whom go on to develop PD or DLB [50], show pathological levels of cardiac MIBG uptake, which is comparable to that of Hoehn and Yahr stage III–V PD patients [40, 51, 52]. This observation strongly suggests that cardiac sympathetic denervation happens very early during the prodromal phase of RBD-positive PD and DLB patients. In general, studies of the association between cardiac denervation and disease stage have shown mixed results with some studies showing a negative correlation between cardiac MIBG signal and Hoehn and Yahr stage and with progressive UPDRS motor scores, whereas other studies failed to detect such correlations [31]. These studies did not control for important phenotypical characteristics such as RBD status or tremor- vs. akinetic-rigid dominant subtypes of PD. It seems probable that degeneration of the peripheral autonomic nervous system is driven more by these phenotypes than by disease stage defined strictly by motor symptom progression.
Symptoms of dysautonomia including orthostatic hypotension correlate poorly with cardiac MIBG uptake. Early PD cases with abnormal MIBG uptake often do not present with orthostatic hypotension [53,54,55]. An early fluorodopamine PET study reported markedly decreased myocardial uptake in all PD patients with orthostatic hypotension, whereas 50% of patients without orthostatic hypotension showed some fluorodopamine retention mostly in the septum [41]. This finding suggests a potential association between the sympathetic imaging markers and orthostatic hypotension but only is discernible when 3D tomographic techniques are applied.
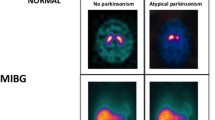
Patients with atypical parkinsonism, including MSA, PSP, and CBD, most often exhibit normal or near-normal cardiac sympathetic imaging [35, 42]. Cardiac MIBG uptake is decreased in 10–20% of MSA subjects, possibly explained by slight to moderately decreased number of tyrosine hydroxylase-immunoreactive cardiac nerve fibers in some MSA cases at postmortem [39, 56]. Some studies reported that nearly all PSP patients show normal cardiac MIBG uptake [56], whereas others reported that significant fractions of PSP populations show decreased MIBG uptake [31, 39]. Recent meta-analyses reported pooled sensitivity and specificity estimates of ~ 85% for separating PD from atypical movement disorders [57, 58].
It should be remembered that loss of sympathetic cardiac innervation is not specific to PD. Many patients with diabetic neuropathy also develop cardiac autonomic neuropathy [59]. Patients with chronic heart failure often display markedly reduced cardiac MIBG signal [60]. Also, several types of medications interfere with cardiac MIBG uptake, including phenylephrine, labetolol, cocaine, some antidepressants, and other drugs [61]. It is mandatory to abstain from these medications before cardiac MIBG imaging.
Imaging the Parasympathetic Nervous System
The parasympathetic nervous system is probably affected very early on in most cases of PD. Pathological α-synuclein inclusions are found in the DMV in the majority of PD patients [62], and approximately 50% of DMV neurons are lost during the cause of the disease [63, 64]. α-Synuclein aggregates can be found in vagal efferents of internal organs [65,66,67], including the myenteric and submucosal plexus of the gastrointestinal tract [68,69,70]. A rostrocaudal gradient of α-synuclein pathology has been reported throughout the gastrointestinal canal [6, 71], and it has been suggested that this distribution parallels the density of efferent vagal innervation [72].
The dual-hit hypothesis suggests that α-synuclein pathology initially forms in the olfactory bulb and the autonomic nerve endings of the gastrointestinal mucosa, and then spreads by retrograde axonal transport through the vagus to the DMV and possibly through the sympathetic pathways to the IML [11, 73, 74]. This idea gained some support from two recent epidemiological studies showing that truncal vagotomy may reduce the risk of PD by 40–50% [75, 76]. Also, α-synuclein inclusions have been detected in gastrointestinal tissues of PD patients up to 20 years prior to the time of diagnosis [7, 9, 77]. Despite the realization that the parasympathetic branch of the autonomic nervous system may be crucial to our understanding of early PD pathogenesis, very little work has been done to develop appropriate imaging markers of this system. An important obstacle to the development of parasympathetic imaging markers is the lack of specific binding targets. The pre- and postganglionic parasympathetic neurons are cholinergic, but so are approximately 70% of the 100 million neurons of the enteric nervous system [78].
Measurements of acetylcholinesterase (AChE) activity have been used for decades to assess cholinergic nerve fiber density including parasympathetic fibers in histological studies [79,80,81]. More recently, immunohistochemical detection of the vesicular acetylcholine transporter (VAChT) has become a preferred method for specific labeling of cholinergic neurons.
We recently validated the PET tracer [11C]donepezil for in vivo quantification of AChE density in peripheral organs and demonstrated that the pattern of [11C]donepezil binding approximates vagal innervation of internal organs. This PET tracer shows no biliary excretion of radiometabolites during a 1-h PET scan, which is an important requisite for quantifying binding to AChE in the upper gastrointestinal tract [82].
So far, two [11C]donepezil PET studies of PD patients have been published. In the first study, 12 early- to moderate-stage PD patients showed a marked [11C]donepezil standard uptake value reduction in the small intestine (35%) and pancreas (30%) in comparison to healthy controls [83] (Fig. 3). In the second study, 19 newly diagnosed PD patients (disease duration 1.5 years) exhibited a somewhat less pronounced decrease in the small intestine (14%), no significant decrease in the pancreas, but a highly significant 22% decrease in the colon [84]. Based on these two cross-sectional studies, we hypothesized that the initial [11C]donepezil signal decrease is seen in the colon and small intestine during the prodromal phase, followed by progressive involvement of the pancreas after the time of diagnosis [84]. Recently, we showed that 22 patients with idiopathic RBD had significantly decreased [11C]donepezil values in the small intestine and colon compared to healthy controls, but again no significant decrease was seen in the pancreas [85••]. This observation largely confirmed our prediction and demonstrates that the cholinergic innervation of the intestine is progressively damaged during the prodromal phase of PD, whereas pancreatic denervation probably manifests during the motor phase.
Interestingly, histological studies of the small and large intestine of guinea pigs and humans have shown that 70–90% of α-synuclein-positive varicosities colocalize with the cholinergic marker VAChT (vesicular acetylcholine transporter), whereas far fewer varicosities colocalize with other major neurotransmitter systems including 5-HT and tyrosine hydroxylase [86, 87]. These observations suggest that cholinergic nerve terminals of the enteric and autonomic nervous systems may be particularly vulnerable to α-synuclein-related damage, and by extension, that cholinergic PET markers such as [11C]donepezil may be optimal to track progressive damage to the autonomic nervous system of the gut.
Recently, ultrasonography was used for the first time to study the vagus nerve size on the neck in PD patients (disease duration 5 years) [88•]. The cross-sectional areas of both the right and left vagus nerves were significantly decreased in PD patients compared to controls. Near-significant inverse correlations were seen between vagal size and Hoehn and Yahr stage (p = 0.06) and also with total gastrointestinal dysfunction score on the SCOPA-AUT questionnaire (p = 0.06). This ultrasonographical marker separated PD from controls with 75–80% sensitivity and specificity and provides independent in vivo evidence that the parasympathetic system is markedly affected in PD.
