Abstract
Moderate traumatic brain injury (MTBI) is poorly defined in the literature and the nomenclature “moderate” is misleading, because up to 15 % of such patients may die. MTBI is a heterogeneous entity that shares many aspects of its pathophysiology and management with severe traumatic brain injury. Many patients who ‘’talk and died’’ are MTBI. The role of neuroimaging is essential for the proper management of these patients. To analyze all aspects of the pathophysiology and management of MTBI, proposing a new way to categorize it considering the clinical picture and neuroimaging findings. We proposed a different approach to the group of patients with Glasgow Coma Scale (GCS) ranging from 9 through 13 and we discuss the rationale for this proposal. Patients with lower GCS scores (9–10), especially those with significant space-occupying lesions on the CT scan, should be managed following the guidelines for severe traumatic brain injury, with ICU observation, frequent serial computed tomography (CT) scanning and ICP monitoring. On the other hand, those with higher range GCS (11–13) can be managed more conservatively with serial neurological examination and CT scans. Given the available evidence, MTBI is an entity that needs reclassification. Large-scale and well-designed studies are urgently needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Illustrative case
A 26-years-old male with no significant medical history suffered an accident on a public road. He was biking without a helmet and struck a horse, 120 km from the main city. The emergency services documented a Glasgow coma scale (GCS) score of 14 points at the scene (E4, V4, M6) without focal neurological symptoms. Blood pressure (BP) was 115/85 mmHg, pulse oximetry 95 % on spontaneous ventilation with a breathing rate of 16 breaths per minute, and heart rate was 66 beats per minute. He was not drunk and had no a history of illicit drug use. He was transported to a regional hospital with a peripheral infusion of crystalloids (saline 0.9 %, 1500 ml) and analgesia without sedation. Paramedics did not note adverse events during the transport. The patient arrived to the emergency room of the hospital with a GCS score of 12 (E3, V3, M6) without pupillary dysfunction, focal neurological signs or abnormal motor response. Vital signs were normal including BP of 122/88 mmHg and pulse oximetry of 94 %. Laboratory and radiological exams were performed according to the ATLS protocol and extracranial injuries were not detected.
Introduction
Traumatic brain injury (TBI) is one of the leading causes of death and disability and affects mainly young adults at their most productive age [1–3]. It has been described as the silent epidemic of our time and has a significant socio-economic impact [3]. Although guidelines have been established for managing patients with mild and severe TBI [4–10] this is not the case for the so-called ‘’moderate TBI.’’ Any literature search makes obvious the wide gap between papers dedicated to mild or severe TBI relative to moderate head injuries [11]. Indeed, many authors include moderate and severe TBI as a single entity [1, 12].
Like severe TBI is much more of a heterogeneous entity with different injury mechanisms, pathophysiology, and neuropathological findings and with a high incidence of intracranial lesions and risk of neurological worsening that may result in a poor functional outcome [1, 12–15].
Several patients who talk and deteriorate or even die (“talk and died”) are initially moderate TBI [16–22]. The epidemiological profile of moderate TBI has changed in recent years [17], but still has high morbidity and mortality rates [13–22]. Therefore, this entity, when adequately assessed, presents significant windows of opportunity to optimize management and improve neurological outcome.
Is the Definition of Moderate TBI Appropriate?
To answer this key question, it is necessary to understand the history. Jennet and Teasdale described the Glasgow Coma Scale (GCS) in 1974 [23]. Since its original description, TBI with GCS scores of 9–12 is considered moderate [24–26]. In the 1980s, different ways to define this group of patients emerged. Annegers et al. suggested to include in the definition a period of loss of consciousness or posttraumatic amnesia of at least 30 min after injury [27]. In one of the largest published series, Rimel et al. added a time period for definitive categorization, suggesting that a TBI should be considered moderate if the GCS was 9–12 points for at least 6-h post-trauma [28]. In 1998 Tabbador et al. defined TBI as moderate when the GCS was between 9 and 11 48 h after injury [29]. Kraus et al. maintained the range of 9–12 points on the GCS, but added as additional criteria that the patient must remain hospitalized for at least 48 h, or should have an abnormal computed tomography (CT) or undergo a neurosurgical procedure [30]. These latter criteria were maintained by Levin et al. who also added the presence of neurological deficit or depressed skull fracture with dural laceration [31]. Stein showed in a review of the relevant literature that at least one third of the patients with a GCS of 13 had intracranial lesions and because of this suggested to include patients with a score of 13 in the “moderate” category, a definition used by others after Stein’s report [32–35].
Factors that Interfere with the Correct Categorization of Moderate TBI
In addition to the lack of uniform criteria for the definition of moderate TBI, it is well known that several confounding factors influence the correct evaluation of the GCS [36]. If such factors are not taken into account, there is a high risk of assigning a patient to an erroneous category. As a frequent example in daily clinical practice, a patient with moderate TBI under the effects of illicit drugs or alcohol might be erroneously included in the severe category [2, 9, 12–14]. Some of the following issues interfere with a correct GCS evaluation are as follows:
-
Lack of experience and training in the use of GCS.
