Abstract
Background
Posterior lumbar interbody fusion (PLIF) surgery represents an effective option to treat degenerative conditions in the lumbar spine. To reduce the drawbacks of the classical technique, we developed a variant, so-called Lateral-PLIF, which we then evaluated through a prospective consecutive series of patients.
Methods
All adult patients treated at our institute with single or double level Lateral-PLIF for lumbar degenerative disease from January to December 2017 were prospectively collected. Exclusion criteria were patients < 18 years of age, traumatic patients, active infection, or malignancy, as well as unavailability of clinical and/or radiological follow-up data. The technique consists of insert the cages bilaterally through the transition zone between the central canal and the intervertebral foramen, just above the lateral recess. Pre- and postoperative (2 years) questionnaires and phone interviews (4 years) assessed pain and functional outcomes. Data related to the surgical procedure, postoperative complications, and radiological findings (1 year) were collected.
Results
One hundred four patients were selected for the final analysis. The median age was 58 years and primary symptoms were mechanical back pain (100, 96.1%) and/or radicular pain (73, 70.2%). We found a high fusion rate (95%). A statistically significant improvement in functional outcome was also noted (ODI p < 0.001, Roland-Morris score p < 0.001). Walking distance increased from 812 m ± 543 m to 3443 m ± 712 m (p < 0.001). Complications included dural tear (6.7%), infection/wound dehiscence (4.8%), and instrument failure (1.9%) but no neurological deterioration.
Conclusions
Lateral-PLIF is a safe and effective technique for lumbar interbody fusion and may be considered for further comparative study validation with other techniques before extensive use to treat lumbar degenerative disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lumbar interbody fusion surgery represents an effective treatment option for a wide range of spinal disorders, including degenerative conditions, isthmic spondylolisthesis, and for revision surgery [19, 43]. The principle is to achieve stable arthrodesis of spinal segments while restoring disk height, segmental lordosis, and load-bearing to anterior structures [53]. Three main approaches have been used, these being anterior (i.e., anterior lumbar interbody fusion, ALIF), lateral (i.e., extreme lateral interbody fusion, XLIF or oblique lumbar interbody fusion, OLIF), and posterior (i.e., posterior or transforaminal interbody fusion, PLIF and TLIF, respectively) [36, 42]. The choice of technique depends on patient characteristics, the specific spinal disease, vertebral level, and the experience and preference of the surgeon [42]. A combination of posterior and antero-lateral techniques (combined approach) has also been advocated in specific cases [4].
Among the posterior approaches, traditional PLIF was frequently used in the past, with acceptable fusion rate and achieves good decompression of neurological structures, especially for discogenic/facetogenic low back pain, foraminal stenosis, recurrent disk herniation, and symptomatic spondylolisthesis [6, 7, 10, 12, 30, 37, 54, 57]. This technique is also biomechanically effective as it provides optimal stabilization of the instrumented spinal segment [5, 59]. However, this technique has been associated with significant dural/neurological complications, in up to 20% of patients, motivating surgeons to move to safer techniques, such as TLIF or XLIF, with less requirement for nerve root manipulation [14, 18, 27, 46].
The transforaminal unilateral approach (TLIF) was developed at the end of the 1990s with the aim of reducing approach-related complications [22]. The principle in TLIF is to use one cage which is inserted via the intervertebral foramen that can be performed as an open or minimally invasive open technique (MIS-TLIF). This reduces muscle injury, dural tears, and nerve retraction. Conversely, it includes unilateral facetectomy and direct foraminal decompression, so it can be used only in selected cases [58].
Taking into account the pros and cons of both techniques (TLIF and PLIF), we developed a modified posterior approach, termed “Lateral-PLIF,” with the aim of reducing their respective technical limitations and difficulties while providing direct posterior and foraminal decompression, as well as achieving good biomechanical stability and fusion. The purpose of the present study was to analyze the efficacy and safety of this technique in a consecutive prospective series of patients who were treated for a wide variety of degenerative diseases of the lumbar spine.
Material and methods
Study design and patient selection
This was a consecutive prospective cohort study performed in a single academic institution. Patients who underwent a single/double level “Lateral-PLIF,” between January and December 2017, for the primary diagnosis of isthmic or degenerative spondylolisthesis, lumbar spinal stenosis (instable), severe degenerative disk disease, or recurrent disk herniation, were selected for the study. Patients with lumbar stenosis were selected for this approach only if they complained of lumbar pain correlated with obvious signs of instability, such as synovial cysts and dislocation > 5 mm on flexion–extension X-ray. Disk herniations were selected for this approach only in case of third recurrence. The indication for surgery was decided by an expert spine surgeon (more than 10 years of experience) in patients who had not responded to conservative treatment modalities. Patients with a history of previous lumbar decompression, microdiscectomy, and single or multilevel instrumentation were included. Exclusion criteria were patients < 18 years of age and patients with trauma, active infection, or malignancy, as well as unavailability of clinical and/or radiological follow-up data.
Clinical examinations, radiologic studies, hospital charts, and office records were all collected. Telephone interviews were carried out to supplement data in the chart.
This study was written in accordance with the PROCEES (Preferred Reporting of Case Series in Surgery) guidelines for surgical series [1].
The study obtained ethics approval (N°21–312) and patients provided informed consent to participate in the study.
Lateral-PLIF—technical note: step-by-step for the procedure (single level)
-
1.
The patient was placed on a suitable spine frame in the prone position.
-
2.
Using a standard midline incision, careful subperiosteal exposure of the posterior bony elements was carried out (i.e., lamina of the 2 adjacent vertebras, posterior facets and pedicular entry zones).
-
3.
Sub-total facetectomy with bilateral resection of the inferior facet of the upper vertebra using an osteotome, and partial removal of the superior facet of inferior vertebra making it flat for insertion of facilizing pedicle screws.
-
4.
Multiaxial pedicle screws were then inserted at the appropriate levels, and correct placement confirmed with biplanar fluoroscopy or CT-guided navigation.