In Vivo Staging of Multiple Pathologies in Prodromal PD
Using the methods reviewed above in combination with other radioisotope and magnetic resonance imaging (MRI) imaging markers, it is now possible to perform comprehensive in vivo staging of neuronal pathologies related to separate Braak stages. In a multimodality imaging study, we compared idiopathic RBD patients with PD patients and healthy controls [85••]. We used [11C]donepezil PET to assess cholinergic (parasympathetic) gut innervation, [123I]MIBG to measure cardiac sympathetic innervation, neuromelanin-sensitive MRI to measure integrity of pigmented neurons of the locus coeruleus [89], and 18F-dihydroxyphenylalanine (DOPA) PET to assess nigrostriatal dopamine storage capacity. The RBD patients displayed fully developed pathology, at the level of diagnosed PD patients, in the parasympathetic and sympathetic nervous systems, and the pigmented cells of the locus coeruleus. In contrast, 70% of RBD subjects had normal FDOPA PET scans of the dopamine system. These findings support that the autonomic nervous systems show early and marked pathology in the prodromal phase of PD at a time where the dopamine system is mostly intact.
Interestingly, a previous study reported that RBD patients display more significant reductions in cardiac MIBG signals compared to Hoehn and Yahr stage I–II PD patients [40], and in our study, the RBD patients also seemed to show larger reductions in the [11C]donepezil signal of the gut compared to early-stage PD. These observations suggest that the RBD-positive phenotype is characterized by more severe denervation of the autonomic nervous system. In support, a recent study showed that 64% of RBD-positive PD patients exhibited phosphorylated α-synuclein pathology in the colon compared to only 13% of RBD-negative PD patients [90•].
Functional Imaging of the GI Tract
Oropharynx and Esophagus
The specific pathophysiological mechanism of dysphagia is still unknown, but basal ganglia dysfunction leading to pharyngeal ridigity and bradykinesia, and peripheral motor and sensory pharyngeal nerve pathology may be causative factors [91]. In the esophagus, dysmotility is most likely caused in part by vagal denervation [92].
In PD, the prevalence of subjectively perceived dysphagia symptoms varies greatly across studies with a pooled estimate of 35% [93,94,95]. Objectively measureable dysfunction is generally more frequent, and the presence of drooling and dysphagia increases the risk of aspiration and respiratory infection [96].
Dysphagia can be quantified with several different imaging methods. Fluoroscopic barium studies can assess swallowing and oropharyngeal transit time by means of continuous X-ray after ingestion of a barium-containing liquid or solid meal [97]. Studies using this methodology reported dysfunction of both oral, pharyngeal, and esophageal phases in up to 91% of PD patients [98, 99]. Pharyngeal constriction dysfunction was reported in 42 and 30% of PD patients in two individual studies, although the predominant feature in the study by Ellerston and colleagues was delayed airway closure with a prevalence of 62% [98, 100]. Another study used multichannel intraluminal impedance manometry (MII) to evaluate pharyngoesophageal function and found esophageal motility and transit abnormalities in up to 67% of PD cases. Also, 95% of 65 PD patients displayed esophageal manometric peristalsis dysfunction [101], and importantly, manometric abnormalities were seen in up to 47% of PD patients with no or only mild dysphagia symptoms [102]. This was supported in a small study using barium-swallow methodology, which showed no correlation between barium-swallow scores and self-reported dysphagia in PD patients [103]. Recently, a study of 184 PD patients proposed videofluoroscopic swallowing as a predictor of poor prognosis, since oropharyngeal dysfunction was found to be associated with the development of aspiration pneumonia detected in 25 patients [104].
Radioisotope scintigraphy can also be used to evaluate dysphagia. Here, transit times through the oropharyngeal and esophageal regions are evaluated from time-activity curves subsequent to gamma camera recording of an ingested radiolabeled liquid or solid meal. Limited data has been published in PD patients, but one study showed significantly delayed esophageal transit time in an early to moderate disease stage patient group compared to control subjects. Also, all 18 PD patients displayed objective scintigraphic or EMG dysfunction in a study by Potulska and colleagues, even though subjective dysphagia symptoms were present in only 13 patients [105].
Dysphagia symptoms have been reported in up to 73% of MSA patients and are also considered characteristic features of patients with PSP and CBD, although based on very limited data [103, 106, 107]. Studies of objective dysphagia in these rare disorders are scarce but generally report a high prevalence of dysfunction. Two barium-swallow studies in PSP patients reported abnormalities in up to 70%, especially in the oral and pharyngeal phase [103, 108]. In MSA patients, esophageal food stagnation was seen in 16/16 patients [109], and a larger study of 59 MSA patients reported videofluoroscopic abnormalities in the majority of participants with MSA-P and MSA-C subtype differences [107]. Also, CBD patients displayed marked barium-swallow abnormalities in 23 of 24 patients, specifically related to multiple swallows to clear a single bolus. This patient group did not report aspiration, and subjective symptoms did not predict objective results [106].
In summary, both subjective dysphagia symptoms and objective dysfunction are frequent in PD, with esophageal dysmotility being the most prevalent feature. In addition, atypical parkinsonian patients also seem to show severe symptomatic and objective abnormalities, mainly related to oral and pharyngeal regions and with potential disease-specific differences. In general, limited association is seen between subjective and objective measures, which seems to be the case across all parkinsonian disorders.
Stomach
Gastric emptying is regulated by vagal innervation and the enteric nervous system as well as by a range of hormones [110]. The pacemaker cells of Cajal provide autonomous regulation of motility, but the central nervous system also influences gastric functions including contractions, volume, and acid secretion [111]. Thus, dysregulation of gastric mechanical function can be caused by multiple factors, but the exact underlying pathophysiology related to PD remains unknown.
Patients with PD report a range of subjective symptoms probably associated with gastroparesis and gastric dysfunction. The prevalence of gastroparesis symptoms in PD such as bloating, nausea, and vomiting is up to 50%, but prevalence estimates vary greatly across studies [110, 112].
The gold standard method for objectively assessing gastric emptying time (GET) is gastric scintigraphy subsequent to ingestion of a radioactive standard solid meal [113]. Scintigraphic GET evaluation in PD was recently reviewed in detail [114]. In short, only a modest delay was seen in PD patients compared to control subjects, and substantial heterogeneity and methodological differences were seen across studies. An early study showed significantly prolonged GET in PD patients with both fluctuating and nonfluctuating motor symptoms compared to controls, but data from another study did not confirm this finding [115, 116]. Another study reported reduced emptying time in PD compared to controls although these PD patients were examined after withdrawal of anti-parkinsonian medication [83]. However, Hardoff et al. did not detect differences in GET between treated and untreated PD patients, which questions the purported influence of levodopa treatment on mechanical stomach emptying [117]. Several studies showed a wide range in reported emptying time within PD patient groups, and also, no firm associations have been described between scintigraphic GET and gastroparesis symptoms or medication status [114, 117, 118].