-
Alcohol (although always remembering the fundamental premise that it is never safe to attribute a depressed level of consciousness solely to alcohol after TBI) [37–39].
-
Sedation, regardless of the drug used, influences the neurological exam. A knowledge of the drugs given is necessary in order to use the appropriate antagonist, if available.
-
Intubation, which eliminates the possibility of obtaining the verbal component of the GCS. This shortcoming of the GCS can be overcome by using the FOUR score [40], but categorization of TBI severity by FOUR score has not been studied thus far.
-
Illicit drugs with the same considerations as for the use of sedatives.
-
Associated trauma, such as maxilo-facial injury that prevents the appropriate assessment of ocular and verbal components.
-
Extracranial secondary insults (hypotension, hypoxemia, hypoglycemia, hyponatremia, etc.) may influence the level of consciousness and should be corrected before the GCS evaluation.
Epidemiology
Serious problems arise because of the wide variability in the definitions of moderate TBI. Additionally, there are many factors that could contribute to an erroneous determination of GCS. For these reasons, it is difficult to analyze and compare different series of patients with moderate TBI.
Despite these limitations, it is estimated that 20 % of hospital admissions for TBI are moderate, with an incidence ranging between 4 and 28 % according to the series [13–15, 25, 26]. Overall, for every 22 mild TBI, there are 2.5 moderates and 1 severe [14]. Moderate TBI mainly affects the young adult population involved in traffic accidents. Many of them are under the effects of alcohol or illicit drugs and the TBI is frequently associated with extracranial injuries [25, 26, 28, 32, 34, 35]. Moderate TBI with significant extracranial injuries has a worse outcome than isolated moderate TBI [41]. Using mnemonics, we can say that in moderate TBI the “rule of 30” holds true. This means that individuals with moderate TBI, have approximately a 30 % chance of having a brain lesion (intra- or extra-axial), a 30 % chance that such injuries progress in their volume or mass effect (new bleeding, rebleeding, edema) and a 30 % chance that these individuals suffer deterioration or worsening in their neurological status [13–15, 25, 26, 28, 32, 34, 35]. Mortality in moderate TBI is around 15 %, while more than half of the victims suffer cognitive sequelae and only 20 % recover without significant dissability [13–15, 25, 26, 28, 34, 35].
Pathophysiology of Moderate TBI
TBI is an extremely heterogeneous disease with various forms of presentation [1, 9, 10, 12–14]. External forces, including direct impact, acceleration–deceleration phenomena, penetrating injuries or shock waves, cause the brain injury [1, 9, 10, 12–14]. Whatever the mechanism, the nature, intensity, and duration of the generated forces are the factors that determine the final pattern and extent of the brain damage. Acute TBI was characterized by Gennarelli as a dynamic process that evolves with time [41]. From the pathophysiological point of view, we can distinguish two basic types of injury known as primary and secondary lesions [1, 9, 10, 12–14, 42]. Primary lesions occur immediately upon impact and at the cellular level they evolve during the early stages of trauma [1, 2, 12–14, 42].
The consequence of these lesions can be functional or structural, focal or diffuse [1, 2, 12–14, 42]. Cell death, axonal injury, and vascular damage microscopically characterize structural primary damage. Diffuse axonal injury (DAI) is the principal example of a primary diffuse brain damage. DAI occurs by inertial loading that generates shearing forces in axons causing physical (primary axotomy) or functional damage; this may evolve to delayed or secondary axotomy [1, 2, 12–14, 42]. Changes in axonal permeability are followed by neuro-filament accumulation, local inflammatory changes, and axonal rupture. Wallerian degeneration of axons and myelin sheets is associated with delayed changes. These changes can take 4–8 weeks and are responsible for the tissue atrophy that is observed months later [1, 2, 12–14, 42]. Severe DAI is macroscopically characterized by the presence of multiple small hemorrhagic lesions in specific brain areas, such as corona radiata, centrum semiovale, subcortical white matter, corpus callosum, posterolateral midbrain, and superior cerebellar peduncles [1, 2, 12–14, 42, 43].
Immediately after trauma, multiple pathways are triggered, almost all of them with neurotoxic effects on glia, neurons and endothelial cells [1, 2, 12–14, 42, 43]. The toxic cascade has different components, such as the release of oxygen free radicals, lipid peroxidation, thrombosis in the microcirculation, inflammation, apoptosis, and edema [1, 2, 12–14]. These phenomena may induce impaired autoregulation, decrease in cerebral blood flow (CBF), ischemic or non-ischemic forms of tissue hypoxia, and intracranial hypertension, [2, 9, 10, 12–14, 42–44].
