Abstract
Objectives
To identify risk factors for poor outcome one year post-mild traumatic brain injury (mTBI).
Design
This study was a prospective observational study using consecutive adult hospital admissions with mTBI.
Subjects
A total of 869 consecutive mTBI patients were enrolled in this study.
Methods
All patients were reviewed by the specialist TBI rehabilitation team at six weeks and one year following mTBI. Demographic and injury data collected included: age, gender, TBI severity and Glasgow Coma Scale (GCS). At twelve months, global outcome was assessed by the Extended Glasgow Outcome Score (GOSE) and participation restriction by the Rivermead Head Injury Follow-up Questionnaire (RHFUQ) via semi-structured interview. An ordinal regression (OR) was used to identify associated factors for poor GOSE outcome and a linear regression for a poor RHFUQ outcome.
Results
In the GOSE analysis, lower GCS (p < 0.001), medical comorbidity (p = 0.027), depression (p < 0.001) and male gender (p = 0.008) were identified as risk factors for poor outcome. The RHFUQ analysis identified: lower GCS (p = 0.002), female gender (p = 0.001) and injuries from assault (p = 0.003) were variables associated with worse social functioning at one year.
Conclusion
mTBI is associated with a significant impact upon the physical health and psychosocial function of affected individuals. The results of this study demonstrate that differences in mTBI outcome can be identified at twelve months post-mTBI and that certain features, particularly GCS, are associated with poorer outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) is a worldwide public health concern due to its high incidence, often referred to as a “silent epidemic” [31]. It is associated with severe morbidity and mortality worldwide, resulting in extensive healthcare expenditure [24]. Mild traumatic brain injuries (mTBIs) account for the vast majority of cases of TBI with a reported incidence of 300 per 10 [41] population [7, 40, 41]. Furthermore, this figure may represent an underestimation as a significant proportion of mTBIs do not engage with healthcare services [7]. Despite often being thought of as a relatively innocuous diagnosis, mTBI is associated with a profound impact on the physical, mental health and quality of life of patients throughout both the acute and chronic phase following the injury. Whilst the healthcare expenditure of mTBI remains lower compared to severe TBI, it is reported to account for up to $13,564 (US dollars) per patient within the first twelve months post-injury [32]. The majority of the expenditure is associated with acute hospital admission; however, a significant proportion is related to the ongoing rehabilitation required beyond twelve months [32]. The elevated incidence of mTBI in addition to the significant healthcare burden emphasises the importance of early identification of risk factors for poor outcome and prompt intervention to ameliorate patient outcome.
Cognitive deficit, emotional lability and physical health complaints including but not limited to headache and fatigue account for the majority of the symptomatic burden within the first 72 h following mTBI [4, 23]. Throughout the first-month recovery, common persistent features include headache, fatigue, sleep disturbance and emotional distress [23, 27, 43]. The majority of the symptomatic burden post-mTBI resolves within the first three months [27, 43]. However, a significant proportion of mTBI patients suffer from persistent cognitive deficit and poor neuropsychological outcomes in addition to physical post-concussion symptoms when compared to controls beyond the first three months of recovery [34, 45]. Moreover, the insidious onset of new symptoms and functional deficit within the first twelve months is reported, with deficits in general health and quality of life in some cases persisting beyond ten years post-injury [45].
The underlying cause of poor outcomes in mTBI is multi-factorial. Numerous patient demographics, injury and clinical factors have been associated with a detrimental impact upon patient outcomes. A key factor remains mTBI severity. The Glasgow Coma Scale (GCS) is the most frequently used measure of TBI severity, where scores of 13–15 are classified as “mild”. Despite being classified as “mild injuries”, increasing injury severity (lower GCS) within the mTBI population (GCS 13–15) is associated with poorer outcomes post-mTBI [11]. Furthermore, other injury severity scores including the Abbreviated Injury Severity Scale (AIS) corroborate the findings that increasing injury severity within the mTBI patient population demonstrates a detrimental impact upon long-term outcomes [37]. However, contrasting reports indicate that the evidence of intracranial pathology on CT imaging at the time of the injury is associated with a detrimental impact upon mTBI outcomes irrespective of GCS score at the time of the injury [6, 18]. Other key risk factors for poor outcome following mTBI include gender, age, lower educational status and evidence of emotional distress at the time of the injury, increasing post-traumatic amnesia (PTA) duration and pre-existing neuropsychiatric complaints [1, 15, 17, 43].
The objective of this prospective observational single-centre study was to assess the association of a number of demographic and injury factors on mTBI outcome at twelve months post-injury, with particular interest in admission GCS.