The recent meta-analysis also compared scintigraphic GET data with outcomes from 13C-sodium breath test studies, an indirect measure of GET [114]. In short, ingested 13C-sodium is emptied from the stomach, absorbed in the jejunum, metabolized in the liver, and expired from the lungs as 13CO2. The concentration of the end product is measured and mathematically converted to a GET estimate [119]. On these breath test measures, PD patients generally show a more severe and marked delay of GET, which was not evident in scintigraphic studies. Given the fact that breath tests are also dependent on other physiological mechanisms like intestinal absorption and liver metabolization, it must be questioned whether the breath test is a valid marker of mechanical gastric emptying [114]. Of note, studies have reported decreased small intestinal absorption in PD using sugar absorption tests. Abnormal intestinal permeability could in part explain the differences seen between scintigraphy and breath test GET [120, 121]. We recently used a magnetic tracking system that generates capsule position and orientation graphs, and here we also saw no difference in GET when comparing PD patients and controls [122]. On the other hand, functional MRI of stomach motility was able to detect significantly decreased amplitude of peristaltic contractions in PD subjects [123]. In summary, standardized multimodality studies are clearly warranted to disentangle the relative contributions of gastric dysmotility and intestinal absorption to upper gastrointestinal dysfunction.
Very few studies have investigated GET disturbances in atypical parkinsonian disorders. A study of 25 MSA patients used 13C-sodium breath tests and showed significantly delayed GET compared to controls [124] . This finding was corroborated in a scintigraphic study of 12 MSA patients [125].
Small Intestine
Very limited data has been published on small intestinal function in PD and other parkinsonian disorders. Small intestinal bacterial overgrowth is a prevalent feature of PD, but the underlying causes are not well understood [93].
In a recent study, gastrointestinal transit time was evaluated in PD using the radio-opaque marker (ROM) technique [126•]. Using CT, it was possible to analyze individual intestinal segments, and retained markers were seen in the small intestine in five of 24 PD patients 24 h after ingestion of the last markers (Fig. 4). This observation indicates that small intestinal dysfunction to some extent is present in early to moderate disease stage PD patients. In support, 22 patients in this PD group were examined using a magnetic tracking system, which measures GI segmental passage. This method also showed significantly increased small intestinal transit time in the PD group compared to controls [122]. Furthermore, a study by Dutkiewicz and colleagues investigated small bowel transit time using serial SPECT scans after ingestion of a 99mTc-containing capsule [127]. Here, orocecal transit time of more than 4 h was seen in 7/10 PD patients compared to < 4 h in all 10 control subjects.
It has been proposed that nonabsorbed tablets can be detected on a CT scan as a sign of small intestinal dysfunction and malabsorption [128]. However, a recent CT study identified unabsorbed tablet residues in only two of 32 examined PD patients, suggesting that such medication malabsorption is not a frequent problem in early- to moderate-stage PD [126•].
In summary, the available data suggest that small intestinal dysfunction may be a common feature in PD, but more studies are needed.
Colon
In PD, constipation symptoms are among the most frequently reported GI nonmotor symptoms, and they often present at the early premotor stage of the disease [3, 129, 130]. The exact underlying mechanisms remain unclear, but cell loss and α-synuclein pathology are seen in both the parasympathetic vagal neurons innervating the upper colon and the neurons of the intermediolateral cell column innervating the lower third of the colon [67, 68, 131]. Also, studies have shown increased permeability, positive fecal markers of intestinal inflammation, and changes in the intestinal microbiota in PD [120, 132•, 133]. The symptom prevalence is on average 50%, but substantial variation in constipation prevalence is seen between studies. It was recently reported that more than 10 different constipation symptom definitions have been used in the PD literature, which probably contributes to the variable findings [134]. Thus, objective markers of colonic function may be a more accurate measure of intestinal involvement in PD.
As previously described, colonic transit time (CTT) can be evaluated by the use of ROM methods. A planar X-ray image is performed 24 h subsequent to ingestion of the last capsule, and the number of retained ROM determined and converted to a transit time estimate [135] (Fig. 5). The literature on CTT in PD was recently reviewed in detail [134]. Figure 6 depicts pooled PD and control ROM data, derived from four different CTT studies, which used identical methodology [126•, 135,136,137]. An optimal cutoff score of 28 ROM yielded a sensitivity of 84% and specificity of 88% for separating patients and controls.
One study showed no CTT difference in PD patients on and off levodopa treatment, respectively. This indicates that colonic dysfunction is predominantly related to disease involvement rather than medical treatment, although this needs to be confirmed in future studies [138]. Also, it has been reported that CTT and subjective constipation symptoms as well as number of weekly bowel movements do not correlate in PD patients [134, 139].
By means of CT scans, the segmental distribution of ROM in the colon can be accurately assessed. A uniform distribution throughout the colon indicates slow transit constipation as opposed to predominant accumulation in the descending and rectosigmoid colon, which indicates outlet obstruction constipation [140]. This distinction is of some relevance, as a large proportion of PD patients report straining for defecation and are known to suffer from anorectal dysfunction such as dyssynergic defecation [141,142,143,144]. Several studies of CTT in PD report a tendency toward delayed transit in the more distal colonic segments rather than general slow transit [126•, 138, 142, 145].
In addition to the ROM technique, colonic volume can be defined on CT or MRI scans and has been proposed as a measure of colonic function [126•, 146]. We recently reported significantly increased colonic volume in a group of 32 PD patients, especially pronounced in the more distal colonic segments (Fig. 7).
The literature on colonic function in atypical parkinsonian disorders is scarce. Constipation has been reported in 55–87% of MSA patients [147, 148]. One small study employed the ROM technique and reported significantly delayed total and rectosigmoid CTT in 14 of 15 MSA patients, compared to controls [149]. This indicates that not only constipation symptoms but also objectively measurable dysfunction is a common feature of MSA, although more data are needed.
Anorectal
Up to 83% of PD patients report straining for defecation, and anorectal complications are among the most frequently self-reported constipation symptoms in this patient group [141, 144, 150]. This indicates that outlet obstruction constipation may be more prevalent than slow transit constipation.
Defecography subsequent to rectally instilled barium contrast medium is a commonly used technique for evaluating anorectal function, where pelvic floor muscle relaxation, emptying rate, and rectal prolapse are assessed on serial X-ray images. Associated anorectal manometry and electromyography (EMG) can provide information on anorectal pressure and contractile function of the puborectalis muscle [140, 150].
Defecography studies have in general reported increased rectal widening, dysfunction of the puborectalis muscle, and increased postdefecation residual volume in PD patients [142, 145, 151, 152]. Also, two studies reported incomplete emptying in about half of the patients, even though this was not evident in PD patients in the off-medication state [143, 150]. In addition, two studies found puborectalis contraction and sphincter muscle abnormalities during straining [142, 143]. Anorectal angle differences between rest and straining correlated with CTT, and thus, it has been suggested that delayed CTT may partly be caused by pelvic floor dyssynergia [145].