Secondary lesions are defined as those that appear minutes, hours or days after injury, aggravating the primary lesions [1, 2, 9, 10, 12–14, 44–47]. Sources of injuries can be intra or extracranial [1, 2, 9, 10, 12–14, 45–48] (Table 1). Because the primary lesion has no specific treatment, the main goal in the management of TBI is the early detection and aggressive treatment of intra- or extracranial secondary injuries [1, 2, 9, 10, 12–14, 45–48]. Among the systemic causes, hypotension and hypoxemia have the greatest impact on outcome [1, 2, 9, 10, 12–14, 45–48]. The presence of both in the early hours after injury significantly increases the possibility of unfavorable results [1, 9, 45, 46].
Intracranial hypertension is one of the most common mechanisms of secondary injury in severe TBI and can also occur in patients with moderate TBI who experience worsening after presentation. It has strongly negative impact on prognosis [1, 2, 9, 10, 12–14, 45–48]. It may be triggered by the development of brain edema, intra or extra-axial hematomas, changes in cerebrospinal fluid (CSF) dynamics and/or increased cerebral blood volume. Whatever the mechanism, high intracranial pressure (ICP) exerts its deleterious effect by triggering ischemia or producing tissue shifts (herniation) that cause compression of brain structures and the brainstem [1, 2, 9, 10, 12–14, 45–48]. Ischemic brain damage is a frequent finding after TBI and may result from cerebral hypoxia (low oxygen delivery), increased oxygen brain requirements (fever, seizures), impaired autoregulation (vasoparalysis) and abnormalities in the microcirculation or at the mitochondrial level [1, 2, 12–14, 49–54]. TBI triggers a vicious circle of neurotoxic phenomena that feeds and strengthens each other and whose final destination is often cell death either by apoptosis or necrosis [1, 2, 12].
Cerebral Contusions: Injuries that Should Activate the Alarm Signal?
Cerebral contusions (CC) are focal parenchymal lesions which are present in more than half of the cases of moderate and severe TBI [55, 56]. They are heterogeneous, varying in appearance from its classical description of ‘’salt and pepper’’ to the formation of a solid hematoma [56, 57]. From a histopathological point of view, CC generated as a result of the initial mechanical impact have two clearly differentiated zones: an irreversible and central necrotic zone called ‘‘core’’ and an active surrounding area, dominated by inflammatory phenomena, edema and neurotoxic cascades [58, 59].
Recently, a positron emission tomography (PET) study demonstrated a progressive decrease in CBF, and cerebral metabolic rates of oxygen (CMRO2) and glucose (CMRglc) in the peri-contusional area [60]. Cerebral oxygen extraction (COE) followed the same pattern and coupling between CBF and CMRO2 remained stable [60]. The authors hypothesized that this area evolves gradually and centrifugally to necrosis. Due to the preservation of COE and the absence of markers of anaerobic metabolism, ischemia was discarded as a cause of this process [60].
The pathophysiological cascades triggered in the peri-contusional area produce profound changes in local osmolality, alter blood brain barrier (BBB) permeability and vascular autoregulation, cause microcirculatory thrombosis and increase vascular reactivity particularly to changes in CO2 [58, 59, 61–63]. All these phenomena develop into a vicious circle of edema, increased ICP, and decreased CBF culminating in energy failure and cell death [58, 59, 61–63].
CC are lesions of dynamic and expansive nature, especially in the first hours after trauma. Progression rates average 45 % [55–62]. Progression may be due to increasing the size of the initial bleeding, emergence of new sites of bleeding initially invisible in CT scan, or development of peri-contusional edema [57, 59]. Several predictors of progression have been identified, including initial contusional size, severity of injury, presence of associated acute subdural hematoma or traumatic subarachnoid hemorrhage, coagulopathy, short interval time between the injury and the CT scan, and need for surgical decompressive procedures [57].
Traditionally, local or systemic disorders of coagulation were considered the cause of the phenomenon of hemorrhagic progression of CC [59]. This paradigm has changed in the light of current knowledge [59]. A hypothesis postulates that the kinetic energy accumulated in the blood vessels as a result of the initial impact may not be enough to rupture them but may be sufficient to start anomalous signals at the molecular level culminating in endothelial dysfunction and failure of the microcirculation with progressive development of petechial hemorrhages that coalesce, increasing the size of the initial contusion [59].
Progression of CC can cause clinical deterioration and death (talk and die) [57–59, 64–66]. In this context, it is important to note that while CC can be located elsewhere, most develop in the temporal and frontal lobes where growth vectors are directed to the brain stem and can cause sudden clinical deterioration or death, sometimes without previous abnormality in the neuromonitoring (e.g., normal ICP) [22, 64, 65].
