Abstract
The authors report a rare case of most likely radiation-induced glioma (RIG) with epithelioid features and the presence of molecular features consistent with RIG. This occurred 70 years after craniofacial brachytherapy. Such a late development of radiation-induced glioblastoma (RIGBM) and the advanced age of presentation for an epithelioid glioblastoma are both unique in the literature. Despite not receiving the full course of adjuvant chemotherapy after surgery and radiotherapy, the patient displayed no signs of recurrence during a 5-year follow-up. RIGBM should be further studied to reveal potential unique clinical and molecular characteristics, as well as to better predict survival and treatment response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiation-induced glioma (RIG) is a well-known cerebral complication that can arise after radiation therapy [8, 10, 11, 14, 15, 20]. Typically, the delay between completing radiation treatment and a RIG diagnosis is 7–19 years [8, 11, 20]. However, in this article, we report on a unique case of a right frontal epithelioid glioblastoma multiforme that was diagnosed more than 70 years after facial brachytherapy treatment and several decades after the patient had developed radiation-induced facial basal cell carcinoma.
Clinical presentation
A 75-year-old woman consulted her general physician for persistent headaches, nausea, and vomiting for 2 weeks. Her Karnofsky index was 70%. The neurological examination was normal. The head computed tomography (CT) scan revealed a right frontal mass for which the patient was referred to the neurosurgical department. The patient’s past medical history revealed a facial angioma treated with brachytherapy at the age of 3 years. She also underwent 25 surgeries for the excision of recurrent radio-induced facial basocellular carcinomas (BCC) developed in previously irradiated sites since her early twenties.
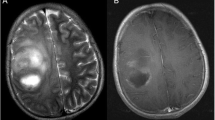
A cerebral magnetic resonance image (MRI) performed upon arrival showed a right frontal mass composed of solid and cystic components located on the ipsilateral orbital roof and contacting the frontal sinus without signs of bony involvement (Fig. 1A–E). A rim of cerebrospinal fluid (CSF) existed between the lesion and the surrounding brain parenchyma (Fig. 1A). A contrast-enhanced image showed avid enhancement of the solid component and rim enhancement around the cystic part (Fig. 1B, C). The fluid attenuation inversion recovery (FLAIR) image showed significant peritumoral brain edema (Fig. 1A). The susceptibility weighted image (SWI) showed some hemorrhagic signs within the tumor (Fig. 1D). The magnetic resonance spectroscopy (MRS) of the peritumoral edema demonstrated a non-tumoral profile (Fig. 1F). There was no restricted diffusion. The MRI was mostly in favor of a high-grade, partially cystic meningioma. The 18F-DOPA positron emission tomography/magnetic resonance image (PET/MRI) was in favor of an aggressive glial tumor with meningeal attachment (Fig. 2).
A Axial T2 FLAIR MRI sequence shows a right hyperintense mass with significant perilesional edema and mass effect. Arrow shows the area of the CSF rim. B T1-weighted MRI with gadolinium shows avid enhancement of the solid part and rim enhancement around the cystic part. The tumor is contacting the posterior wall of the frontal sinus. C Coronal T1 MRI with gadolinium. Arrow points to the area of contact between the tumor and the orbital roof. D SWI shows intratumoral hemorrhagic spots demonstrated by an arrow. E MRS of the peritumoral edema. The square shows the area of edema analyzed. Asterisk sign on cystic part of the tumor
Simultaneous 18F-DOPA positron emission tomography/Magnetic resonance images. A Anterior and D Lateral views show an extensive right frontal lesion on the maximum intensity projection in the anterior and lateral views. B Axial view shows intense and heterogeneous uptake in the periphery of the tumor (arrow) (SUVmax 12.3; tumor/striatum ratio 3.4) and absence of uptake in the central part of the lesion. C FLAIR sequence shows an extensive edema and mass effect (solid arrows) as well as an ischemic sequellae over the left superior temporal gyrus (dashed arrow). E Post-gadolinium T1 sequence and fusion with PET F show perfect matching between the peripheral viable components and central necrotic areas
This right-handed patient underwent a unilateral right frontal craniotomy, guided by neuronavigation (Medtronic, Minnesota, USA). Intraoperatively, a clear separation between the solid tumor and the macroscopically healthy surrounding brain parenchyma was encountered so that en bloc resection after the dissection of the meningeal attachment was performed.