-
5.
An inter-laminar distractor may be applied at this stage, at the base of the spinous processes, to optimize exposure and enlarge the working space.
-
6.
Midline ligamentous structures were generally resected but the adjacent spinous processes kept intact. The tip and the medial part of the superior facet were then resected sufficiently to expose the proximal part of the intervertebral foramen (Fig. 1), i.e., the transition zone between the central canal and the lateral canal, just above the lateral recess (Figs. 1 and 2). When necessary, the lateral recess was opened more using a Kerrison rongeur to decompress the passing nerve root. If additional central decompression was required, partial laminectomy and flavectomy could be performed.
-
7.
Exposure of the underlying disk space was facilitated by the removal of the lateral ligamentum flavum and of underlying fatty tissue, while preserving fatty tissue surrounding the nerve root. Epidural bleeding was frequently encountered at this point. Once hemostasis was achieved, the disk was exposed between the dural sac medially and the foraminal root laterally, just above the lateral recess, with no or only minimal retraction of the neurological structures. A scalpel blade was then used to create a rectangular window in the annulus in the transitional zone (Fig. 3), corresponding to the lateral part of the central canal and the medial part of the IV foramen. This is a more medial cage insertion trajectory than TLIF, which avoids the risk of foraminal root injury. At the same time, the trajectory is lateral enough to avoid greater retraction on the dural sac as in standard PLIF. A nerve root retractor placed around the dural sac was used to protect that structure, but only limited retraction was typically necessary (Fig. 4C).
-
8.
Specialized straight and angled osteotomes, pituitary rongeurs, rasps, and curettes were used to elevate and remove disk material. Intervertebral distraction (Figs. 2 and 4C) was applied on one side, starting at 6/7 mm and increasing up to 11/12 mm, so that discectomy can be commenced easily and safely on the contralateral side, very gradually. The distraction was performed progressively, each 1 mm alternating from one side to the other. Endplate preparation was completed from each side of the dural sac.
-
9.
The disk space was sized for an appropriate interbody cage (most frequently 11/12 mm height, 8/10° lordosis, and 20/25 mm length cages were inserted). The anterior aspect of the disk space and two cages were both packed with bone graft. This could involve the use of local bone or bone substitutes, depending on the specific clinical situation.
-
10.
The two cages were inserted into the interbody space and pushed forwards using a straight impactor (Figs. 4C and 5).
-
11.
After cage placement, the rods were then contoured in slight lordosis and placed inside the screw heads with the application of segmental compression to optimize restoration of local lordosis.
-
12.
The dura was protected with a collagen sponge and additional bone graft then placed along the rods and on the decorticated lamina.
-
13.
Planar fluoroscopy (or intraoperative CT scan) was used to confirm the correct placement of the cages, pedicle screws, and rods.
-
14.
Closure was undertaken in a standard fashion
Transitional zone (red square). The drawing shows the different zones to perform the discectomy and to place the cage, according to the surgical techniques. Blue square corresponds to the area of standard PLIF technique. Green square to the TLIF technique. Red square to the “Lateral-PLIF”: it is the safe transition zone between the central canal and the proximal part of IV foramen
Case example. A Preoperative sagittal T2 MRI shows degenerative spondylolisthesis L4-L5 of a patient who suffers for lumbar and radicular pain. B Lateral spine radiograph confirms spinal instability with worse listhesis at stand-up position. C Intraoperative image after screw and left cage inserted. Intervertebral distraction is applied at the right side. Mild retraction of dural sac (indicated by ★) is performed on the left side to show the cage. D Immediate sagittal CT scan shows the final result: the cage inserted, reduction of listhesis and supralaminar morselized bone autograft. ▲ left L4 nerve root; ★ dural sac
Postoperative recovery did not differ substantially from other standard fusion procedures. Mobilization was usually undertaken over the first few days, and fusion healing was expected in a 6-month to 1-year time frame.
Surgical data
Operative findings including operative time, blood loss and intraoperative complications (for example dural tear or nerve injury), details of the implants (cage height, length, and lordosis), and number of levels were also collected.
Clinical evaluation
Pre- and postoperative clinical examinations, comorbidities, and previous surgeries were recorded. The follow-up review consisted of office visits at 3 months, 1 year and as long as 2 years post-surgery, and a phone interview at 4 years.
Patients were asked if they would choose the surgery again, based on the degree of perceived improvement or deterioration in pain and function compared to their preoperative status.
Patients were also asked to complete pre- and postoperative questionnaires at 4 months and 2 years, assessing pain (medication use) and ability to perform activities of daily living (ADLs), including lifting, walking, standing, sitting, work status, and social activities.
The questionnaires were based on the Oswestry Disability Index (ODI), visual analog scale (VAS), and Roland-Morris. The ODI was used to categorize patients into 3 classes of disability: class I from 0 to 20, class II from 21 to 40, and class III from 41 to 100, reflecting minimal, moderate, and severe disability, respectively.
Postoperative data included early and long-term complication rate, early (30 day) and follow-up (2 years) readmission and reoperation rate. Early complications were defined as wound dehiscence, deep tissue infection, hematoma, and implant malposition, all requiring revision surgery in the first 30 days; and pneumonia, urinary tract infection or retention, deep venous thrombosis (DVT), and anemia requiring transfusion. Long-term complications include permanent motor deficit, instrument failure, pseudarthrosis, and adjacent segment disease (ASD).
Radiological evaluation
Follow-up visits included clinical assessment and imaging evaluation, looking for mechanical stability and fusion. Successful fusion was assessed on the postop CT scan at 1 year and was defined as the presence of bridging trabecular bone between the fused vertebrae, and the absence of any radiolucent zones spanning > 50% of the implant-vertebral interface. Two independent neurosurgeons assessed the image data. In the event of disagreement concerning fusion healing, a third independent assessment was obtained.