Special Problems: Bi-frontal Contusions
Bi-frontal contusions are present in about 20 % of cases of moderate TBI with CC [22, 55, 64, 65]. Because the mechanism of injury for this group of patients is frequently sagittal brain acceleration/deceleration, focal contusions involving the inferior part of the frontal lobes are the result of contact with the rough surface of the orbital roof [22, 42, 55, 56, 64, 65]. Hemorrhagic swelling of these contusions may disrupt the median forebrain bundle, gyrus rectus, and anterior hypothalamic nuclei. These structures are involved in behavior control and thus their injury almost always causes changes in personality, volition, motivation, judgment, and social interactions [42, 55, 56, 64, 65].
When these contusions swell, the brain is displaced posteriorly causing “square shift” and abrupt deterioration because of descent of the brain stem into the posterior fossa with stretching and deformity of the small perforating blood vessels of the basilar artery [22, 64, 65]. When this occurs, death is a major risk due to respiratory arrest, sudden coma, and autonomic changes [22, 54, 64, 65]. These lesions are also very often associated with disturbances of sodium and water metabolism (e.g., diabetes insipidus or SIADH) [22, 55, 56, 64, 65].
Management of patients with large bi-frontal contusions has always presented special problems for neurosurgeons. Aggressive surgical resection of these contusions may worsen the late neurological deficit and neuropsychological consequences [22, 55, 56, 64, 65, 67]. Also, surgical decompression requires bi-frontal decompressive craniotomy and cutting of the falx and sagittal sinus on the frontal cranial fossa [22, 87]. This is a major procedure, caries hemorrhage risk, and requires delayed cranioplasty 2 to 3 months later [87]. The risk of this surgical procedure must, therefore, be balanced against risk of death due to herniation.
The timing of deterioration is variable, but brain edema will usually peak around the 5th to the 10th day, with resolution of the swelling after this time [22, 55, 56, 64, 65, 67]. Therefore, most neurosurgeons advise serial observation for up to 2 weeks in an intensive care unit environment for these patients [67]. Serial CT scanning every 2 to 3 days is necessary to exclude progression of the lesions. When the patients are unable to obey commands, are very restless, or deteriorate, many experts favor the use of intracranial pressure monitoring with titrated osmotherapy [22, 55, 56, 64, 65, 67]. If this is used, then decompressive craniotomy becomes mandatory if intracranial pressure stays elevated despite medical treatment [67]. Particular problems are posed by the patient with progressive swelling, especially posterior shift of the third ventricle, but preserved capacity to obey commands. For these patients, prophylactic decompressive bi-frontal craniectomy is usually preferable to the risk of sudden death or permanent disability that can result from rapid herniation [67].
Diffuse Axonal Injury (DAI) and Moderate TBI
Classically, DAI has been considered to be a major neuropathological feature of severe, not mild or moderate TBI. Although neuropathological series that include mild and moderate TBI brains are few, these studies have shown that DAI occurs even in mild TBI, but to a lesser extent [68, 69]. Most experts now agree that almost all TBI is usually accompanied by some degree of DAI. This also provides a basis for the cumulative damage from multiple concussions, now termed “Chronic traumatic encephalopathy” (CTE) [68–70]. In support of this concept, Blatter et al. showed that moderate-mild TBI patients (GCS 15–13) demonstrated 50 ml average brain volume loss by 6 weeks using MRI imaging [71]. All these patients recovered to go to rehabilitation [71]. Another study found a mean of 33 ml volume loss on follow-up brain imaging of patients presenting with CT abnormalities and GCS 12–15 [72]. MRI tractography may provide additional useful information. There have been no evidence-based effective treatments for DAI. The management of this condition should be directed to avoid secondary insults, ICP control and when indicated decompressive bi-frontal craniectomy due to the risk of sudden death or permanent disability that can result from rapid herniation [67].
Epidural and Subdural Hematomas in Moderate TBI
Epidural hematomas (EDH) can be seen in 8–30 % of patients with moderate TBI [73–75]. Commonly, they are located in temporo-parietal regions as a result of linear skull fracture with damage of the middle meningeal artery [67]. Venous origin of EDH occurs in one-quarter of the cases [76]. EDH in the elderly is rare, due to adherence of dura to the skull. Mortality is approximates 10 % [67]. EDH represents neurosurgical emergency, as severe brain compression can develop rapidly from high-pressure arterial bleeding. All EDH of more than 30 ml should be evacuated regardless of GCS, and patients with coma and pupillary changes should undergo surgery as soon as possible [67]. Patients with GCS >8 and EDH <30 cm3, thickness <15-mm, midline shift <5 mm, and no focal deficits can be managed non-operatively with serial CT scanning and close neurological observation [67].
Acute Subdural hematomas (ASDH) have been reported in up to 30–50 % of patients with moderate TBI [77, 78]. The elderly population is particularly susceptible due to increased fragility of vessel walls, falls and greater use of antithrombotic and anticoagulants agents [67]. Compared with EDH, the degree of underlying brain damage associated with ASDH is more severe, and mortality rates are greater especially in older patients with poor initial GCS, and other associated brain or systemic injuries [67, 79, 80]. ASDH with thickness greater than 10 mm or midline shift greater than 5 mm should undergo to surgical evacuation regardless of GCS score [67]. For comatose patients with less than a 10-mm-thick lesion or less than 5 mm of midline shift, indications for surgical evacuation include a decrease of GCS by 2 or more points, ICP >20 mmHg, or asymmetric or fixed and dilated pupils [67]. Patients with less severe ASDH can be monitored clinically; after 7–10 days an ASDH may liquefy, to become drainable with burr-holes, thus avoiding the major morbidity of craniotomy [81].