Distinct EMG anal sphincter abnormalities and dysfunction are seen in both MSA and PSP, and although some PD patients can resemble MSA patients, sphincter abnormalities are most commonly seen in later-stage PD [153]. Thus, it has been proposed that severe involvement during the first 5 years of symptom onset can potentially differentiate MSA from PD [154]. Libelius and colleagues showed EMG dysfunction in up to 75% of MSA patients, and Stocchi et al. reported reduced resting anal and maximal contraction pressure in about half of the MSA group [153]. In a comprehensive videomanometry study, dysfunction was reported in 93% of MSA patients, including significant abnormalities in anal pressure during squeezing, phasic rectal contraction, and residual feces compared to controls [149]. Thus, anorectal dysfunction appears to be a prevalent feature in MSA, although based on limited data.
Conclusions
Radioisotope-based imaging techniques can noninvasively estimate the progressive involvement of the autonomic nervous system in PD and other movement disorders. Nearly all late-stage PD patients exhibit marked sympathetic denervation on cardiac 123I-MIBG scintigraphy, whereas most patients with atypical parkinsonism have normal MIBG uptake. 11C-donepezil PET studies have disclosed a clear loss of intestinal signal in prodromal PD and in manifest PD cases, which is most likely explained by progressive parasympathetic denervation. Recently, ultrasonography has also demonstrated significant reduction in the size of the cervical vagus nerves in PD patients.
Alternative imaging methods have demonstrated dysfunction in all parts of the gastrointestinal canal in PD, but very few studies have explored gastrointestinal dysfunction in atypical movement disorders.
In PD, objective dysfunction as measured with imaging methods is considerably more frequent than subjective gastrointestinal symptoms. Relying solely on subjective symptoms in PD research may therefore underestimate the degree and extent of gastrointestinal involvement. Thus, objective imaging markers of autonomic degeneration and its functional consequences hold potential to improve our understanding of PD pathophysiology and the identification of distinct PD subtypes characterized by differential involvement of different neurotransmitter systems.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Orimo S, Ghebremedhin E, Gelpi E. Peripheral and central autonomic nervous system: does the sympathetic or parasympathetic nervous system bear the brunt of the pathology during the course of sporadic PD? Cell Tissue Res. 2018;373(1):267–86 Excellent recent review on the degree of autonomic nervous system damage in PD.
Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD, et al. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology. 2001;57:456–62.
Adams-Carr KL, Bestwick JP, Shribman S, Lees A, Schrag A, Noyce AJ. Constipation preceding Parkinson’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2015;86:e4.193–e4.
Noyce AJ, Lees AJ, Schrag AE. The prediagnostic phase of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2016;87:871–8.
Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White III CL, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702.
Gelpi E, Navarro-Otano J, Tolosa E, Gaig C, Compta Y, Rey MJ, et al. Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Mov Disord. 2014;29:1010–8.
Hilton D, Stephens M, Kirk L, Edwards P, Potter R, Zajicek J, et al. Accumulation of alpha-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol. 2014;127:235–41.
Shannon KM, Keshavarzian A, Dodiya HB, Jakate S, Kordower JH. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov Disord. 2012;27:716–9.
Stokholm MG, Danielsen EH, Hamilton-Dutoit SJ, Borghammer P. Pathological alpha-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann Neurol. 2016;79:940–9.
Uchihara T, Giasson BI. Propagation of alpha-synuclein pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol. 2016;131:49–73.
Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm (Vienna). 2003;110:517–36.
Krassioukov AV, Bygrave MA, Puckett WR, Bunge RP, Rogers KA. Human sympathetic preganglionic neurons and motoneurons retrogradely labelled with DiI. J Auton Nerv Syst. 1998;70:123–8.
Hasler WL. Approach to the patient with gas and bloating. In: Yamada T, editor. Gastroenterology. Philadelphia: Lippincott Williams & Wilkins; 2003. p. 195–219.
Schubert ML, Peura DA. Control of gastric acid secretion in health and disease. Gastroenterology. 2008;134:1842–60.
Sumikura H, Takao M, Hatsuta H, Ito S, Nakano Y, Uchino A, et al. Distribution of alpha-synuclein in the spinal cord and dorsal root ganglia in an autopsy cohort of elderly persons. Acta Neuropathol Commun. 2015;3:57.
Braak H, Sastre M, Bohl JR, de Vos RA, Del Tredici K. Parkinson’s disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol. 2007;113:421–9.
Wakabayashi K, Takahashi H. The intermediolateral nucleus and Clarke’s column in Parkinson’s disease. Acta Neuropathol. 1997;94:287–9.
Del Tredici K, Braak H. Spinal cord lesions in sporadic Parkinson’s disease. Acta Neuropathol. 2012;124:643–64.
Orimo S, Uchihara T, Nakamura A, Mori F, Kakita A, Wakabayashi K, et al. Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain. 2008;131:642–50.
Gray F, Vincent D, Hauw JJ. Quantitative study of lateral horn cells in 15 cases of multiple system atrophy. Acta Neuropathol. 1988;75:513–8.
Wenning GK, Tison F, Ben Shlomo Y, Daniel SE, Quinn NP. Multiple system atrophy: a review of 203 pathologically proven cases. Mov Disord. 1997;12:133–47.
Nishie M, Mori F, Fujiwara H, Hasegawa M, Yoshimoto M, Iwatsubo T, et al. Accumulation of phosphorylated alpha-synuclein in the brain and peripheral ganglia of patients with multiple system atrophy. Acta Neuropathol. 2004;107:292–8.
Sone M, Yoshida M, Hashizume Y, Hishikawa N, Sobue G. alpha-Synuclein-immunoreactive structure formation is enhanced in sympathetic ganglia of patients with multiple system atrophy. Acta Neuropathol. 2005;110:19–26.
Orimo S, Kanazawa T, Nakamura A, Uchihara T, Mori F, Kakita A, et al. Degeneration of cardiac sympathetic nerve can occur in multiple system atrophy. Acta Neuropathol. 2007;113:81–6.
Iwasaki Y, Yoshida M, Hashizume Y, Hattori M, Aiba I, Sobue G. Widespread spinal cord involvement in progressive supranuclear palsy. Neuropathology. 2007;27:331–40.
Kikuchi H, Doh-ura K, Kira J, Iwaki T. Preferential neurodegeneration in the cervical spinal cord of progressive supranuclear palsy. Acta Neuropathol. 1999;97:577–84.
Wakabayashi K, Hayashi S, Morita T, Shibasaki Y, Watanabe Y, Takahashi H. Neurofibrillary tangles in the peripheral sympathetic ganglia of non-Alzheimer elderly individuals. Clin Neuropathol. 1999;18:171–5.
Orimo S, Amino T, Itoh Y, Takahashi A, Kojo T, Uchihara T, et al. Cardiac sympathetic denervation precedes neuronal loss in the sympathetic ganglia in Lewy body disease. Acta Neuropathol. 2005;109:583–8.