Histopathology and immunohistochemistry showed tumoral proliferation of glial origin invading the leptomeninges. It was composed of large cells of epithelioid aspect with abundant eosinophilic cytoplasm. Areas of palisading necrosis and neo-angiogenesis were observed (Fig. 3). Tumor cells were positive for Olig2 and for perivascular reticulin. The estimated proliferation index was 10%. The immunohistochemical analyses were negative for IDH1, IDH2, R132H, BRAF, FGFR1, FGFR3, H3F3A, TERT, or V600E mutations. ATRX expression was maintained. Homozygous deletion of CDKN2A was associated with mutations in TP53 and PIK3CA. Cytogenetic analysis using fluorescence in situ hybridization showed no amplification of EGFR on chromosome 7 (7p11.2) or loss of heterozygosity at 10q related to the PTEN locus (10q23).
The patient received adjuvant conformational external-beam radiotherapy at a dose of 40 Gy in 15 fractions associated with concurrent temozolomide. Adjuvant temozolomide was discontinued after one cycle because of grade 4 thrombocytopenia (33 × 109/L). Five years after treatment, the patient is alive with no signs of tumor progression.
Discussion
This clinical case of epithelioid glioblastoma presents some atypical and even unique features. Firstly, it is the only reported case in which two radiation-induced tumor types, BCC and glioblastoma, occurred with different timelines after facial brachytherapy. This case advocates for long-term follow-up of patients who received brachytherapy during childhood. Secondly, the development of RIGBM more than 70 years post-irradiation is the longest delay reported in the literature [8, 10, 11, 15]. Thirdly, the long remission period observed in this case was unexpected for an E-GBM. Lastly, both MRI and preoperative findings were consistent with high-grade meningioma: meningeal attachment and peritumoral CSF rim on MRI and the long delay after irradiation.
The patient’s glioblastoma was classified as a radiation-induced tumor according to the widely used Cahen et al.’s criteria for radiation-induced tumors and by the molecular features of RIG described in recent studies attempting to define it [2, 4, 5, 18, 19]. According to Cahen et al., the tumor was developed in a previously irradiated region with a latency period between irradiation and tumor development, a different histology from the original irradiated lesion, and there was no other disease predisposing to the development of the tumor [2]. According to molecular features, the molecular analyses of the presented case share many of the frequent genetic alterations of RIG mainly TP53 mutation, CDKN2A deletion, and the absence of mutations in IDH1, IDH2, H3F3A, and the TERT promoter [4, 5, 18]. In comparison to RIG described in the literature, our case possesses various particularities. First, it is the first reported case of a radiation-induced cerebral tumor after definitive brachytherapy to the head and neck. In addition to Cahen et al.’s criteria, the occurrence of radiation-induced BCC is another argument for the radiation-induced character of the patient’s glioblastoma [6, 17]. Second, the dose gradient of brachytherapy means that the meninges and brain parenchyma were exposed to a low dose. A large retrospective study of irradiated infants showed that sites receiving low radiation doses are the zones with a predilection for radiation-induced tumors [6]. Notably, we did not have access to the dosimetry and distribution of the dose of the brachytherapy that was received 70 years ago. In whatever way, the use of older radiation techniques is a risk factor for radiation-induced secondary malignancies [7]. Presuming that the area of the patient’s E-GBM was out-of-field during facial brachytherapy 70 years ago, one study showed that out-of-field cells are at risk of delayed radiation-induced tumorigenesis after a latency period of up to 40 years by the formation of discreet DNA damages that are often presumed to be non-consequential [9]. Third, it is the first reported case where two radiation-induced tumors occur in the same patient with different timelines. The difference in delay between the radio-induced BCC and the RIGBM could be explained by the difference in cellular turnover between the skin and brain parenchyma [3, 17]. To rule out the rare possibility of direct extension or perineurial spread of multiple facial BCCs, it is important to consider that E-GBMs and BCCs have distinct cellular origins. Therefore, the expression of glial markers, as described previously, is characteristic for E-GBMs, while epithelial markers such as pan-cytokeratin were not expressed in this case.