Methods
This prospective observational study was conducted with the specialist neurosciences department of the Sheffield Teaching Hospital (STH) Trust. The study population was derived from the Sheffield Brain Injury after Trauma (SHEFBIT) cohort. The SHEFBIT cohort encompasses all adult patients over the age of 18, admitted to the Sheffield Teaching Hospital Trust with a diagnosis of TBI between 2010 and 2017. Paediatric patients are not included within the SHEFBIT cohort as they receive care in a different hospital within the region. A diagnosis of TBI was made by the Common Data Elements criteria [30]. The SHEFBIT cohort is fully representative of adult TBI patients with regard to: patient demographics, injury aetiology, socioeconomic status (SES) and injury severity. The patient cohort used within this study was limited to patients who received a diagnosis of mTBI which was defined as a GCS score on admission of 13–15. In line with the United Kingdom (UK) National Institute for Health and Care Excellence (NICE) guidance for managing TBI, patients should be admitted for observation if their GCS has not returned to 15 regardless of negative imaging investigations.
All patients within the study population were reviewed with 24 h of their admission following TBI. During this initial review, key injury and demographic data were collated. All study participants were then reviewed twice within a specialist neurorehabilitation clinic throughout the twelve-month observational period. The first follow-up appointment took place at six weeks, with a final review at twelve months post-injury. Demographic data including age, gender, ethnicity and zip (postal) code were recorded. In addition to demographic factors, clinical data including GCS at the time of injury, aetiology and a review of CT imaging at the time of the injury were recorded. The Trauma Audit and Research Network Classification (TARN) criteria were used to define aetiology. This classifies TBI aetiology as: Fall, Road Traffic Collision (RTC), Assault, Sport Injuries and Other (including falls greater than 2 m, etc.) [22]. A record of alcohol intoxication at the time of injury was taken from the medical admission record. Employment history was taken from the medical notes. With regard to this study, employment history was classified as employed (including students in full-time education) and unemployed. A full employment history allowed for an assessment of socioeconomic class using the United Kingdom (UK) Office of National Statistics Socio-Economic Classification (NS-SEC) [8]. The NS-SEC comprises eight levels according to work type. With regard to this study, a ninth level was added to account for full-time students. Ethnicity was recorded as a binary outcome: White and non-White. An assessment of pre-existing medical comorbidity was conducted using the Cumulative Illness Rating Scale (CIRS) where a threshold of a score > 10 was used to establish significant medical comorbidity [16]. The need for Home Support prior to injury, including residential care and a formal package of care, was recorded as a binary outcome.
All study participants underwent CT imaging at the time of admission. All CT scans were assessed using a binary assessment, “no abnormality detected” or “abnormal CT Scan”. “Abnormal CT Scan” was an umbrella term used to identify any CT scan with an evident abnormality including skull fractures, cerebral contusions and intra-cerebral haemorrhage. A diagnosis of depression was recorded for patients who scored greater than eight on the Hospital Anxiety and Depression Scale (HADS), measured at 12 months post-mTBI.
A secondary review was conducted at twelve months post-injury, and outcome data were generated at this time point. Two outcome measures were used: the Extended Glasgow Outcome Scale (GOSE) and the Rivermead Head Injury Follow-up Questionnaire (RHFUQ). The GOSE remains the primary measure of global outcome following TBI, classifying outcome into eight categories: Death, Vegetative State, Severe Disability (Upper and Lower), Moderate Disability (Upper and Lower) and Good Recovery (Upper and Lower) [44]. GOSE outcome was generated using a semi-structured interview within the specialist neurorehabilitation clinic. The RHFUQ provides a measure of psychosocial function or participation restriction in patients following brain injury (previously called Handicap). The RHFUQ generates a score out of 40 points, where an increasing score is representative of worse psychosocial function [10]. The RHFUQ is a widely validated tool for assessment of outcome post-TBI [10].
Statistical analyses were conducted using the Statistical Package for Social Sciences (SPSS) 25. Initial univariate analyses were conducted to assess baseline correlations between patient demographics (age and gender), clinical factors (TBI aetiology, severity and CT identified pathology) and outcome measures at 1 year (GOSE and RHFUQ). Furthermore, t-tests were conducted between GCS at the time of injury, grouped (GCS 13 + 14 vs 15) and age. An ordinal regression (OR) was generated to assess the predictive factors of GOSE score at twelve months post-mTBI. Within the OR, GOSE score was grouped as Death, Vegetative State, Severe Disability (Upper and Lower) as one outcome group with the remaining groups assessed separately. This change was made as the vast majority of mTBI make a good recovery with the poorer outcomes relatively unexpected. A linear regression (LR) was used to assess the predictive factors of RHFUQ score. The RHFUQ does not allow for assessment of patients who died throughout the first twelve months. Therefore, an adjusted patient cohort of 833 survivors was used for RHFUQ analysis.