Goldstein DS, Chang PC, Eisenhofer G, Miletich R, Finn R, Bacher J, et al. Positron emission tomographic imaging of cardiac sympathetic innervation and function. Circulation. 1990;81:1606–21.
Wong KK, Raffel DM, Bohnen NI, Altinok G, Gilman S, Frey KA. 2-Year natural decline of cardiac sympathetic innervation in idiopathic Parkinson disease studied with 11C-hydroxyephedrine (HED) PET. J Nucl Med. 2016.
Orimo S, Yogo M, Nakamura T, Suzuki M, Watanabe H. Brain imaging in Aging Special Issue of Ageing Research Reviews I—meta-iodobenzylguanidine (MIBG) cardiac scintigraphy in alpha-synucleinopathies. Ageing Res Rev. 2016;30:122–33.
Sakakibara R, Tateno F, Kishi M, Tsuyusaki Y, Terada H, Inaoka T. MIBG myocardial scintigraphy in pre-motor Parkinson’s disease: a review. Parkinsonism Relat Disord. 2014;20:267–73.
Chung EJ, Kim SJ. (123)I-metaiodobenzylguanidine myocardial scintigraphy in lewy body-related disorders: a literature review. J Mov Disord. 2015;8:55–66. https://doi.org/10.14802/jmd.15015.
Goldstein DS, Holmes C, Kopin IJ, Sharabi Y. Intra-neuronal vesicular uptake of catecholamines is decreased in patients with Lewy body diseases. J Clin Invest. 2011;121:3320–30.
Orimo S, Ozawa E, Nakade S, Sugimoto T, Mizusawa H. (123)I-metaiodobenzylguanidine myocardial scintigraphy in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1999;67:189–94.
Yoshita M, Hayashi M, Hirai S. Decreased myocardial accumulation of 123I-meta-iodobenzyl guanidine in Parkinson’s disease. Nucl Med Commun. 1998;19:137–42.
Slaets S, Van Acker F, Versijpt J, Hauth L, Goeman J, Martin JJ, et al. Diagnostic value of MIBG cardiac scintigraphy for differential dementia diagnosis. Int J Geriatr Psychiatry. 2015;30:864–9.
Tateno F, Sakakibara R, Kishi M, Ogawa E, Terada H, Ogata T, et al. Sensitivity and specificity of metaiodobenzylguanidine (MIBG) myocardial accumulation in the diagnosis of Lewy body diseases in a movement disorder clinic. Parkinsonism Relat Disord. 2011;17:395–7.
Nagayama H, Hamamoto M, Ueda M, Nagashima J, Katayama Y. Reliability of MIBG myocardial scintigraphy in the diagnosis of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2005;76:249–51.
Kashihara K, Imamura T, Shinya T. Cardiac 123I-MIBG uptake is reduced more markedly in patients with REM sleep behavior disorder than in those with early stage Parkinson’s disease. Parkinsonism Relat Disord. 2010;16:252–5.
Goldstein DS, Holmes CS, Dendi R, Bruce SR, Li ST. Orthostatic hypotension from sympathetic denervation in Parkinson’s disease. Neurology. 2002;58:1247–55.
Kashihara K, Ohno M, Kawada S, Okumura Y. Reduced cardiac uptake and enhanced washout of 123I-MIBG in pure autonomic failure occurs conjointly with Parkinson’s disease and dementia with Lewy bodies. J Nucl Med. 2006;47:1099–101.
Goldstein DS, Holmes C, Sullivan P, Mash DC, Sidransky E, Stefani A, et al. Deficient vesicular storage: a common theme in catecholaminergic neurodegeneration. Parkinsonism Relat Disord. 2015;21:1013–22.
Kwon SH, Yoon JK, Yoon JH, Lee SJ, Jo KS, Lee DH, et al. The utility of segmental analysis in cardiac I-123 MIBG SPECT in Parkinson’s disease. Nucl Med Mol Imaging. 2015;49:298–302.
Oh JK, Choi EK, Song IU, Kim JS, Chung YA. Comparison of I-123 MIBG planar imaging and SPECT for the detection of decreased heart uptake in Parkinson disease. J Neural Transm (Vienna). 2015;122:1421–7.
Odagiri H, Baba T, Nishio Y, Iizuka O, Matsuda M, Inoue K, et al. On the utility of MIBG SPECT/CT in evaluating cardiac sympathetic dysfunction in Lewy body diseases. PLoS One. 2016;11:e0152746.
Saiki S, Hirose G, Sakai K, Kataoka S, Hori A, Saiki M, et al. Cardiac 123I-MIBG scintigraphy can assess the disease severity and phenotype of PD. J Neurol Sci. 2004;220:105–11.
Nomura T, Inoue Y, Hogl B, Uemura Y, Kitayama M, Abe T, et al. Relationship between (123)I-MIBG scintigrams and REM sleep behavior disorder in Parkinson’s disease. Parkinsonism Relat Disord. 2010;16:683–5.
Kim JS, Park HE, Park IS, Oh YS, Ryu DW, Song IU, et al. Normal ‘heart’ in Parkinson’s disease: is this a distinct clinical phenotype? Eur J Neurol. 2017;24:349–56.
Iranzo A, Tolosa E, Gelpi E, Molinuevo JL, Valldeoriola F, Serradell M, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol. 2013;12:443–53.
Miyamoto T, Miyamoto M, Inoue Y, Usui Y, Suzuki K, Hirata K. Reduced cardiac 123I-MIBG scintigraphy in idiopathic REM sleep behavior disorder. Neurology. 2006;67:2236–8.
Knudsen K, Fedorova TD, Hansen AK, Sommerauer M, Otto M, Svendsen KB, et al. In-vivo staging of pathology in REM sleep behaviour disorder: a multimodality imaging case-control study. Lancet Neurol. 2018;17:618–28. https://doi.org/10.1016/S1474-4422(18)30162-5.
Braune S, Reinhardt M, Schnitzer R, Riedel A, Lucking CH. Cardiac uptake of [123I]MIBG separates Parkinson’s disease from multiple system atrophy. Neurology. 1999;53:1020–5.
Reinhardt MJ, Jungling FD, Krause TM, Braune S. Scintigraphic differentiation between two forms of primary dysautonomia early after onset of autonomic dysfunction: value of cardiac and pulmonary iodine-123 MIBG uptake. Eur J Nucl Med. 2000;27:595–600.
Berganzo K, Tijero B, Somme JH, Llorens V, Sanchez-Manso JC, Low D, et al. SCOPA-AUT scale in different parkinsonisms and its correlation with (123) I-MIBG cardiac scintigraphy. Parkinsonism Relat Disord. 2012;18:45–8.
Yoshita M. Differentiation of idiopathic Parkinson’s disease from striatonigral degeneration and progressive supranuclear palsy using iodine-123 meta-iodobenzylguanidine myocardial scintigraphy. J Neurol Sci. 1998;155:60–7.
Treglia G, Cason E, Stefanelli A, Cocciolillo F, Di Giuda D, Fagioli G, et al. MIBG scintigraphy in differential diagnosis of parkinsonism: a meta-analysis. Clin Auton Res. 2012;22:43–55.