Relevance of Neuroimaging in the Evaluation of Moderate TBI
CT scan is the radiological modality of choice in the acute phase of TBI, due to its wide availability and speed of acquisition [1, 9, 82–88]. It can be done repeatedly; it is cost-effective and can be performed in mechanically ventilated or monitored patients as well as in patients with pacemakers or metallic prostheses [1, 9, 82–88]. CT scan can identify the type and severity of the injury; it is very sensitive for detecting blood collections and has prognostic value [1, 9, 82].
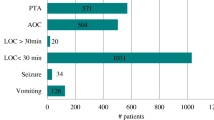
Heterogeneity characterizes the neuroimaging findings of moderate TBI (Fig. 1) [1, 13–15, 25, 28, 32, 34, 35]. Different series report an ‘‘abnormal’’ CT scan in more than half of patients with a moderate TBI [1, 13–15, 25, 28, 32, 34, 35]. Intracranial lesions (contusions, subdural hematoma and traumatic subarachnoid hemorrhage) triple the chances of a bad neurological outcome [87]. In addition approximately, one-third of these lesions progress and contribute to neurological deterioration [14, 15, 35]. Therefore, the CT scan is an essential tool in assessing extent, location, pathophysiology, severity and evolution of injury, and the information it provides is crucial to guide therapeutic decisions [82–88]. Traditionally, Marshall’s classification has been used in most clinical trials involving moderate or severe TBI [83]. This classification relies on the lesion volume, state of basal cisterns, degree of midline shift and whether or not blood collections have been surgically evacuated (Fig. 2). It helps in predicting the neurological outcome and the likelihood of developing intracranial hypertension [83]. Other scales (like the Rotterdam scale) have been proposed, but are used much less frequently and have not been extensively validated [86, 87].
Tomographic classification system of severe TBI. (I) Diffuse injury type I (normal); (II) Diffuse injury II (open basal cisterns, midline centered and small lesions <25 cc); (III) Diffuse injury III (absent basal cisterns); (IV) Diffuse injury IV (midline shift >5 mm without focal lesion >25 cc); Non-evacuated Mass: intra or extra-axial lesions >25 cc not surgically evacuated. Evacuated mass lesion. The image shows incidence of intracranial hypertension according to injury and associated outcomes in a series of 96 patients. Modified with author permission from [104]
The CT scan findings of our illustrative case are shown in Fig. 3 a. Bilateral fronto-basal contusions less than 25 cc were found together with cisternal effacement and traumatic subarachnoid hemorrhage. The CT scan was classified as a diffuse injury type III according to Marshall Classification [83]. These patients have a 63 % probability of developing intracranial hypertension and 55.6 % risk of bad outcome [83]. The probability of death or poor outcome at 6 months using the IMPACT prognostic calculator (www.tbi-impact.org), was 27 and 36 %, respectively. The neurosurgeon on call decided upon a conservative approach with ICP monitoring and serial CT scans to guide treatment.
Illustrative case. A 26-year-old male admitted to our institution with a GCS score of 12 (E3, V3, M6) without any pupillary asymmetry, focal neurological signs or abnormal motor response. a Left On the left, CT scan on admission showed bilateral frontobasal hemorrhagic contusions with a total volume <25 cc. Severe effacement of the basal cisterns. Patient was classified as a Diffuse injury III in the Marshall’s classification. On the right is shown the control CT scan 24 h after admission when ICP was 45 mmHg. Peri-contusional edema has increased significantly. b Left Because non-response to first level therapies to control intracranial hypertension bifrontal decompressive craniectomy was performed. Right Evolutive CT scan at 6th day after surgery
Is There a Role for ICP Monitoring in Moderate TBI?
The Brain Trauma Foundation guidelines recommend ICP monitoring for the management of severe TBI (i.e., GCS 3–8) with abnormal CT scan (Class II recommendation) and in select cases with normal CT scan (Class III recommendation) [9]. No specific recommendations for ICP monitoring are provided for moderate TBI cases. In practice ICP is monitored in a minority of patients with moderate TBI (8–20 % in different series) and high ICP has been reported in close of 50 % of these monitored cases [15, 25, 27, 28, 35] (Table 2).
In our institutions, we consider ICP monitoring in moderate TBI patients who fulfill one or more of the following criteria:
-
a)
Postoperative period after removal of acute subdural hematoma or multiple CC. In these cases, sudden changes in ICP could signal hemorrhages due to decompression or reperfusion, new extra-axial collections or worsening brain swelling [9].