This study was conducted within the STH under the ethical approval of both the Sheffield Hospital Trust (ref: STH16208) and the University of Sheffield Ethics Committee (ref: 008,315).
Results
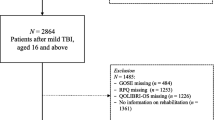
A total of 869 individuals with mTBI were recruited into the SHEFBIT cohort between 2010 and 2017. A total of 21 patients were lost to follow-up throughout the twelve-month study period. A further 15 patients died within the first year post-TBI. As the GOSE includes an outcome for death, the final study population within the GOSE analysis encompassed 848 individuals; however, as the RHFUQ does not facilitate an assessment of participants who died, final cohort in the RHFUQ model includes 833 individuals. Median age was 39 years (interquartile range 25,54). The majority of participants were male (65.0%) and identified as “White British” (93.4%). The final patient demographics and initial injury data are presented in Table 1. The study cohort seemed representative of all TBI with regard to aetiology, with Falls the most common cause (38.4%), followed by RTC (26.3%), Assault (18.0%), Other (9.6%) and Sport (7.7%). With regard to TBI severity, GCS 15 at the time of injury accounted for the majority of cases (54.5%), followed by GCS 14 (30.2%) and GCS 13 (15.3%). The majority of participants (63.7%) had a normal CT head at the time of presentation. Median inpatient length of stay was 2 days (range 1–94 days).
In terms of the GOSE, the majority of study participants (57.6%) of patients made a “Good” recovery at twelve months, with over a third of the total patient cohort (34.8%) making full recovery (“Good Upper”). The full distribution of GOSE outcome at twelve months is detailed in Table 2. Within the final study population, mean RHFUQ score was 7.94 (SD 8.33). As the RHFUQ is unable to assess participants who were dead at twelve months, an analysis was conducted between the RHFUQ final patient population and those excluded from the analysis; the only significant difference between the groups was age.
Independent t-tests between grouped GCS (GCS13 + 14 vs 15) demonstrated that there was no significant difference between groups with regard to age (p = 0.912). Moreover, there was a statistically significant relationship between lower admission GCS and increased incidence of CT pathology at the time of the injury (p = < 0.01) and increased likelihood of alcohol intoxication at the time of injury (p < 0.01). There was no significant relationship between grouped GCS score and pre-existing medical comorbidity (p = 0.984). The results of the univariate analyses are displayed in Table 3.
In univariate analysis, grouped GCS had a statistically significant relationship with GOSE score at 12 months (p < 0.01) and RHFUQ score (p < 0.01).
The primary outcome measure used in this study was GOSE at twelve months post-injury, and an ordinal regression (OR) was used to assess the risk factors associated with poor GOSE outcome. Within the OR model, significant relationships were found between GOSE score and GCS (p < 0.001), significant medical comorbidity (p = 0.027), male gender (0.008), and depression (p < 0.001). There were no statistically significant relationships between age (p = 0.791), alcohol intoxication at the time of injury (p = 0.053), initial employment (p = 0.207) ethnicity (p = 0.300), aetiology (p = 0.188), social class (p = 0.982) and CT abnormality (p = 0.514). This model was highly significant (p < 0.001), -2 log likelihood value was 2642.30, Chi-squared value was 4440.96 and Nagelkerke pseudo-R [24] value was 0.196 (Table 4).
A linear regression (LR) was conducted to assess factors associated with higher RHFUQ score, therefore worsening psychosocial function, at twelve months. Significant relationships were found between RHFUQ score and GCS (p = 0.002), Female gender (p < 0.001), Depression (p < 0.001), Assault aetiology compared to Falls (p = 0.003) and Assault aetiology compared to Sports injuries (p = 0.035). No significant association was demonstrated with: Ethnicity (p = 0.609), Age (p = 0.671), CT abnormality (p = 0.354), Alcohol intoxication (p = 0.483), Significant medical comorbidity (p = 0.790), Assault aetiology vs RTC (p = 0.534), Other causes (p = 0.546), Home Support (p = 0.215) and Initial Employment (p = 0.280). The LR was highly significant (p < 0.001) with an adjusted R-square value of 0.510 (Table 5).