Orimo S, Suzuki M, Inaba A, Mizusawa H. 123I-MIBG myocardial scintigraphy for differentiating Parkinson’s disease from other neurodegenerative parkinsonism: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2012;18:494–500.
Jang W, Kim JS, Cho JW, Ahn JY, Choi YY, Kim HT. Thyroid MIBG uptake in Parkinson’s disease with diabetes mellitus. Clin Auton Res. 2013;23:221–4.
Merlet P, Pouillart F, Dubois-Rande JL, Delahaye N, Fumey R, Castaigne A, et al. Sympathetic nerve alterations assessed with 123I-MIBG in the failing human heart. J Nucl Med. 1999;40:224–31.
Jacobson AF, Travin MI. Impact of medications on mIBG uptake, with specific attention to the heart: comprehensive review of the literature. J Nucl Cardiol. 2015;22:980–93.
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211.
Gai WP, Blumbergs PC, Geffen LB, Blessing WW. Age-related loss of dorsal vagal neurons in Parkinson’s disease. Neurology. 1992;42:2106–11.
Eadie MJ. The pathology of certain medullary nuclei in parkinsonism. Brain. 1963;86:781–92.
Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396:67–72.
Greene JG. Causes and consequences of degeneration of the dorsal motor nucleus of the vagus nerve in Parkinson’s disease. Antioxid Redox Signal. 2014;21:649–67.
Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol. 1988;76:217–21.
Lebouvier T, Neunlist M, Bruley des Varannes S, Coron E, Drouard A, N'Guyen JM, et al. Colonic biopsies to assess the neuropathology of Parkinson’s disease and its relationship with symptoms. PLoS One. 2010;5:e12728.
Pouclet H, Lebouvier T, Coron E, Des Varannes SB, Neunlist M, Derkinderen P. A comparison between colonic submucosa and mucosa to detect Lewy pathology in Parkinson’s disease. Neurogastroenterol Motil. 2012;24:e202–5.
Del Tredici K, Braak H. Spinal cord lesions in sporadic Parkinson’s disease. Acta Neuropathol. 2012;124:643–64.
Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White III CL, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702.
Hopkins DA, Bieger D, de Vente J, Steinbusch WM. Vagal efferent projections: viscerotopy, neurochemistry and effects of vagotomy. Prog Brain Res. 1996;107:79–96.
Hawkes CH, Del Tredici K, Braak H. Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol. 2007;33:599–614.
Borghammer P. How does Parkinson’s disease begin? Perspectives on neuroanatomical pathways, prions, and histology. Mov Disord. 2018;33:48–57.
Svensson E, Horvath-Puho E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol. 2015;78:522–9.
Liu B, Fang F, Pedersen NL, Tillander A, Ludvigsson JF, Ekbom A, et al. Vagotomy and Parkinson’s disease risk: a Swedish register-based matched cohort study. Mov Disord. 2016;31:154.
Ito S, Takao M, Hatsuta H, Kanemaru K, Arai T, Saito Y, et al. Alpha-synuclein immunohistochemistry of gastrointestinal and biliary surgical specimens for diagnosis of Lewy body disease. Int J Clin Exp Pathol. 2014;7:1714–23.
Anlauf M, Schafer MK, Eiden L, Weihe E. Chemical coding of the human gastrointestinal nervous system: cholinergic, VIPergic, and catecholaminergic phenotypes. J Comp Neurol. 2003;459:90–111.
Giacobini E. Cholinesterases and cholinesterase inhibitors. London: Martin Dunitz Ltd; 2000.
Schmid W, van der Zypen E, Keller H. Die Wirkung einer subtotalen Vagotomie auf den Plexus myentericus (Auerbach) verschiedener Darmabschnitte. Acta Anat (Basel). 1979;104:36–51.
Pauza DH, Saburkina I, Rysevaite K, Inokaitis H, Jokubauskas M, Jalife J, et al. Neuroanatomy of the murine cardiac conduction system: a combined stereomicroscopic and fluorescence immunohistochemical study. Auton Neurosci. 2013;176:32–47.
Gjerloff T, Jakobsen S, Nahimi A, Munk OL, Bender D, Alstrup AK, et al. In vivo imaging of human acetylcholinesterase density in peripheral organs using 11C-donepezil: dosimetry, biodistribution, and kinetic analyses. J Nucl Med. 2014;55:1818–24.
Gjerloff T, Fedorova T, Knudsen K, Munk OL, Nahimi A, Jacobsen S, et al. Imaging acetylcholinesterase density in peripheral organs in Parkinson’s disease with 11C-donepezil PET. Brain. 2015;138:653–63.
Fedorova T, Seidelin LB, Knudsen K, Schacht AC, Geday J, Pavese N, et al. Decreased intestinal acetylcholinesterase in early Parkinson’s disease: an 11C-donepezil PET study. Neurology. 2017;88(8):775–81.
•• Knudsen K, Fedorova TD, Hansen AK, Sommerauer M, Otto M, Svendsen KB, et al. In-vivo staging of pathology in REM sleep behaviour disorder: a multimodality imaging case-control study. Lancet Neurol. 2018;17:618–28 Comprehensive multi-modality imaging study which demonstrated fully developed pathology in the autonomic nervous system but relatively intact nigrostriatal dopaminergic innervation in patients with idiopathic RBD.
Sharrad DF, de Vries E, Brookes SJ. Selective expression of alpha-synuclein-immunoreactivity in vesicular acetylcholine transporter-immunoreactive axons in the guinea pig rectum and human colon. J Comp Neurol. 2013;521:657–76.
Sharrad DF, Gai WP, Brookes SJ. Selective coexpression of synaptic proteins, alpha-synuclein, cysteine string protein-alpha, synaptophysin, synaptotagmin-1, and synaptobrevin-2 in vesicular acetylcholine transporter-immunoreactive axons in the guinea pig ileum. J Comp Neurol. 2013;521:2523–37.
• Tsukita K, Taguchi T, Sakamaki-Tsukita H, Tanaka K, Suenaga T. The vagus nerve becomes smaller in patients with Parkinson’s disease: a preliminary cross-sectional study using ultrasonography. Parkinsonism Relat Disord. 2018. https://doi.org/10.1016/j.parkreldis.2018.06.002 The first study to demonstrate reduced vagal nerve diameter in PD patients using ultrasonography.
Keren NI, Taheri S, Vazey EM, Morgan PS, Granholm AC, Aston-Jones GS, et al. Histologic validation of locus coeruleus MRI contrast in post-mortem tissue. NeuroImage. 2015;113:235–45.
• Leclair-Visonneau L, Clairembault T, Coron E, Le Dily S, Vavasseur F, Dalichampt M, et al. REM sleep behavior disorder is related to enteric neuropathology in Parkinson disease. Neurology. 2017;89:1612–8 This study showed that RBD-positive PD patients exhibit significantly more pathological alpha-synuclein pathology in the intestine compared to RBD-negative cases.