The preoperative and postoperative lumbar and local lordosis (see below) were measured on standing lateral radiograph by an experienced spine surgeon (CB) using an image diagnostic program (Centricity™, GE Healthcare) and compared between each group. Lumbar lordosis was measured using the Cobb method, between the upper endplate of L1 and the upper endplate of S1.
Local lordosis was measured between two upper end plates of two adjacent vertebrae (Fig. 6). For double level fusion (L4-S1), the local lordosis was measured between upper L4-S1 endplates. The values for local lordosis were divided into 3 groups: ≤ 5°, between 6° and 10°, and > 10°.
Disk space height was measured and compared on lateral radiographs using the method outlined by Goldstein et al. [21].
Statistical analysis
Continuous variables were calculated as mean and standard deviation (SD); categorical variables provided as numbers and frequencies. Categorical variables were compared using chi-square or Fisher’s exact test analysis, while continuous variables were compared using Mann–Whitney U test and independent sample t-test. Pearson’s correlation test was used for the evaluation of correlations between continuous variables. A two-tailed α level of 0.05 was used for statistical testing. All statistical analyses were performed using SPSS v20, IBM.
Results
General data
A total of 133 consecutive patients underwent Lateral-PLIF approach in 2017 at our institute. Among them, 25 patients did not meet inclusion criteria and 4 presented with an incomplete clinical and/or radiological follow-up. Finally, 104 patients were selected for the final analysis. Mean age was 57.8 ± 10.5 years, with a slight majority of males (54 patients, 51.9%).
In terms of significant surgical antecedents, 22 (21.15%) patients had been already operated for a spinal pathology and 5 (4.81%) had already undergone fusion surgery.
Table 1 summarizes patient baseline characteristics, surgical indications, and comorbidities.
Surgical procedures
Eighty-eight (84.6%) patients underwent single level fusion, while the remaining 16 (15.4%) had double level fusion (see Table 2). The mean operative time (OT) was 165.6 ± 47.5 min for single level and 225.1 ± 56.4 min for double level surgery. The average estimated blood loss for single level surgery was 551.6 ± 405.9 ml, and 857.5 ± 447.1 ml for double level surgery. No significant correlation was observed between blood loss (p = 0.108), OT (p = 0.292), and complications.
Clinical findings
Preoperative clinical symptoms are presented in Table 1. The main preoperative symptoms were lower back pain (100 patients, 96.1%) and radicular pain (73, 70.2%). Twenty-three (23/104, 22%) patients had motor deficit, which ranged between 2 and 4 of the MRC (Medical Research Council) scale, and 33/104 presented sensory deficit (31.7%).
At follow-up, a consistent reduction in pain symptomatology was reported. Specifically, 58/100 (58%) and 60/73 (82%) presented a major improvement in back and radicular pain, respectively. Fourteen (14/23, 60.8%) patients with a motor deficit recovered completely, 7/23 showed improvement, and 2/23 remained stable. Sensory deficit also improved in 22 of 33 patients at follow-up (31.7% vs 10.6%), and 2/5 patients had reduced sphincter dysfunction at follow-up (4.8% vs 1.9%).
At the 2-year ambulatory visit, a total of 88 (84.6%) patients reported satisfaction with the improvement after surgery and the remaining 16 (15.4%) did not report a change in their condition. At the 4-year phone interview, satisfaction with surgery was confirmed in 80% of patients. No patients reported a worsening of their condition. Only one patient had undergone spinal cord stimulation implant surgery to treat persistent neuropathic pain.
Complications
Seven early complications (see Table 2) required revision surgery: 5 (4.81%) cases of infection/wound dehiscence, 2 (1.92%) for malposition of pedicle screws, and 1 (0.96%) iatrogenic arterial injury during discectomy (endovascular repair). Nine minor complications were treated conservatively: 7 (6.73%) cases of dural tear and 1 (0.96%) deep hematoma not associated with neurological worsening.
During follow-up, we found asymptomatic screw failure in 2 patients (1.92%), and a total of three patients (2.9%) underwent revision surgery with proximal extension of fusion (one for instrument failure and the other 2 due to ASD).
No cauda equina syndrome, malposition or migration of cage, LCR leak, or DVTs were observed. No new motor deficit was reported.
No significant correlation was observed between comorbidities (p = 0.235), previous surgery (p = 0.092), and complications.
Functional outcomes
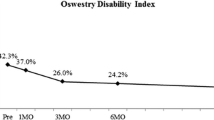
Preoperative VAS showed a mean value of 7.7 ± 1.3 [5–10], ODI was 49.4 ± 12.5 [22–82], and Roland-Morris score had a mean value of 14.9 ± 4.8 [3–24]. At the latest follow-up, the mean value for VAS was 3.2 ± 2.6 [0–8] and ODI was 29.2 ± 17.1 [4–62], while the Roland-Morris score was 8.4 ± 6.4 [0–19].As reported in Table 3, all disability index values showed a statistically significant reduction at 3- and 12-month follow-up compared to preoperative evaluations.
Regarding the 3 classes of ODI score specified above (see methods), we found a significant decrease in the number of patients with severe disability, or class III, at 12-month follow-up (79.4% vs 20.9%, p < 0.0001). Consequently, patient numbers in class I (0 vs 33.3%) and class II (20.6 vs 45.8) increased. The changes in the different ODI classes are shown in Table 3.
Walking distance was also evaluated. Distance (reported as time) by patient was converted to meters using healthy walking speed [22]. In the preoperative evaluation, patients reported being able to walk a mean distance of 812 m ± 891 m whereas this increased to a mean of 3443 m ± 712 m at the last postoperative consultation. This improvement in walking distance was statistically significant (p < 0.001).
Radiological outcome
The radiological outcomes for the cohort are shown in Table 4. Postoperative CT scans at 1 year confirmed radiographic fusion in 95% of patients. No subsidence or sinking of the interbody cages into the vertebral bodies was observed.