-
b)
GCS of 9–11 and CC (temporal or bifrontal) without surgical intervention. In these instances, ICP monitoring can help recognize progression of the contusions [9, 64–66].
-
c)
Diffuse injury type III. Due to the high probability of intracranial hypertension and poor outcome, ICP monitoring in these cases is indispensable [42, 83].
-
d)
General anesthesia for emergency non-cranial surgery. Especially in the presence of large mass lesions under conservative treatment (epidural, subdural hematomas, CC) [9]. ICP monitoring is indicated in these cases due to loss of clinical evaluation and to evaluate effects of anesthetics on cerebrovascular autoregulation [9].
-
e)
Concomitant severe chest trauma requiring deep sedation, high PEEP levels, recruitment maneuvers or prone ventilation [88, 89]. In these cases, monitoring is important because utilization of protective ventilation with low tidal volumes can cause hypercapnia, cerebral vasodilation and increased ICP [88, 89]. Utilization of high levels of PEEP may worsen intracranial hypertension by hindering adequate cerebral venous return [88, 89].
-
f)
Concomitant intra-abdominal compartment syndrome. This syndrome is associated with intracranial hypertension [90].
-
g)
Prolonged traumatic shock. These patients have increased risk of cerebral edema.
Talk and Die Patients
The term ‘‘talk and die,’’ was first used by Reilly in 1975 to describe a subset of patients with head injuries who presented with a verbal component of the GCS score of three or higher and who, given their clinical presentation, were thought to have sustained a survivable head injury, but subsequently deteriorated and died due to potentially reversible causes [91]. Most of these patients are included in the moderate TBI category [16–22].
Since that time, the understanding of the pathophysiology and treatment of head injuries has evolved, and different studies have analyzed the incidence and potential causative factors of ‘‘talk and die’’ [16, 20, 92–94]. Age, GCS, CT scan findings, delays in obtaining neuroimages and performing definitive surgical treatment, coagulopathy, thrombocytopenia, and severity of injury are among these factors [20, 92–95].
Reilly et al. made a description of 66 patients with TBI who had talked at some time after the injury but then expired [91]. 25 % of these patients did not have intracranial hematoma at necropsy. Most of these patients had raised ICP, and the most common reason was peri-contusional swelling [91]. Almost half of the non-hematoma cases had ischemic or hypoxic brain damage. Fatality without intracranial hypertension was most often due to meningitis [91]. The authors concluded that morbidity and mortality in these patients might be reduced by early diagnosis and more aggressive treatment of raised ICP [91].
More recent studies have shown that patients who “talk and die” are most frequently adult men and the most common mechanisms of trauma are falls, motor vehicle accidents and violence [18, 91]. In these studies, the average GCS at admission to emergency department was 14 and the most frequent intracranial injuries were acute subdural hematoma, diffuse cerebral edema and CC [16–22, 92–95]. In about 14 % of these patients with GCS 13 at admission, initial CT was normal but became abnormal during hospitalization, especially because of development of diffuse cerebral edema [91].
Most studies highlight that delay in the care of these patients contributed to death [16–22, 91–95]. Among the most important factors are: delays in diagnosis of lesion through CT scan, delays in the transfer to a specialized center, failure to identify risk factors for deterioration, inadequate prevention of secondary injury, inappropriate correction of underlying coagulopathy and loss of the opportunity for definitive neurosurgical treatment [21].
Management Strategies
In the emergency room, we should emphasize the ABC of resuscitation and ATLS guidelines [96] for the assessment of associated injuries, and preventing or aggressively correcting secondary insults, such as hypoxia and hypotension [1, 9, 10, 96–98]. The GCS score should be determined and a head CT scan should be obtained. Multidisciplinary consultation should be guided by the accompanying injuries with urgent triage of injuries requiring surgery. Patients should be admitted to the intensive care unit and followed with serial physical examinations and also CT scans when necessary (especially in type II and III diffuse lesions and in cases with sizable contusions). Transcranial Doppler (TCD) can be useful during initial valoration [99, 100]. Establishing algorithms and protocols for evaluation and treatment is strongly advisable. Invasive ICP monitoring should be considered for selected cases as previously discussed. Patients with diffuse injury type III should be treated as a severe TBI, and consequently we recommend sedation, analgesia, mechanical ventilation, physiological neuroprotection, anticonvulsant prophylaxis and an algorithm to manage intracranial hypertension [1, 9, 10].
Guidelines for surgical treatment are not firmly established [67]. The surgical decision must be supported by pathophysiological reasoning and findings from neuroimaging and neuromonitoring. Space occupying lesions that cause neurological deterioration, intracranial hypertension refractory to medical treatment and radiological signs of mass effect (midline shift, cisternal effacement or compression) are associated with poor outcome if not surgically evacuated [67]. Recommendations for the surgical treatment of traumatic parenchymal lesions have been proposed; however these are based on a low level of evidence [67]. In Fig. 4, we depicted an algorithm for Moderate TBI management.