Discussion
The primary aim of this single-centre, prospective observational study was to assess the impact of admission GCS on outcome of mTBI at twelve months. An additional aim was to identify other risk factors associated with poor mTBI outcome. Outcome was determined with both a global measure of function (GOSE) and a measure of psychosocial function (RHFUQ). This was a very large prospective study with a very low attrition rate (2.4%) at 1 year achieved by persistent attempts at follow-up. The demographic data are representative of the mTBI population including a predominance of male patients (64.98%), high number of falls, majority of participants with a normal CT head (63.7%) and presenting with GCS 15 (54.5%) [27, 40, 41, 43]. The primary measure of mTBI outcome was conducted using the GOSE at twelve months. Around a third of patients made a complete recovery within the twelve-month follow-up period. This is a slightly lower prevalence compared to the UPFRONT study, where 56% of cases were reported to make a complete recovery [43]. However, we only enrolled mTBI patients who were admitted to hospital, compared to the UPFRONT study where 39% of patients were not hospitalised secondary to their injuries and had a far lower incidence of CT abnormality 15% compared to 36% within this study. These differences may account for the disparity between our results and those of the UPFRONT study.
For both the GOSE OR model and the RHFUQ LR model, lower admission GCS was associated with poorer mTBI outcome. Within the context of mTBI, lower GCS has previously been reported with poorer outcomes [11]. Moreover, with regard to overall TBI outcome and predicting mortality, classifying TBI injuries with GCS of 13 as “moderate” rather than “mild” produces statistical models with a greater predictive outcome in TBI [13, 29]. This demonstrates that the disparity in outcomes within the mTBI patient population may be addressed by a re-classification of injuries, which may facilitate more appropriate intervention and follow-up, ameliorating patient outcomes.
Other literature has focussed upon other measures of mTBI severity including CT scan abnormality, duration of post-traumatic amnesia (PTA), Abbreviated Injury Severity Score (AIS) and LOS [15, 37]. CT abnormality, often referred to as “complicated” mTBI, is associated with a detrimental impact on both short- and long-term mTBI outcome [6, 18]. However, it is known that abnormalities on CT are associated with lower GCS (13/14) score [18]. This was confirmed in this study, where GCS 13 + 14 injuries demonstrated an increased incidence of CT pathology. This relationship may explain why CT abnormality was not an associated factor for either GOSE or RHFUQ. The relationship between admission GCS and CT abnormality may result in the discrepancies reported between different studies regarding the relative importance of either GCS or CT abnormality when predicting mTBI outcome. Furthermore, study methodology may facilitate differences in study outcome. As commonly in mTBI studies, umbrella terms including “CT abnormality” or “Complicated mTBI” are used to encompass all CT injuries from bony to parenchymal injuries. The lack of differentiation of severity stratification of CT injury in mTBI may be responsible for the conflicting reports of the impact of CT-identified abnormality upon mTBI outcome; it has been demonstrated that in particular intra-cerebral injuries, including number of haemorrhagic contusions, are associated with poorer mTBI outcome [19]. Moreover, the impact of CT abnormality may be overestimated in studies which include both hospitalised and non-hospitalised mTBI given the clinical need for periods of observation for patients with abnormal CT images. There is clear heterogeneity within mTBI presentation, clinical care and outcomes and a delineation between the hospitalised and non-hospitalised mTBI populations within study design may be required, as hospitalisation secondary to mTBI is a known risk factor for increased post-concussion symptoms following mTBI [42].
This study demonstrates the strong association of psychiatric illness upon mTBI outcome. It is well documented that a previous psychiatric history, including depression, is a risk factor for poor global and symptomatic outcome post-TBI [5, 20]. Moreover, it is known that increasing emotional distress at the time of TBI and throughout the early phase of recovery is associated with poor mTBI outcome [43].
We found no significant association of social class on mTBI outcome. There is great variation in the reported impact of social class and socioeconomic status (SES) upon mTBI outcome. The UPFRONT study, using educational level as a marker of SES, reports that an increased educational level infers a protective impact upon mTBI; others show no effect [36, 43]. The methodological differences between studies, with particular reference to the measure of SES, allow for great variation in results and therefore present difficulties in making comparisons between groups. Furthermore, this study reports no difference in mTBI outcome with ethnicity. Whilst the pre-existing literature reports a detrimental impact upon outcomes secondary to ethnicity and race, this is associated with differences in health insurance status [28]. However, it is difficult to make comparisons within the context of this study as the patient population is relatively homogenous (93% White British) in addition to taking place in a state-funded healthcare system rather than an insurance-based system.