The mean postoperative improvement in local lordosis was 4.2° ± 1.5°, overall. The values for local lordosis were divided into 3 groups according to the angle: ≤ 5°, 6°–10°, and > 10°. Despite the group with local lordosis > 10° being the largest at the preoperative stage, there was a significant increase after surgery (p = 0.029). Regarding the specific level, the correction of local lordosis was statistically significant at the L4-L5 level (9.9° ± 4.9° vs 13.0° ± 4.1°; p = 0.016), but not at the L3-L4 level (7° ± 3.5° vs 11.3° ± 4.0°).
An increase in lumbar lordosis was observed but was not significantly different (51° ± 12.9° vs 52.3° ± 12.0°).
Lastly, the average increase in disk height after surgery was 9 mm, showing a significant difference (p = 0.0001) compared with preoperative disk height.
Discussion
This study introduces a more lateral variation of the standard PLIF technique, which we term “Lateral-PLIF,” representing a modern way of performing the PLIF procedure. Our results are based on a large prospective series of 104 patients, who were selected for baseline characteristics and indications, in a tertiary spine referral center over a 1-year period (2017). Long-term follow-up (2 and 4 years) ensured thorough evaluation of clinical outcomes.
According to our findings, the “Lateral-PLIF” technique appeared to be a safe and effective technique for posterior interbody arthrodesis for the treatment of a wide range of degenerative diseases of the lumbar spine.
We observed an improvement in lumbar and radicular symptoms in most patients as well as a major improvement in motor and sensory function in the sub-set of patients with preoperative deficits. The significant improvement of functional/pain scores at follow-up, consistently repeated, showed the reliability of this technique. Walking performances in patients improved significantly, as well. In fact, the mean VAS score dropped from 7.7 ± 1.3 preoperatively to 3.2 ± 2.6 at last follow-up. Moreover, we found a significant reduction in ODI and Roland-Morris scores at both early (3 months) and long-term (2 and 4 year) follow-up. Regarding the 3 categories of ODI scores, we saw a significant decrease in the number of patients with severe or class III disability at 12-month follow-up (79.4% vs 20.9%).
Additionally, radiological outcomes were encouraging, with a mean improvement in regional lordosis of 4.2° ± 1.5°, especially at the L4-L5 levels.
The observed fusion rate appeared to be optimal (i.e., 95% at 1-year imaging follow-up) and consistent with previous results for short segment fixation surgery [26, 42, 59, 60]. This measure has been shown to have a good correlation with long-term outcomes in several studies [26].
In this study, good correction of local lordosis, similar to or better than previously reported series [34, 45], was achieved, although the majority of patients presented preoperatively with > 10° local lordosis. A statistically significant and effective increase in disk height was also observed. Though lumbar lordosis did not show major improvement postoperatively, this is a common finding after the limited posterior interbody fusion approach and thus it represents one of most important advances of anterior (ALIF) and lateral (OLIF/XILIF) techniques, which, on the contrary, offer the possibility of limited undirected decompression when used alone [5, 16, 34, 36, 42].
The rate of complications was low, with only one iatrogenic arterial injury during discectomy (< 1%) which was amenable to endovascular repair. Compared to previous series which analyzed other posterior techniques, a lower rate of durotomy and nerve dysfunction was found in our study [8, 21, 27]. Other complications were minor and had no appreciable effect on surgical outcomes. This was also reflected by patient satisfaction with surgery, which was confirmed in 80% of patients at last follow-up, and is comparable to other fusion techniques [3, 14, 33, 38]. Estimated operative time and blood loss were also similar to standard PLIF series [7, 21, 37, 38].
Pros and cons of Lateral-PLIF
From the initial description provided by Cloward [12] in 1940, the PLIF technique has evolved with advances in methods of spinal segmental fusion [10, 11, 20, 29, 48, 49]. The main advantage of traditional PLIF is the bilateral approach, which offers the opportunity to achieve optimal cleaning of the disk space and permits the placement of two cages into the disk space. To date, the main concern with this approach is the extent of neural retraction required, with potential nerve root injury, dural tear, and epidural fibrosis leading to chronic radiculopathy [13, 17, 25, 44, 53, 60]. The direct posterior surgical access to the disk space spares the facet joints but requires, however, significant retraction of the thecal sac from each side, and this is associated with up to 20% of the complications reported in the literature [32, 35, 47]. Moreover, PLIF is neither effective nor safer when repeat surgeries are performed, and when the spinal canal is already extensively opened by a wide laminectomy [39]. Recent studies have documented significantly higher rates of neurologic injury (2–5%) and dural tear (6–10%) [14, 18, 21, 27, 46] than was found in our series. In fact, only 7/104 (6.73%) cases of intradural dural tear were found in our study but after careful duroplasty using sutures or sealant patch (such as TachoSil), none of these was associated with a postoperative CSF leak. It is likely that the more lateral access allowed better maneuverability to identify a dural tear and deal with it. We did not observe any new postoperative neurological deficits in our series. In the Lateral-PLIF technique, as mentioned above, the bilateral facetectomy gives access to the transitional zone (Figs. 3 and 7), between the central canal and the foramen, just above the lateral recess, providing safe access to the disk and thus permitting excessive root and dural sac retraction to be avoided. On the other hand, the midline incision and the wide bilateral subperiosteal dissection results in a greater postoperative pain and, probably, postoperative in-hospital stay compared to unilateral access.
To address the limitations of PLIF, in 1998, Harms and Jeszenszky reported a new technique via a unilateral transforaminal route to achieve the insertion of a single interbody cage (banana-shaped) packed with bone graft and termed TLIF [23, 42]. The main advantage of this approach was the insertion of the implant through a safer zone for the dural sac medially, using a single surgical corridor that reduced access-associated muscle injury [3, 8, 24, 27, 40] and dural complications [14, 25, 31, 38] but also limited the discectomy and preparation of endplates. It can be used only in selected cases where there is no bilateral foraminal stenosis [14, 36]. It has also been supposed that the single cage reduces the fusion surface and limits the control of 3D stability, compared to 2 cages, and presents more difficult placement in some cases [2, 9, 51].