Mu L, Sobotka S, Chen J, Su H, Sanders I, Adler CH, et al. Altered pharyngeal muscles in Parkinson disease. J Neuropathol Exp Neurol. 2012;71:520–30.
Cannon WB. Oesophageal peristalsis after bilateral vagotomy. Am J Phys. 1907;19:436–44.
Fasano A, Visanji NP, Liu LW, Lang AE, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015;14:625–39.
Miller N, Allcock L, Hildreth AJ, Jones D, Noble E, Burn DJ. Swallowing problems in Parkinson disease: frequency and clinical correlates. J Neurol Neurosurg Psychiatry. 2009;80:1047–9.
Kalf JG, de Swart BJ, Bloem BR, Munneke M. Prevalence of oropharyngeal dysphagia in Parkinson’s disease: a meta-analysis. Parkinsonism Relat Disord. 2012;18:311–5.
Lin CW, Chang YC, Chen WS, Chang K, Chang HY, Wang TG. Prolonged swallowing time in dysphagic parkinsonism patients with aspiration pneumonia. Arch Phys Med Rehabil. 2012;93:2080–4.
Logemann J. Measurements of swallow from videofluoroscopic studies, 2 edn. Texas Pro-ed. 1993.
Leopold NA, Kagel MC. Pharyngo-esophageal dysphagia in Parkinson’s disease. Dysphagia. 1997;12:11–8 discussion 9-20.
Bushmann M, Dobmeyer SM, Leeker L, Perlmutter JS. Swallowing abnormalities and their response to treatment in Parkinson’s disease. Neurology. 1989;39:1309–14.
Ellerston JK, Heller AC, Houtz DR, Kendall KA. Quantitative measures of swallowing deficits in patients with Parkinson’s disease. Ann Otol Rhinol Laryngol. 2016;125:385–92.
Suttrup I, Suttrup J, Suntrup-Krueger S, Siemer ML, Bauer J, Hamacher C, et al. Esophageal dysfunction in different stages of Parkinson’s disease. Neurogastroenterol Motil. 2016:1–7.
Sung HY, Kim JS, Lee KS, Kim YI, Song IU, Chung SW, et al. The prevalence and patterns of pharyngoesophageal dysmotility in patients with early stage Parkinson’s disease. Mov Disord. 2010;25:2361–8.
Johnston BT, Castell JA, Stumacher S, Colcher A, Gideon RM, Li Q, et al. Comparison of swallowing function in Parkinson’s disease and progressive supranuclear palsy. Mov Disord. 1997;12:322–7.
Tomita S, Oeda T, Umemura A, Kohsaka M, Park K, Yamamoto K, et al. Video-fluoroscopic swallowing study scale for predicting aspiration pneumonia in Parkinson’s disease. PLoS One. 2018;13:e0197608.
Potulska A, Friedman A, Krolicki L, Spychala A. Swallowing disorders in Parkinson’s disease. Parkinsonism Relat Disord. 2003;9:349–53.
Grunho M, Sonies B, Frattali CM, Litvan I. Swallowing disturbances in the corticobasal syndrome. Parkinsonism Relat Disord. 2015;21:1342–8.
Lee HH, Seo HG, Kim KD, Lee SH, Lee WH, Oh BM, et al. Characteristics of early oropharyngeal dysphagia in patients with multiple system atrophy. Neurodegener Dis. 2018;18:84–90.
Litvan I, Sastry N, Sonies BC. Characterizing swallowing abnormalities in progressive supranuclear palsy. Neurology. 1997;48:1654–62.
Taniguchi H, Nakayama H, Hori K, Nishizawa M, Inoue M, Shimohata T. Esophageal involvement in multiple system atrophy. Dysphagia. 2015;30:669–73.
Marrinan S, Emmanuel AV, Burn DJ. Delayed gastric emptying in Parkinson’s disease. Mov Disord. 2014;29:23–32.
Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–43.
Verbaan D, Marinus J, Visser M, van Rooden SM, Stiggelbout AM, van Hilten JJ. Patient-reported autonomic symptoms in Parkinson disease. Neurology. 2007;69:333–41.
Donohoe KJ, Maurer AH, Ziessman HA, Urbain JL, Royal HD, Martin-Comin J, et al. Procedure guideline for adult solid-meal gastric-emptying study 3.0. J Nucl Med Technol. 2009;37:196–200.
Knudsen K, Szwebs M, Hansen AK, Borghammer P. Gastric emptying in Parkinson’s disease—a mini-review. Parkinsonism Relat Disord. 2018. https://doi.org/10.1016/j.parkreldis.2018.06.003.
Evans MA, Broe GA, Triggs EJ, Cheung M, Creasey H, Paull PD. Gastric emptying rate and the systemic availability of levodopa in the elderly parkinsonian patient. Neurology. 1981;31:1288–94.
Djaldetti R, Baron J, Ziv I, Melamed E. Gastric emptying in Parkinson’s disease: patients with and without response fluctuations. Neurology. 1996;46:1051–4.
Hardoff R, Sula M, Tamir A, Soil A, Front A, Badarna S, et al. Gastric emptying time and gastric motility in patients with Parkinson’s disease. Mov Disord. 2001;16:1041–7.
Krygowska-Wajs A, Cheshire WP Jr, Wszolek ZK, Hubalewska-Dydejczyk A, Jasinska-Myga B, Farrer MJ, et al. Evaluation of gastric emptying in familial and sporadic Parkinson disease. Parkinsonism Relat Disord. 2009;15:692–6.
Tanaka Y, Kato T, Nishida H, Yamada M, Koumura A, Sakurai T, et al. Is there a delayed gastric emptying of patients with early-stage, untreated Parkinson’s disease? An analysis using the 13C-acetate breath test. J Neurol. 2011;258:421–6.
Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, et al. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One. 2011;6:e28032.
Davies KN, King D, Billington D, Barrett JA. Intestinal permeability and orocaecal transit time in elderly patients with Parkinson’s disease. Postgrad Med J. 1996;72:164–7.
Knudsen K, Haase AM, Fedorova TD, Bekker AC, Ostergaard K, Krogh K, et al. Gastrointestinal transit time in Parkinson’s disease using a magnetic tracking system. J Parkinsons Dis. 2017;7:471–9.
Unger MM, Hattemer K, Moller JC, Schmittinger K, Mankel K, Eggert K, et al. Real-time visualization of altered gastric motility by magnetic resonance imaging in patients with Parkinson’s disease. Mov Disord. 2010;25:623–8.
Tanaka Y, Kato T, Nishida H, Yamada M, Koumura A, Sakurai T, et al. Is there delayed gastric emptying in patients with multiple system atrophy? An analysis using the (13)C-acetate breath test. J Neurol. 2012;259:1448–52.
Thomaides T, Karapanayiotides T, Zoukos Y, Haeropoulos C, Kerezoudi E, Demacopoulos N, et al. Gastric emptying after semi-solid food in multiple system atrophy and Parkinson disease. J Neurol. 2005;252:1055–9.