This study also found no relationship between age and mTBI outcome in either model. Whilst age is a well-known risk factor for poor outcome following TBI, the impact of age on mTBI outcome remains unclear [26]. The UPFRONT study demonstrated that increasing age was associated with an improved recovery although this effect was found to interact with educational level, demonstrating the difficulty in isolating a single significant risk factor in mTBI [43]. However, the TRACK-TBI study failed to find a relationship between age and mTBI outcome [27].
Similarly, there was no association between alcohol intoxication and mTBI outcome, measured using either the GOSE or the RHFUQ. Within the pre-existing literature, there have been conflicting reports surrounding the “protective” impact of alcohol intoxication on TBI outcome [3]. However, this “protective” impact is considered to be a manifestation of numerous conflicting factors, including an initial lower GCS score secondary to acute alcohol intoxication [14, 39]. The phenomenon is manifest in this study where acute alcohol intoxication was inversely related to GCS at the point of injury.
Medical comorbidity is known to be associated with poorer TBI outcome [17, 38]. An association was demonstrated within the GOSE, but not within RHFUQ.
The role of gender in TBI outcome is debated although more studies find that women experience poorer global outcomes compared with men. However, the opposite has also been found [2, 12, 21]. Furthermore, with respect to specific TBI sequelae, in particular symptomatic outcome, it has been demonstrated that in the early phase of mTBI recovery, female gender is associated with greater symptomatic burden, although no difference in return to work was found [1]. These results corroborate the findings in the RHFUQ model which demonstrates that female gender is related to an increased RHFUQ score inferring that female gender is associated with poorer social functioning at twelve months post-mTBI. However, in the primary GOSE analysis, male gender was associated with a worse outcome at twelve months. The discrepancy between the two outcome results may lie in the differences between the measures themselves. The RHFUQ is a linear measure which may facilitate assessment of more subtle differences between groups, in this case gender. Furthermore, the heterogeneity of the mTBI population and the interaction between numerous patient and injury factors provides great difficulty in predicting mTBI outcome.
Unemployment at the time of injury had a negative association on GOSE score but none on RHFUQ. This discrepancy may be secondary to relationships between employment status and other known risk factors for poor TBI outcome. For example, patients of increasing age are less likely to be employed at the time of injury.
TBI aetiology and GOSE were not associated at twelve months. But in the RHFUQ model, an aetiology of assault was associated with worse outcome compared with falls and sports injuries. A potential explanation for this is that TBI aetiologies associated with increasing emotional distress at the time of injury, such as assault or road traffic collisions, are associated with poorer mTBI outcome [33, 43]. Furthermore, within the sensitivity binary logistic analysis using a binary GOSE outcome, assault again was found to be associated with a negative impact upon outcome at 12 months.
Within this study, two statistical analyses were conducted using two different outcomes measures. The RHFUQ is a truly linear outcome measure used to assess psychosocial function, previously called handicap, whereas the GOSE is an ordinal measure of global function following TBI. Whilst a number of factors, including GCS, were found to have similar impacts upon both outcome measures, some discrepancies were found between the two models, in particular the impact of gender and underlying TBI aetiology on mTBI outcome. This is demonstrated in the pre-existing literature where the impact of gender upon outcome has been shown to produce conflicting results according to the outcome measure used.
Key strengths of this prospective observational study include the large patient cohort and low attrition rate throughout the twelve-month study period. Furthermore, the SHEFBIT cohort is fully representative of adult hospitalised mTBI, with regard to age, underlying aetiology and injury severity. Whilst the majority of the patients within this study had an admission GCS score of 15, there is adequate spread of GCS score to make inferences regarding the impact of TBI severity within the context of mTBI. Moreover, all cases were managed within a single-centre specialist neurorehabilitation clinic resulting in good continuity of care and reducing inter-observer bias. Furthermore, due to the heterogeneous nature of mTBI outcome, underlying aetiology, risk factors and severity, producing robust statistical models with appropriate predictive power remains a difficulty. Within this study, both statistical models demonstrated high statistical significance with Nagelkerke scores of 0.19 and 0.51. Acute prognostic models looking mainly at severe TBI only predict around 0.25–0.35 of the variance, suggesting that most of the outcome after TBI still needs to be attributed to other factors [9, 25, 35].
In summary, mTBI encompasses a large heterogeneous population which accounts for the majority of all TBI [43]. The heterogeneity of the mTBI patient population with regard to demographics, injury factors, hospitalisation status and outcomes results in difficulty in making fully comprehensive statistical models to fully predict outcome. However, this study examined two different outcomes which demonstrate the significant association of mTBI severity, gender, neuropsychiatric status and significant medical comorbidity upon twelve-month mTBI outcome.