For this reason, we have developed our technique to attempt to maintain a simultaneous bilateral approach allowing interbody distraction to be achieved on one side during insertion of the cage on the contralateral side. This important point explains why inserted cages were higher, 11/12 mm on average, compared to in classic TLIF (9/10 mm) [15, 28, 56]. Cage-like implants (polyether ether ketone—PEEK or titanium cages) meet the mechanical requirements for PLIF by serving both a mechanical function and a biological (bone growth) function. They are cubic or cylindrical in shape and are placed in pairs, resulting in a greater surface area for fusion and a better distribution of load. This type of implant provides higher immediate stability, especially for lateral bending motion, which is not usually found in the TLIF technique [52, 59]. Using our technique, placement of the cages was optimal; no malposition was encountered, as opposed to the TLIF technique in which optimal positioning of the cage is more challenging. At L5S1, the pelvic position and morphology may prevent good positioning of the cage in the L5-S1 disk space using the TLIF technique [36, 40]. Conversely, no difficulties were found in this segment using the Lateral-PLIF approach.
Even though the bilateral disk access may provide a better preparation of the vertebral endplates and immediate stability, it can result in a longer operative time compared to the unilateral single-cage approach, with potential increase of general complications (bleeding, infections, DVT, etc.).
In terms of decompression, with Lateral-PLIF, removal of spinous processes, partial laminotomy of the upper vertebra, and bilateral foraminotomy can be performed as needed to allow effective central and bilateral canal opening. Conversely, the TLIF technique spares the contralateral lamina, facet joint, and pars interarticularis, limiting decompression of neural structures. This “bone resection,” in our opinion, also allows the segmental lordosis to be corrected by removal of all the bony contacts posteriorly between the two adjacent vertebrae. Moreover, it also allows an increase in the bone surface for fusion by opening the posterior facet joints on each side [50, 55]. Morselized autogenous bone obtained from the posterior elements is then packed anteriorly in the intervertebral space, inside the cage [41] and posteriorly around the rods on the decorticated posterior elements to improve the likelihood of arthrodesis.
While a bilateral facetectomy could appear unnecessary in the context of unilateral pathology, such as recurrent disk herniation, instances of pure unilateral disease in patients needing lumbar arthrodesis are uncommon. Even when symptoms are confined to one side, a contralateral foraminal and lateral canal decompression is frequently required to prevent postoperative contralateral radiculopathy. Moreover, opting for a bilateral approach offers several advantages, including more effective disk cleaning and endplate preparation, improved intervertebral distraction, and enhanced fusion outcomes. These benefits are applicable irrespective of the uni/bilateral nature of the pathology and the need for decompression.
Future perspectives
We believe that further improvements in this technique can be made in the near future by applying minimally invasive approaches. Appropriate decompression and circumferential (360°) fusion, with a reduction of muscle injury and complications of open surgery, will be made possible with developments of MIS instruments, material innovations, and factors that stimulate bone growth.
Further comparative studies and randomized clinical trials (RCT) with previous technique could validate the outcomes and allow widespread use of the Lateral-PLIF approach.
Limitations
The present study does present some limitations. Despite being a large prospective series, this is the experience of a single tertiary spinal center. The surgical procedures were performed by different spinal surgeons with different levels of skill and experience. The design of the study limited direct comparisons with other techniques, specifically standard PLIF and TLIF. Additionally, missing data and patients lost to follow-up may have contributed to statistical bias. Lastly, interobserver variability in data analysis could also introduce further bias to the study.
The primary objective of the present study was to provide a comprehensive description of the technique. Although some numerical limitation can be argued, our prospective cohort of 104 patients already represents a significant sample size. However, we are retrospectively collecting data from all patients who underwent surgery with this technique from 2017 to 2023, with the aim of providing more robust conclusions with an extended follow-up period.
Conclusion
Our results suggest that Lateral-PLIF is a safe and effective technique with wide applicability to achieve lumbar fusion, restoring appropriate disk height and correct lordosis and providing excellent fusion rates. Lumbar interbody fusion achieved by this approach appears to offer the benefits of both TLIF and PLIF procedures while reducing their respective limitations and technical difficulties. Without significantly increasing either the global operative time or blood loss, this procedure led to satisfactory clinical and radiological outcomes with a low complication rate. Further comparative RCT are now needed to validate final outcomes before the widespread use of this approach.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and from corresponding authors (F.C.) upon reasonable request.