Our patient was admitted to the neurointensive care unit and was intubated, sedated and mechanically ventilated. Neurosurgery was consulted and an intraparenchymal ICP monitor was inserted. The initial ICP was 63 mmHg with a good response to first level therapeutic measures (osmotherapy and mild hyperventilation). Hours later, the patient required gradual increase in the level of therapeutic intensity. ICP became refractory to first level therapies 56 h after admission. A new CT scan showed an increase in the peri-contusional edema and effacement of the basal cisterns (Fig. 3 b). Bi-frontal decompressive craniectomy was performed (Fig. 3 c and d). ICP normalized after surgery, allowing gradual reduction of the medical measures used for ICP control. Five days after surgery, ICP monitoring was discontinued. Mechanical ventilation was withdrawn 7 days after injury. The patient recovered gradually with no residual focal neurologic deficits. He was discharged from the neurointensive care unit on day 13 with a GCS score of 15. He was discharged home 3 weeks after injury. A titanium cranioplasty was performed 1 month later.
Prognosis and Outcome
The reported mortality rates in moderate TBI are around 15 % [15, 35]. Approximately 75 % of these deaths occur in individuals with GCS score 9 or 10, whereas the group with GCS score ranging between 11 and 13 has a more benign clinical course and better outcomes [13, 14]. Furthermore, over 50 % of the survivors demonstrate psychological and neurocognitive deficits highlighted by disturbances in personality, behavior, chronic headaches, and memory disorders. These disturbances compromise daily life activities and impede or delay reintegration to social and work environments [13–15, 25, 26, 28, 29, 34, 35]. Elderly and pediatric patients are most susceptible populations to these sequelea [13–15, 25, 26, 28, 34, 35].
Multiple and varied predictors of poor outcome have been identified in moderate TBI. In one study, age over 45 years, delayed initiation of enteral feeding and pneumonia were associated with greater length of stay and with cognitive-behavioral and functional long-term disability [26]. In another study with prospective evaluation at 6 months, poor outcome was associated basal skull fracture, traumatic subarachnoid hemorrhage, subdural hematoma, coagulopathy, lower GCS score and lesion type on CT scan according to a European modification of Marshall’s classification [34]. In a prospective multicenter Italian study of 315 patients with moderate TBI, the factors associated with poor outcome were early seizures and medical complications in the group of patients with GCS 11–13 and a score ≤4 points in motor component of GCS among patients with GCS 9–10 [35].
Prognostic models such as IMPACT or CRASH have been extensively validated and apply to patients with moderate TBI. The variables included in the calculators are clinical (age, GCS, pupil status, presence of hypoxia and hypotension), CT scan findings and certain laboratory parameters such as glucose and hemoglobin levels [101, 102]. The CRASH score also incorporates adjustment according to the specific country where the patient resides [101].
Certain biomarkers such as S100β protein, increase in blood and cerebrospinal fluid in response to injury and may have prognostic value [103]. Many studies have shown significant association between S100β levels and adverse outcome in moderate and severe TBI [103].
Two months after hospital discharge, the patient underwent multidisciplinary evaluation. Although he had a normal neurological examination, neuropsychological tests showed bradyphrenia, attention and memory deficits, and flat emotional response with abnormal social behavior.
Refining the Categorization of TBI Severity: A Proposal for a New Classification Scheme
It is clear that a highly variable and heterogeneous cohort of patients is included within the category of moderate TBI. Within this spectrum, there are patients (especially those with lower levels of alertness) who have a high possibility of harboring or developing intracranial lesions that may cause serious and potentially life-threatening consequences. Thus, although most individuals survive the initial injury, the risks in these cases should not be underestimated. The death rate can reach 15 %, and the likelihood of neurological disability is considerable.
Applying the term ‘‘moderate’’ to characterize patients with TBI who have alterations in the level of consciousness, but are not comatose, may be deceiving by conveying the impression that these injuries are not that serious and therefore do not need aggressive therapy. In fact, available evidence indicates that individuals with GCS scores of 9 and 10 share pathophysiology, lesion types, and prognosis similar to those with severe TBI [1, 2, 12–14] [35]. Meanwhile, patients with GCS 11–13 can also have substantial risk of subsequent neurological deterioration, particularly if the initial CT scan is abnormal. Therefore, we propose revisiting the classification of TBI by incorporating patients with GCS 9–10 and abnormal CT scan into the severe TBI category and using the term ‘‘potentially severe TBI” to classify patients with GCS 9–10 and normal CT scan and those with GCS 11–13 with abnormal CT scan. The purpose of this reclassification and terminology change is to increase the sensitivity of the ‘’alarm signal’’ to avoid underestimating the severity of non-comatose but high risk patients Fig. 5.
References
Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–41.