Conclusion
Whilst the majority of mTBI patients make a good recovery within the first twelve months post-injury, a significant proportion of those affected continue to have impaired global and social functioning at twelve months. Risk factors for worse outcomes at twelve months include: lower GCS at the time of the injury, significant pre-existing medical comorbidity, female gender and a diagnosis of depression. With regard to the classification of injury severity in TBI, the results of this study demonstrate that tangible differences in mTBI outcome can be measured at twelve months post-mTBI between patients according to lower GCS.
References
Bazarian JJ, Blyth B, Mookerjee S, He H, McDermott MP (2010) Sex differences in outcome after mild TBI. J Neurotrauma 27(3):527–539. https://doi.org/10.1089/neu.2009.1068
Berry C, Ley EJ, Tillou A, Cryer G, Margulies DR, Salim A (2009) The effect of gender on patients with moderate to severe head injuries. J Trauma 67(5):950–953
Brickley MR, Shepherd JP (1995) The relationship between alcohol intoxication, injury severity and Glasgow Coma Score in assault patients. Injury 26(5):311–314. https://doi.org/10.1016/0020-1383(95)00034-7
Bloom BM, Kinsella K, Pott J, Patel HC, Harris T, Lecky F, Pearse R (2017) Short-term neurocognitive and symptomatic outcomes following mild TBI: A prospective multi-centre observational cohort study. Brain Inj 31(3):304–311. https://doi.org/10.1080/02699052.2016.1256501
Booker J, Sinha S, Choudhari K, Dawson J, Singh R (2019) Predicting functional recovery after mild TBI: the SHEFBIT cohort. Brain Inj 33(9):1158–1164. https://doi.org/10.1080/02699052.2019.1629626
Borgaro SR, Prigatano GP, Kwasnica C, Rexer JL (2003) Cognitive and affective sequelae in complicated and uncomplicated mild TBI. Brain Inj 17(3):189–198. https://doi.org/10.1080/0269905021000013183
Cassidy JD, Carroll LJ, Peloso PM, Borg J, von Holst H, Holm L, Kraus J, Coronado VG; WHO Collaborating Centre Task Force on Mild TBI (2004) Incidence, risk factors and prevention of mild TBI: results of the WHO Collaborating Centre Task Force on Mild TBI. J Rehabil Med. (43 Suppl):28–60. https://doi.org/10.1080/16501960410023732.
Chandola T, Jenkinson C (2000) The new UK National Statistics Socio-Economic Classification (NS-SEC); investigating social class differences in self-reported health status. J Pub Health Med 22:182–190
Crash Trial Collaborators MRC, Perel P, Arango M, Clayton T, Edwards P, Komolafe E, Poccock S, Roberts I, Shakur H, Steyerberg E, Yutthakasemsunt S (2008) Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ (Clinical research ed) 336(7641):425–429. https://doi.org/10.1136/bmj.39461.643438.25
Crawford S, Wenden FJ, Wade DT (1996) The Rivermead head injury follow up questionnaire: A study of a new rating scale and other measures to evaluate outcome after head injury. J Neurol Neurosurg Psychiatry 60(5):510–514
Dikmen S, Machamer J, Temkin N (2017) Mild TBI: Longitudinal Study of Cognition, Functional Status, and Post-Traumatic Symptoms. J Neurotrauma 34(8):1524–1530. https://doi.org/10.1089/neu.2016.4618
Farace, E., & Alves, W. M (2000). Do women fare worse: a metaanalysis of gender differences in TBI outcome, Journal of Neurosurgery, 93(4), 539–545. Retrieved Sep 25, 2020, from https://thejns.org/view/journals/j-neurosurg/93/4/article-p539.xml
Godoy DA, Rubiano A, Rabinstein AA et al (2016) Moderate TBI: The Grey Zone of Neurotrauma. Neurocrit Care 25:306–319. https://doi.org/10.1007/s12028-016-0253-y
Hadjizacharia P, O’Keeffe T, Plurad DS, Green DJ, Brown CV, Chan LS, Demetriades D, Rhee P (2011) Alcohol exposure and outcomes in trauma patients. Eur J Trauma Emerg Surg 37(2):169–175. https://doi.org/10.1007/s00068-010-0038-5\