Abbreviations
- ALIF:
-
Anterior lumbar interbody fusion
- CT:
-
Computed tomography
- ODI:
-
Oswestry Disability Index
- MIS:
-
Minimally invasive technique
- OLIF:
-
Oblique lumbar interbody fusion
- PLIF:
-
Posterior lumbar interbody fusion
- PROCEES:
-
Preferred Reporting of Case Series in Surgery
- RX:
-
Conventional plan radiography
- TLIF:
-
Transforaminal lumbar interbody fusion
- XLIF:
-
Extreme lateral lumbar interbody fusion
- VAS:
-
Visual analogic scale
References
Agha RA, Sohrabi C, Mathew G, Franchi T, Kerwan A, O’Neill N (2020) The PROCESS 2020 guideline: updating consensus Preferred Reporting Of CasESeries in Surgery (PROCESS) guidelines. Int J Surg 84:231–235. https://doi.org/10.1016/j.ijsu.2020.11.005
Aoki Y, Yamagata M, Ikeda Y, Nakajima F, Ohtori S, Nakagawa K, Nakajima A, Toyone T, Orita S, Takahashi K (2012) A prospective randomized controlled study comparing transforaminal lumbar interbody fusion techniques for degenerative spondylolisthesis: unilateral pedicle screw and 1 cage versus bilateral pedicle screws and 2 cages. J Neurosurg Spine 17:153–159. https://doi.org/10.3171/2012.5.Spine111044
Audat Z, Moutasem O, Yousef K, Mohammad B (2012) Comparison of clinical and radiological results of posterolateral fusion, posterior lumbar interbody fusion and transforaminal lumbar interbody fusion techniques in the treatment of degenerative lumbar spine. Singapore Med J 53:183–187
Barrey CY, Boissiere L, D’Acunzi G, Perrin G (2013) One-stage combined lumbo-sacral fusion, by anterior then posterior approach: clinical and radiological results. Eur Spine J 22(Suppl 6):S957-964. https://doi.org/10.1007/s00586-013-3017-9
Barrey C, Darnis A (2015) Current strategies for the restoration of adequate lordosis during lumbar fusion. World J Orthop 6:117–126. https://doi.org/10.5312/wjo.v6.i1.117
Brantigan JW (1994) Pseudarthrosis rate after allograft posterior lumbar interbody fusion with pedicle screw and plate fixation. Spine (Phila Pa 1976) 19:1271–1279. https://doi.org/10.1097/00007632-199405310-00014
Brantigan JW, Steffee AD, Lewis ML, Quinn LM, Persenaire JM (2000) Lumbar interbody fusion using the Brantigan I/F cage for posterior lumbar interbody fusion and the variable pedicle screw placement system: two-year results from a Food and Drug Administration investigational device exemption clinical trial. Spine (Phila Pa 1976) 25:1437–1446. https://doi.org/10.1097/00007632-200006010-00017
Chi KY, Cheng SH, Kuo YK, Lin EY, Kang YN (2021) Safety of lumbar interbody fusion procedures for degenerative disc disease: a systematic review with network meta-analysis of prospective studies. Global Spine J 11:751–760. https://doi.org/10.1177/2192568220938024
Cho JH, Hwang CJ, Lee DH, Lee CS (2021) Clinical and radiological outcomes in patients who underwent posterior lumbar interbody fusion: comparisons between unilateral and bilateral cage insertion. BMC Musculoskelet Disord 22:963. https://doi.org/10.1186/s12891-021-04852-y
Cloward RB (1985) Posterior lumbar interbody fusion updated. Clin Orthopaed Related Res 193:16–19
Cloward RB (1981) Spondylolisthesis: treatment by laminectomy and posterior interbody fusion. Clin Orthopaed Related Res 154:74–82
Cloward RB (1953) The treatment of ruptured lumbar intervertebral discs by vertebral body fusion. I. Indications, operative technique, after care. J Neurosurg 10:154–168. https://doi.org/10.3171/jns.1953.10.2.0154
Cole CD, McCall TD, Schmidt MH, Dailey AT (2009) Comparison of low back fusion techniques: transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr Rev Musculoskelet Med 2:118–126. https://doi.org/10.1007/s12178-009-9053-8
de Kunder SL, van Kuijk SMJ, Rijkers K, Caelers I, van Hemert WLW, de Bie RA, van Santbrink H (2017) Transforaminal lumbar interbody fusion (TLIF) versus posterior lumbar interbody fusion (PLIF) in lumbar spondylolisthesis: a systematic review and meta-analysis. Spine J 17:1712–1721. https://doi.org/10.1016/j.spinee.2017.06.018
Du L, Sun XJ, Zhou TJ, Li YC, Chen C, Zhao CQ, Zhang K, Zhao J (2017) The role of cage height on the flexibility and load sharing of lumbar spine after lumbar interbody fusion with unilateral and bilateral instrumentation: a biomechanical study. BMC Musculoskelet Disord 18:474. https://doi.org/10.1186/s12891-017-1845-1
Fallatah S, Wai E, Baily CS (2013) The value of adding posterior interbody fusion in the surgical treatment of degenerative lumbar spine disorders: a systematic review. Int J Spine Surg 7:e24-28. https://doi.org/10.1016/j.ijsp.2013.01.003
Fraser RD (1995) Interbody, posterior, and combined lumbar fusions. Spine (Phila Pa 1976) 20:167–177. https://doi.org/10.1097/00007632-199512151-00016
Ghobrial GM, Theofanis T, Darden BV, Arnold P, Fehlings MG, Harrop JS (2015) Unintended durotomy in lumbar degenerative spinal surgery: a 10-year systematic review of the literature. Neurosurg Focus 39:E8. https://doi.org/10.3171/2015.7.Focus15266
Ghogawala Z, Dziura J, Butler WE, Dai F, Terrin N, Magge SN, Coumans JV, Harrington JF, Amin-Hanjani S, Schwartz JS, Sonntag VK, Barker FG 2nd, Benzel EC (2016) Laminectomy plus fusion versus laminectomy alone for lumbar spondylolisthesis. N Engl J Med 374:1424–1434. https://doi.org/10.1056/NEJMoa1508788
Gjessing MH (1951) Osteoplastic anterior fusion of the lower lumbar spine in spondylolisthesis, localized spondylosis, and tuberculous spondylitis. Acta Orthop Scand 20:200–213. https://doi.org/10.3109/17453675108991168