The risk of developing a secondary malignancy is increased in younger patients and in females [7, 14, 15]. There is no correlation between the age of primary radiotherapy or the radiation dose and the delay in the occurrence of RIG [8]. The latency period for RIG is variable across case series and is usually several years, with a mean range period described between 7 and 19 years [11, 15, 19]. Nevertheless, latency periods as high as 30–40 years post-radiation have been described [14, 20]. The calculated cumulative incidence of intracranial tumor post-irradiation at 30 years was 3–8.5% [10, 14]. The latency period post-radiation and the subsequent development of tumors appear to correlate with tumor behavior, with later occurring tumors (> 35 years after radiation) more likely to be benign [14, 20]. In our case, however, the patient received brachytherapy at the age of 3 and developed cerebral malignancy 70 years later. The longest delay reported in the literature was 61 years after an initial tinea capitis irradiation during infancy [16].
E-GBM is a subtype of glioblastoma introduced into the 2016 World Health Organization (WHO) classification of central nervous system tumors, characterized by the presence of large epithelioid cells with abundant eosinophilic cytoplasm, vesicular chromatin, and prominent nucleoli [13]. E-GBMs, which mostly affect children and young adults, are more aggressive than conventional glioblastomas [1]. Surprisingly, our patient had a remission of 5 years despite not receiving adjuvant chemotherapy. Of note, E-GBMs are often not suspected to be high-grade gliomas on imaging studies due to the sharp circumscription and leptomeningeal dissemination, which are commonly present at diagnosis and raise suspicion for a high-grade meningioma or a metastatic lesion [1]. In our case, the initial suspected diagnosis was a high-grade meningioma due to the circumscribed aspect of the tumor, the presence of a CSF rim between the tumor and adjacent brain parenchyma, the adherence of the tumor to the ipsilateral orbital floor and frontal sinus, and the absence of signs of tumor infiltration of the adjacent brain on diffusion MRI and MRS.
A specific molecular pattern for RIG has not yet been established [4, 5, 18]. However, the most common genetic alterations in RIGs include the absence of IDH1 and IDH2 mutations, PDGFRA or TP53 mutations, PDGFRA or CDK4 amplifications, and CDKN2A deletion, along with 1q gain, 1p loss, and 13q loss [4, 5, 18]. In our case, we found a mutation of TP53 associated with CDKN2A deletions, which are typical of RIG. The absence of mutations of IDH1, IDH2, TERT promoter, H3F3A, or BRAF observed in our case is common in RIG [4, 5, 11, 18].
The introduction of temozolomide in routine practice led to an increase in median survival [8]. Patients with RIGBM who received multimodality treatment (surgery, radiotherapy, and chemotherapy in combination) had a median survival of 18 months with a 2-year survival rate of 28.5% [19]. Ongoing research is investigating the outcomes of sporadic glioblastoma and RIGBM [12].
Conclusion
We reported on the first RIGBM occurring 70 years after facial brachytherapy and several decades after several radiation-induced facial cellular carcinomas. This case underlines the importance of favoring non-radiation-based treatment for young children and highlights the need for long-term follow-up.
Abbreviations
- WHO:
-
World Health Organization
- CT:
-
computed tomography
- MRI:
-
magnetic resonance imaging
- MRS:
-
magnetic resonance spectroscopy
- FLAIR:
-
fluid attenuation inversion recovery
- SWI:
-
susceptibility-weighted image
- PET:
-
positron-emission tomography
- E-GBM:
-
epithelioid glioblastoma
- RIG:
-
radiation-induced glioma
- RIGBM:
-
radiation-induced glioblastoma
References
Broniscer A, Tatevossian RG, Sabin ND, Klimo P Jr, Dalton J, Lee R, Gajjar A, Ellison DW (2014) Clinical, radiological, histological and molecular characteristics of paediatric epithelioid glioblastoma. Neuropathol Appl Neurobiol 40:327–336. https://doi.org/10.1111/nan.12093
Cahan WG, Woodard HQ, Higinbotham NL, Stewart FW, Coley BL (1998) Sarcoma arising in irradiated bone: report of eleven cases. Cancer 82:8–34. https://doi.org/10.1002/(sici)1097-0142(19980101)82:1<8::aid-cncr3>3.0.co;2-w