Hart T, Novack TA, Temkin N, Barber J, Dikmen SS, Diaz-Arrastia R, Ricker J, Hesdorffer DC, Jallo J, Hsu NH, Zafonte R (2016) Duration of Posttraumatic Amnesia Predicts Neuropsychological and Global Outcome in Complicated Mild TBI. J Head Trauma Rehabil 31(6):E1–E9. https://doi.org/10.1097/HTR.0000000000000210
Hudson C, Fortin M, Vanasse A (2005) Cumulative illness rating scale was a reliable and valid index in a family practice context. J Clin Epidemiol 58(6):603–608
Humphries TJ, Ingram S, Sinha S, Lecky F, Dawson J, Singh R (2020) The effect of socioeconomic deprivation on 12 month TBI (TBI) outcome. Brain Inj 34(3):343–349. https://doi.org/10.1080/02699052.2020.1715481
Iverson GL, Lange RT, Wäljas M, Liimatainen S, Dastidar P, Hartikainen KM, Soimakallio S, Ohman J (2012) Outcome from Complicated versus Uncomplicated Mild TBI. Rehabil Res Pract 2012:415740. https://doi.org/10.1155/2012/415740
Jacobs B, Beems T, Stulemeijer M, van Vugt AB, van der Vliet TM, Borm GF, Vos PE (2010) Outcome prediction in mild traumatic brain injury: age and clinical variables are stronger predictors than CT abnormalities. J Neurotrauma 27(4):655–668. https://doi.org/10.1089/neu.2009.1059
Juanita A. Haagsma, Annemieke C. Scholten, Teuntje M.J.C. Andriessen, Pieter E. Vos, Ed F. Van Beeck, and Suzanne Polinder.Journal of Neurotrauma.Jun 2015.853–862. https://doi.org/10.1089/neu.2013.3283
Kraus JF, Peek-Asa C, McArthur D (2000) The independent effect of gender on outcomes following TBI: A preliminary investigation. Neurosurg Focus 8(1):1–7
Lecky F, Woodford M, Yates D (2000) Trends in trauma care in England and wales 1989–97. Lancet 355(9217):1771–1775
Lundin A, de Boussard C, Edman G, Borg J (2006) Symptoms and disability until 3 months after mild TBI. Brain Inj 20(8):799–806. https://doi.org/10.1080/02699050600744327
Ma VY, Chan L, Carruthers KJ (2014) Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: Stroke, spinal cord injury, TBI, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pa. Arch Phys Med Rehabil 95(5):986–95.e1
Maas AI, Murray GD, Roozenbeek B, Lingsma HF, Butcher I, McHugh GS, Weir J, Lu J, Steyerberg EW, International Mission on Prognosis Analysis of Clinical Trials in Traumatic Brain Injury (IMPACT) Study Group (2013) Advancing care for traumatic brain injury: findings from the IMPACT studies and perspectives on future research. Lancet Neurology 12(12):1200–1210. https://doi.org/10.1016/S1474-4422(13)70234-5
Marquez CD, de la Plata D, Hart T, Hammond FM, Frol AB, Hudak A, Whyte J (2008) Impact of age on long-term recovery from TBI. Arch Phys Med Rehabil 89(May):896–903. https://doi.org/10.1016/j.apmr.2007.12.030
McMahon P, Hricik A, Yue JK, Puccio AM, Inoue T, Lingsma HF, Beers SR, Gordon WA, Valadka AB, Manley GT, Okonkwo DO, Investigators TRACK-TBI (2014) Symptomatology and functional outcome in mild TBI: results from the prospective TRACK-TBI study. J Neurotrauma 31(1):26–33. https://doi.org/10.1089/neu.2013.2984
McQuistion K, Zens T, Jung HS, Beems M, Leverson G, Liepert A, Scarborough J, Agarwal S (2016) Insurance status and race affect treatment and outcome of traumatic brain injury. J Surg Res 205(2):261–271. https://doi.org/10.1016/j.jss.2016.06.087
Mena JH, Sanchez AI, Rubiano AM, Peitzman AB, Sperry JL, Gutierrez MI, Puyana JC (2011) Effect of the modified Glasgow Coma Scale score criteria for mild TBI on mortality prediction: comparing classic and modified Glasgow Coma Scale score model scores of 13. J Trauma 71(5):1185–1193. https://doi.org/10.1097/TA.0b013e31823321f8
Menon DK, Schwab K, Wright DW, Maas AI (2010) Position statement: Definition of TBI. Arch Phys Med Rehabil 91(11):1637–1640
Mollayeva T, Mollayeva S, Colantonio A (2018) TBI: sex, gender and intersecting vulnerabilities. Nat Rev Neurol 14:711–722