• Knudsen K, Fedorova TD, Bekker AC, Iversen P, Ostergaard K, Krogh K, et al. Objective colonic dysfunction is far more prevalent than subjective constipation in Parkinson’s disease: a colon transit and volume study. J Parkinsons Dis. 2017;7:359–67 This study demonstrated that objective, imaging-based markers of colonic dysfunction is considerably more prevalent than the subjective constipation symptoms experienced by the patients.
Dutkiewicz J, Szlufik S, Nieciecki M, Charzynska I, Krolicki L, Smektala P, et al. Small intestine dysfunction in Parkinson’s disease. J Neural Transm (Vienna). 2015;122:1659–61.
Kimura Y, Kamada Y, Kimura S. A patient with numerous tablets remaining in the stomach even 5 hours after ingestion. Am J Emerg Med. 2008;26:118.e1–2.
Svensson E, Henderson VW, Borghammer P, Horvath-Puho E, Sorensen HT. Constipation and risk of Parkinson’s disease: a Danish population-based cohort study. Parkinsonism Relat Disord. 2016;28:18–22.
Iranzo A, Stefani A, Serradell M, Marti MJ, Lomena F, Mahlknecht P, et al. Characterization of patients with longstanding idiopathic REM sleep behavior disorder. Neurology. 2017;89:242–8.
Del Tredici K, Braak H. Lewy pathology and neurodegeneration in premotor Parkinson’s disease. Mov Disord. 2012;27:597–607.
• Schwiertz A, Spiegel J, Dillmann U, Grundmann D, Burmann J, Fassbender K, et al. Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Parkinsonism Relat Disord. 2018;50:104–7 This recent study analyzed blood and stool samples and demonstrated increased intestinal inflammation and permeability in PD.
Mertsalmi TH, Aho VTE, Pereira PAB, Paulin L, Pekkonen E, Auvinen P, et al. More than constipation—bowel symptoms in Parkinson’s disease and their connection to gut microbiota. Eur J Neurol. 2017;24:1375–83.
Knudsen K, Krogh K, Ostergaard K, Borghammer P. Constipation in Parkinson’s disease: subjective symptoms, objective markers, and new perspectives. Mov Disord. 2017;32:94–105.
Abrahamsson H, Antov S, Bosaeus I. Gastrointestinal and colonic segmental transit time evaluated by a single abdominal X-ray in healthy subjects and constipated patients. Scand J Gastroenterol Suppl. 1988;152:72–80.
Jost WH, Schimrigk K. Cisapride treatment of constipation in Parkinson’s disease. Mov Disord. 1993;8:339–43.
Jost WH, Schrank B. Defecatory disorders in de novo parkinsonians—colonic transit and electromyogram of the external anal sphincter. Wien Klin Wochenschr. 1998;110:535–7.
Tateno F, Sakakibara R, Yokoi Y, Kishi M, Ogawa E, Uchiyama T, et al. Levodopa ameliorated anorectal constipation in de novo Parkinson’s disease: the QL-GAT study. Parkinsonism Relat Disord. 2011;17:662–6.
Ashraf W, Pfeiffer RF, Park F, Lof J, Quigley EM. Constipation in Parkinson’s disease: objective assessment and response to psyllium. Mov Disord. 1997;12:946–51.
Steele SR, Mellgren A. Constipation and obstructed defecation. Clin Colon Rectal Surg. 2007;20:110–7.
Damian A, Adler CH, Hentz JG, Shill HA, Caviness JN, Sabbagh MN, et al. Autonomic function, as self-reported on the SCOPA-autonomic questionnaire, is normal in essential tremor but not in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18:1089–93.
Sakakibara R, Odaka T, Uchiyama T, Asahina M, Yamaguchi K, Yamaguchi T, et al. Colonic transit time and rectoanal videomanometry in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2003;74:268–72.
Mathers SE, Kempster PA, Swash M, Lees AJ. Constipation and paradoxical puborectalis contraction in anismus and Parkinson’s disease: a dystonic phenomenon? J Neurol Neurosurg Psychiatry. 1988;51:1503–7.
Visser M, Marinus J, Stiggelbout AM, Van Hilten JJ. Assessment of autonomic dysfunction in Parkinson’s disease: the SCOPA-AUT. Mov Disord. 2004;19:1306–12.
Wang CP, Sung WH, Wang CC, Tsai PY. Early recognition of pelvic floor dyssynergia and colorectal assessment in Parkinson’s disease associated with bowel dysfunction. Color Dis. 2013;15:e130–7.
Nilsson M, Sandberg TH, Poulsen JL, Gram M, Frokjaer JB, Ostergaard LR, et al. Quantification and variability in colonic volume with a novel magnetic resonance imaging method. Neurogastroenterol Motil. 2015;27:1755–63.
Stocchi F, Badiali D, Vacca L, D'Alba L, Bracci F, Ruggieri S, et al. Anorectal function in multiple system atrophy and Parkinson’s disease. Mov Disord. 2000;15:71–6.
Zhang L, Cao B, Ou R, Wei QQ, Zhao B, Yang J, et al. Non-motor symptoms and the quality of life in multiple system atrophy with different subtypes. Parkinsonism Relat Disord. 2017;35:63–8.
Sakakibara R, Odaka T, Uchiyama T, Liu R, Asahina M, Yamaguchi K, et al. Colonic transit time, sphincter EMG, and rectoanal videomanometry in multiple system atrophy. Mov Disord. 2004;19:924–9.
Edwards LL, Quigley EM, Harned RK, Hofman R, Pfeiffer RF. Characterization of swallowing and defecation in Parkinson’s disease. Am J Gastroenterol. 1994;89:15–25.
Chiu CM, Wang CP, Sung WH, Huang SF, Chiang SC, Tsai PY. Functional magnetic stimulation in constipation associated with Parkinson’s disease. J Rehabil Med. 2009;41:1085–9.
Cadeddu F, Bentivoglio AR, Brandara F, Marniga G, Brisinda G, Maria G. Outlet type constipation in Parkinson’s disease: results of botulinum toxin treatment. Aliment Pharmacol Ther. 2005;22:997–1003.
Libelius R, Johansson F. Quantitative electromyography of the external anal sphincter in Parkinson’s disease and multiple system atrophy. Muscle Nerve. 2000;23:1250–6.
Vodusek DB. Sphincter EMG and differential diagnosis of multiple system atrophy. Mov Disord. 2001;16:600–7.
Funding
The work was financially supported by a grant from the Lundbeck Foundation.
Author information
Authors and Affiliations
Contributions
Both authors participated in writing the first draft and edited and approved the final version.
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Borghammer has received consultancies from F. Hoffmann – La Roche and grant support from the Lundbeck and Jascha Foundations. Dr. Knudsen has nothing to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Neuroimaging
Rights and permissions
About this article
Cite this article
Knudsen, K., Borghammer, P. Imaging the Autonomic Nervous System in Parkinson’s Disease. Curr Neurol Neurosci Rep 18, 79 (2018). https://doi.org/10.1007/s11910-018-0889-4
Published:
DOI: https://doi.org/10.1007/s11910-018-0889-4