Goldstein CL, Macwan K, Sundararajan K, Rampersaud YR (2016) Perioperative outcomes and adverse events of minimally invasive versus open posterior lumbar fusion: meta-analysis and systematic review. J Neurosurg Spine 24:416–427. https://doi.org/10.3171/2015.2.Spine14973
Hackenberg L, Halm H, Bullmann V, Vieth V, Schneider M, Liljenqvist U (2005) Transforaminal lumbar interbody fusion: a safe technique with satisfactory three to five year results. Eur Spine J 14:551–558. https://doi.org/10.1007/s00586-004-0830-1
Harms J, Rolinger H (1982) A one-stager procedure in operative treatment of spondylolistheses: dorsal traction-reposition and anterior fusion (author’s transl). Z Orthop Ihre Grenzgeb 120:343–347. https://doi.org/10.1055/s-2008-1051624
Hsieh PC, Koski TR, O’Shaughnessy BA, Sugrue P, Salehi S, Ondra S, Liu JC (2007) Anterior lumbar interbody fusion in comparison with transforaminal lumbar interbody fusion: implications for the restoration of foraminal height, local disc angle, lumbar lordosis, and sagittal balance. J Neurosurg Spine 7:379–386. https://doi.org/10.3171/spi-07/10/379
Humphreys SC, Hodges SD, Patwardhan AG, Eck JC, Murphy RB, Covington LA (2001) Comparison of posterior and transforaminal approaches to lumbar interbody fusion. Spine (Phila Pa 1976) 26:567–571. https://doi.org/10.1097/00007632-200103010-00023
Jin-Tao Q, Yu T, Mei W, Xu-Dong T, Tian-Jian Z, Guo-Hua S, Lei C, Yue H, Zi-Tian W, Yue Z (2015) Comparison of MIS vs. open PLIF/TLIF with regard to clinical improvement, fusion rate, and incidence of major complication: a meta-analysis. Eur Spine J 24:1058–1065. https://doi.org/10.1007/s00586-015-3890-5
Katz AD, Mancini N, Karukonda T, Greenwood M, Cote M, Moss IL (2019) Approach-based comparative and predictor analysis of 30-day readmission, reoperation, and morbidity in patients undergoing lumbar interbody fusion using the ACS-NSQIP dataset. Spine (Phila Pa 1976) 44:432–441. https://doi.org/10.1097/brs.0000000000002850
Kepler CK, Rihn JA, Radcliff KE, Patel AA, Anderson DG, Vaccaro AR, Hilibrand AS, Albert TJ (2012) Restoration of lordosis and disk height after single-level transforaminal lumbar interbody fusion. Orthop Surg 4:15–20. https://doi.org/10.1111/j.1757-7861.2011.00165.x
Kunze B, Drasseck T, Kluba T (2011) Posterior and transforaminal lumbar interbody fusion (PLIF/TLIF) for the treatment of localised segment degeneration of lumbar spine. Z Orthop Unfall 149:312–316. https://doi.org/10.1055/s-0030-1250689
Kuslich SD, Ulstrom CL, Griffith SL, Ahern JW, Dowdle JD (1998) The Bagby and Kuslich method of lumbar interbody fusion. History, techniques, and 2-year follow-up results of a United States prospective, multicenter trial. Spine (Phila Pa 1976) 23:1267–1278. https://doi.org/10.1097/00007632-199806010-00019
Lan T, Hu SY, Zhang YT, Zheng YC, Zhang R, Shen Z, Yang XJ (2018) Comparison between posterior lumbar interbody fusion and transforaminal lumbar interbody fusion for the treatment of lumbar degenerative diseases: a systematic review and meta-analysis. World Neurosurg 112:86–93. https://doi.org/10.1016/j.wneu.2018.01.021
Launay O, Perrin G, Barrey C (2016) Instrumented PLIF in lumbar degenerative spine: principles, indications, technical aspects, results, complications and pitfalls. In. pp 407–420. https://doi.org/10.1007/978-3-662-47756-4_31
Liu X, Wang Y, Qiu G, Weng X, Yu B (2014) A systematic review with meta-analysis of posterior interbody fusion versus posterolateral fusion in lumbar spondylolisthesis. Eur Spine J 23:43–56. https://doi.org/10.1007/s00586-013-2880-8
Martin CT, Niu S, Whicker E, Ward L, Yoon ST (2020) Radiographic factors affecting lordosis correction after transforaminal lumbar interbody fusion with unilateral facetectomy. Int J Spine Surg 14:681–686. https://doi.org/10.14444/7099
Mehta VA, McGirt MJ, Garcés Ambrossi GL, Parker SL, Sciubba DM, Bydon A, Wolinsky JP, Gokaslan ZL, Witham TF (2011) Trans-foraminal versus posterior lumbar interbody fusion: comparison of surgical morbidity. Neurol Res 33:38–42. https://doi.org/10.1179/016164110x12681290831289
Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ (2015) Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 1:2–18. https://doi.org/10.3978/j.issn.2414-469X.2015.10.05
Nakai S, Yoshizawa H, Kobayashi S (1999) Long-term follow-up study of posterior lumbar interbody fusion. J Spinal Disord 12:293–299
Ohrt-Nissen S, Carreon LY, Andresen AK, Andersen M, Udby P (2022) Clinical and patient-reported outcomes after posterior versus transforaminal lumbar interbody fusion-a propensity score-matched cohort study on 422 patients with 2-year follow-up. Spine(Phila Pa 1976) 47:180–185. https://doi.org/10.1097/brs.0000000000004215
Park MS, Ju YS, Moon SH, Kim TH, Oh JK, Lim JK, Kim CH, Chung CK, Chang HG (2019) Repeat decompression and fusions following posterolateral fusion versus posterior/transforaminal lumbar interbody fusion for lumbar spondylosis: a national database study. Sci Rep 9:4926. https://doi.org/10.1038/s41598-019-41366-z
Phan K, Thayaparan GK, Mobbs RJ (2015) Anterior lumbar interbody fusion versus transforaminal lumbar interbody fusion—systematic review and meta-analysis. Br J Neurosurg 29:705–711. https://doi.org/10.3109/02688697.2015.1036838
Postacchini F (1999) Surgical management of lumbar spinal stenosis. Spine (Phila Pa 1976) 24:1043–1047. https://doi.org/10.1097/00007632-199905150-00020
Reid PC, Morr S, Kaiser MG (2019) State of the union: a review of lumbar fusion indications and techniques for degenerative spine disease. J Neurosurg Spine 31:1–14. https://doi.org/10.3171/2019.4.Spine18915