Pavlov V, Thompson-Leduc P, Zimmer L, Wen J, Shea J, Beyhaghi H, Toback S, Kirson N, Miller M (2019) Mild TBI in the United States: demographics, brain imaging procedures, health-care utilization and costs. Brain Inj 33(9):1151–1157. https://doi.org/10.1080/02699052.2019.1629022
Ponsford J, Willmott C, Rothwell A, Cameron P, Kelly A, Nelms R (2000) Factors influencing outcome following mild traumatic brain injury in adults. J Int Neuropsychol Soc 6(5):568–579. https://doi.org/10.1017/S135561770065
Rabinowitz AR, Li X, McCauley SR, Wilde EA, Barnes A, Hanten G, Mendez D, McCarthy JJ, Levin HS (2015) Prevalence and Predictors of Poor Recovery from Mild Traumatic Brain Injury. J Neurotrauma 32(19):1488–1496. https://doi.org/10.1089/neu.2014.3555
Raj R, Bendel S, Reinikainen M, Kivisaari R, Siironen J, Lång M, Skrifvars M (2013) Hyperoxemia and long-term outcome after traumatic brain injury. Crit Care 17(4):R177. https://doi.org/10.1186/cc12856
Rassovsky Y, Levi Y, Agranov E, Sela-Kaufman M, Sverdlik A, Vakil E (2015) Predicting long-term outcome following traumatic brain injury (TBI). J Clin Exp Neuropsychol 37(4):354–366. https://doi.org/10.1080/13803395.2015.1015498
Røe C, Sveen U, Alvsåker K, Bautz-Holter E (2009) Post-concussion symptoms after mild TBI: influence of demographic factors and injury severity in a 1-year cohort study. Disabil Rehabil 31(15):1235–1243. https://doi.org/10.1080/09638280802532720
Singh R, Humphries T, Mason S, Lecky F, Dawson J, Sinha S (2018) The incidence of anosmia after TBI: the SHEFBIT cohort. Brain Inj 32(9):1122–1128. https://doi.org/10.1080/02699052.2018.1483028
Shahin H, Gopinath SP, Robertson CS (2010) Influence of alcohol on early Glasgow Coma Scale in head-injured patients. J Trauma 69 (5):1176–81–discussion1181. https://doi.org/10.1097/TA.0b013e3181ec2b11
Styrke J, Stålnacke BM, Sojka P, Björnstig U (2007) Traumatic brain injuries in a well-defined population: epidemiological aspects and severity. J Neurotrauma 24(9):1425–1436. https://doi.org/10.1089/neu.2007.0266
Tagliaferri F, Compagnone C, Korsic M, Servadei F, Kraus J (2005) A systematic review of brain injury epidemiology in Europe. Acta Neurochir (Wien) 148(3):255–268
Taylor HG, Dietrich A, Nuss K, Wright M, Rusin J, Bangert B, Minich N, Yeates KO (2010) Post-concussive symptoms in children with mild traumatic brain injury. Neuropsychology 24(2):148–159. https://doi.org/10.1037/a0018112
van der Naalt J, Timmerman ME, de Koning ME, van der Horn HJ, Scheenen ME, Jacobs B, Hageman G, Yilmaz T, Roks G, Spikman JM (2017) Early predictors of outcome after mild TBI (UPFRONT): an observational cohort study. Lancet Neurol 16(7):532–540. https://doi.org/10.1016/S1474-4422(17)30117-5
Weir J, Steyerberg EW, Butcher I, Lu J, Lingsma HF, McHugh GS, Roozenbeek B, Maas AIR, Murray GD (2012) Does the extended Glasgow outcome scale add value to the conventional Glasgow outcome scale? J Neurotrauma 29(1):53–58. https://doi.org/10.1089/neu.2011.2137
Zumstein MA, Moser M, Mottini M, Ott SR, Sadowski-Cron C, Radanov BP, Zimmermann H, Exadaktylos A (2011) Long-term outcome in patients with mild TBI: a prospective observational study. J Trauma 71(1):120–127. https://doi.org/10.1097/TA.0b013e3181f2d670
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
Ethical approval was obtained from Sheffield Hospital Trust (ref: STH16208) and the University of Sheffield Ethics Committee (ref: 008315)
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Brain trauma
Rights and permissions
About this article
Cite this article
Humphries, T.J., Sinha, S., Dawson, J. et al. “Can differences in hospitalised mild traumatic brain injury (mTBI) outcomes at 12 months be predicted?”. Acta Neurochir 164, 1435–1443 (2022). https://doi.org/10.1007/s00701-022-05183-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-022-05183-0