Dasu A, Toma-Dasu I (2017) Models for the risk of secondary cancers from radiation therapy. Phys Med 42:232–238. https://doi.org/10.1016/j.ejmp.2017.02.015
Deng MY, Sturm D, Pfaff E, Sill M, Stichel D, Balasubramanian GP, Tippelt S, Kramm C, Donson AM, Green AL (2021) Radiation-induced gliomas represent H3-/IDH-wild type pediatric gliomas with recurrent PDGFRA amplification and loss of CDKN2A/B. Nat Commun 12:1–11
DeSisto J, Lucas JT, Xu K, Donson A, Lin T, Sanford B, Wu G, Tran QT, Hedges D, Hsu C-Y (2021) Comprehensive molecular characterization of pediatric radiation-induced high-grade glioma. Nat Commun 12:1–16
Diallo I, Haddy N, Adjadj E, Samand A, Quiniou E, Chavaudra J, Alziar I, Perret N, Guérin S, Lefkopoulos D (2009) Frequency distribution of second solid cancer locations in relation to the irradiated volume among 115 patients treated for childhood cancer. Int J Radiat Oncol Biol Phys 74:876–883
Dracham CB, Shankar A, Madan R (2018) Radiation induced secondary malignancies: a review article. Radiat Oncol J 36:85
Elsamadicy AA, Babu R, Kirkpatrick JP, Adamson DC (2015) Radiation-induced malignant gliomas: a current review. World Neurosurg 83:530–542. https://doi.org/10.1016/j.wneu.2014.12.009
Goy E, Tomezak M, Facchin C, Martin N, Bouchaert E, Benoit J, De Schutter C, Nassour J, Saas L, Drullion C (2022) The out-of-field dose in radiation therapy induces delayed tumorigenesis by senescence evasion. Elife 11:e67190
Hijiya N, Hudson MM, Lensing S, Zacher M, Onciu M, Behm FG, Razzouk BI, Ribeiro RC, Rubnitz JE, Sandlund JT (2007) Cumulative incidence of secondary neoplasms as the first event after treatment of childhood acute lymphoblastic leukemia increases over 30 years. JAMA 6:585–590
Kajitani T, Kanamori M, Saito R, Watanabe Y, Suzuki H, Watanabe M, Kure S, Tominaga T (2018) Three case reports of radiation-induced glioblastoma after complete remission of acute lymphoblastic leukemia. Brain Tumor Pathol 35:114–122. https://doi.org/10.1007/s10014-018-0316-1
Louis DN, Aldape K, Brat DJ, Capper D, Ellison DW, Hawkins C, Paulus W, Perry A, Reifenberger G, Figarella-Branger D (2017) Announcing cIMPACT-NOW: the consortium to inform molecular and practical approaches to CNS tumor taxonomy. Springer
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Pettorini BL, Park YS, Caldarelli M, Massimi L, Tamburrini G, Di Rocco C (2008) Radiation-induced brain tumours after central nervous system irradiation in childhood: a review. Childs Nerv Syst 24:793–805. https://doi.org/10.1007/s00381-008-0631-7
Prasad G, Haas-Kogan DA (2009) Radiation-induced gliomas. Expert Rev Neurother 9:1511–1517. https://doi.org/10.1002/pmic.200800802
Simmons NE, Laws ER Jr (1998) Glioma occurrence after sellar irradiation: case report and review. Neurosurgery 42:172–178. https://doi.org/10.1097/00006123-199801000-00038
Toma-Dasu I, Wojcik A, Kjellsson Lindblom E (2017) Risk of second cancer following radiotherapy. Phys Med 42:211–212. https://doi.org/10.1016/j.ejmp.2017.10.004
Whitehouse JP, Howlett M, Federico A, Kool M, Endersby R, Gottardo NG (2021) Defining the molecular features of radiation-induced glioma: a systematic review and meta-analysis. Neurooncol Adv 3:vdab109
Yamanaka R, Hayano A, Kanayama T (2018) Radiation-induced gliomas: a comprehensive review and meta-analysis. Neurosurg Rev 41:719–731. https://doi.org/10.1007/s10143-016-0786-8
Yeh H, Matanoski GM, Wang N, Sandler DP, Comstock GW (2001) Cancer incidence after childhood nasopharyngeal radium irradiation: a follow-up study in Washington County, Maryland. Am J Epidemiol 153:749–756. https://doi.org/10.1093/aje/153.8.749
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
The manuscript does not contain clinical studies or patient data.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mohamed Aziz Cherif and Loganadane Gokoulkrichenane contributed equally as second authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bugdadi, A., Cherif, M.A., Loganadane, G. et al. Epithelioid glioblastoma diagnosed 70 years after craniofacial radiotherapy. Acta Neurochir 165, 2769–2774 (2023). https://doi.org/10.1007/s00701-023-05637-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-023-05637-z