Resnick DK, Choudhri TF, Dailey AT, Groff MW, Khoo L, Matz PG, Mummaneni P, Watters WC 3rd, Wang J, Walters BC, Hadley MN (2005) Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 7: intractable low-back pain without stenosis or spondylolisthesis. J Neurosurg Spine 2:670–672. https://doi.org/10.3171/spi.2005.2.6.0670
Rosenberg WS, Mummaneni PV (2001) Transforaminal lumbar interbody fusion: technique, complications, and early results. Neurosurgery 48:569–574. https://doi.org/10.1097/00006123-200103000-00022
Rothrock RJ, McNeill IT, Yaeger K, Oermann EK, Cho SK, Caridi JM (2018) Lumbar lordosis correction with interbody fusion: systematic literature review and analysis. World Neurosurg 118:21–31. https://doi.org/10.1016/j.wneu.2018.06.216
Said E, Abdel-Wanis ME, Ameen M, Sayed AA, Mosallam KH, Ahmed AM, Tammam H (2022) Posterolateral fusion versus posterior lumbar interbody fusion: a systematic review and meta-analysis of randomized controlled trials. Global Spine J 12:990–1002. https://doi.org/10.1177/21925682211016426
Sakaura H, Yamashita T, Miwa T, Ohzono K, Ohwada T (2013) Outcomes of 2-level posterior lumbar interbody fusion for 2-level degenerative lumbar spondylolisthesis. J Neurosurg Spine 19:90–94. https://doi.org/10.3171/2013.4.Spine12651
Salehi SA, Tawk R, Ganju A, LaMarca F, Liu JC, Ondra SL (2004) Transforaminal lumbar interbody fusion: surgical technique and results in 24 patients. Neurosurgery 54:368–374. https://doi.org/10.1227/01.neu.0000103493.25162.18
Schnee CL, Freese A, Ansell LV (1997) Outcome analysis for adults with spondylolisthesis treated with posterolateral fusion and transpedicular screw fixation. J Neurosurg 86:56–63. https://doi.org/10.3171/jns.1997.86.1.0056
Schwab F, Blondel B, Chay E, Demakakos J, Lenke L, Tropiano P, Ames C, Smith JS, Shaffrey CI, Glassman S, Farcy JP, Lafage V (2014) The comprehensive anatomical spinal osteotomy classification. Neurosurgery 74:112–120. https://doi.org/10.1227/NEU.0000000000000182o
Seo DK, Kim MJ, Roh SW, Jeon SR (2017) Morphological analysis of interbody fusion following posterior lumbar interbody fusion with cages using computed tomography. Medicine (Baltimore) 96:e7816. https://doi.org/10.1097/md.0000000000007816
Sim HB, Murovic JA, Cho BY, Lim TJ, Park J (2010) Biomechanical comparison of single-level posterior versus transforaminal lumbar interbody fusions with bilateral pedicle screw fixation: segmental stability and the effects on adjacent motion segments. J Neurosurg Spine 12:700–708. https://doi.org/10.3171/2009.12.Spine09123
Stonecipher T, Wright S (1989) Posterior lumbar interbody fusion with facet-screw fixation. Spine (Phila Pa 1976) 14:468–471. https://doi.org/10.1097/00007632-198904000-00026
Suk SI, Lee CK, Kim WJ, Lee JH, Cho KJ, Kim HG (1997) Adding posterior lumbar interbody fusion to pedicle screw fixation and posterolateral fusion after decompression in spondylolytic spondylolisthesis. Spine (Phila Pa 1976) 22:210–219. https://doi.org/10.1097/00007632-199701150-00016
Tye EY, Alentado VJ, Mroz TE, Orr RD, Steinmetz MP (2016) Comparison of clinical and radiographic outcomes in patients receiving single-level transforaminal lumbar interbody fusion with removal of unilateral or bilateral facet joints. Spine (Phila Pa 1976) 41:E1039-e1045. https://doi.org/10.1097/brs.0000000000001535
Wang H, Chen W, Jiang J, Lu F, Ma X, Xia X (2016) Analysis of the correlative factors in the selection of interbody fusion cage height in transforaminal lumbar interbody fusion. BMC Musculoskelet Disord 17:9. https://doi.org/10.1186/s12891-016-0866-5
Wimmer C, Krismer M, Gluch H, Ogon M, Stöckl B (1999) Autogenic versus allogenic bone grafts in anterior lumbar interbody fusion. Clin Orthop Relat Res:122–126. https://doi.org/10.1097/00003086-199903000-00015
Xiao YX, Chen QX, Li FC (2009) Unilateral transforaminal lumbar interbody fusion: a review of the technique, indications and graft materials. J Int Med Res 37:908–917. https://doi.org/10.1177/147323000903700337
Xu H, Tang H, Guan X, Jiang F, Xu N, Ju W, Zhu X, Zhang X, Zhang Q, Li M (2013) Biomechanical comparison of posterior lumbar interbody fusion and transforaminal lumbar interbody fusion by finite element analysis. Neurosurgery 72:21–26. https://doi.org/10.1227/NEU.0b013e3182742a69
Zhang BF, Ge CY, Zheng BL, Hao DJ (2016) Transforaminal lumbar interbody fusion versus posterolateral fusion in degenerative lumbar spondylosis: a meta-analysis. Medicine (Baltimore) 95:e4995. https://doi.org/10.1097/md.0000000000004995
Author information
Authors and Affiliations
Contributions
Conceptualization: C.B.; methodology: C.B. and G.C.; investigation: F.C. and I.Z.; data curation: G.C., F.C., A.V., I.Z.; formal analysis: G.C., A.V., R.C.; writing original draft preparation, G.C., and C.B.; writing—review and editing, G.C., F.C., and C.B.; visualization, D.T. and D.C.; supervision: C.B.; project administration, C.B.; all authors have read and agreed to the submitted version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Ethics committee approval has been provided (N°21–312).
Informed consent
The authors state that all the patients gave their informed consent.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Capo, G., Calvanese, F., Vandenbulcke, A. et al. Lateral-PLIF for spinal arthrodesis: concept, technique, results, complications, and outcomes. Acta Neurochir 166, 123 (2024). https://doi.org/10.1007/s00701-024-06024-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00701-024-06024-y