Zammit C, Knight WA. Severe traumatic brain injury in adults. Emerg Med Pract. 2013;15:1–28.
Humphreys I, Wood RL, Phillips CJ, Macey S. The costs of traumatic brain injury: a literature review. Clinicoecon Outcomes Res. 2013;26(5):281–7.
New South Wales Motor Accident Authority Guidelines for mild traumatic brain injury following closed head injury. Sydney, Australia: New South Wales Motor Accident Authority; 2008.
Defense and Veterans Brain Injury Center Updated mTBI clinical guidance. Washington, DC: Defense and Veterans Brain Injury Center; 2008. www.dvbic.org/pdfs/mTBI_recs_for_CONUS.pdf. Accessed 2012 Jan 20.
MTBI Guidelines Development Team Guidelines for mild traumatic brain injury and persistent symptoms. Toronto, ON: Ontario Neurotrauma Foundation; 2010. www.onf.org/documents/Guidelines%20for%20Mild%20Traumatic%20Brain%20Injury%20and%20Persistent%20Symptoms.pdf. Accessed 2012 Jan 25.
The Management of Concussion/mTBI Working Group VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. Washington, DC: Department of Veterans Affairs and Department of Defense; 2009.
Marshall S, Bayley M, McCullagh S, Velikonja D, Berrigan L. Clinical practice guidelines for mild traumatic brain injury and persistent symptoms. Can Fam Physician. 2012;58(257–67):e128–40.
Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(Suppl 1):S1–106.
Maas AI, Dearden M, Teasdale GM, Braakman R, Cohadon F, Iannotti F, et al. EBIC-guidelines for management of severe head injury in adults. European Brain Injury Consortium. Acta Neurochir (Wien). 1997;139:286–94.
Frattalone AR, Ling GS. Moderate and severe traumatic brain injury: pathophysiology and management. Neurosurg Clin N Am. 2013;24:309–19.
Colohan AR, Oyesiku NM. Moderate head injury: an overview. J Neurotrauma. 1992;9(Suppl 1):S259–64.
Timmonds SD, Winestone JS. Moderate brain injury. In: Jallo J, Lotus C, editors. Neurotrauma and critical care. Stuttgart: Thieme Medical; 2009. p. 208–19.
Andriessen TM, Horn J, Franschman G, van der Naalt J, Haitsma I, Jacobs B, et al. Epidemiology, severity classification, and outcome of moderate and severe traumatic brain injury: a prospective multicenter study. J Neurotrauma. 2011;28:2019–31.
Reilly PL. Brain injury: the pathophysiology of the first hours. Talk and die revisited. J Clin Neurosci. 2001;8:398–403.
Lobato RD, Rivas JJ, Gomez PA, Castañeda M, Cañizal JM, Sarabia R, et al. Head-injured patients who talk and deteriorate into coma. Analysis of 211 cases studied with computerized tomography. J Neurosurg. 1991;75:256–61.
Ratanalert S, Chompikul J, Hirunpat S. Talked and deteriorated head injury patients: how many poor outcomes can be avoided? J Clin Neurosci. 2002;9:640–3.
Rockswold GL, Pheley PJ. Patients who talk and deteriorate. Ann Emerg Med. 1993;22:1004–7.
Dunn LT, Fitzpatrick MO, Beard D, Henry JM. Patients with a head injury who talk and die in the 1990s. J Trauma. 2003;54:497–502.
Tan JE, Ng I, Lim J, Wong HB, Yeo TT. Patients who talk and deteriorate: a new look at an old problem. Ann Acad Med Singap. 2004;33:489–93.
Petersen EC, Chesnut RM. Talk and die revisited: bifrontal contusions and late deterioration. J Trauma. 2011;71:1588–92.
Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–4.
Ko DY. Clinical evaluation of patients with head trauma. Neuroimag Clin N Am. 2002;12:165–74.
Fearnside M, McDougall P. Moderate head injury: a system of neurotrauma care. Aust NZJ Surg. 1998;68:58–64.
Vitaz TW, Jenks J, Raque GH, Shields CB. Outcome following moderate traumatic brain injury. Surg Neurol. 2003;60:285–91 discussion 291.
Annegers JF, Grabow JD, Kurland LT, Laws ER Jr. The incidence, causes, and secuelar trends of head trauma in Olmsted County, Minnesota, 1935-1974. Neurology. 1980;30:912–9.
Rimel RW, Giordani B, Barth JT, Jane JA. Moderate head injury: completing the clinical spectrum of brain trauma. Neurosurgery. 1982;11:344–51.
Tabaddor K, Mattis S, Zazula T. Cognitive sequelae and recovery course after moderate and severe head injury. Neurosurgery. 1984;14:701–8.
Kraus JF, Black MA, Hessol N, Ley P, Rokaw W, Sullivan C, et al. The incidence of acute brain injury and serious impairment in a defined population. Am J Epidemiol. 1984;119:186–201.
Levin HS, Goldstein FC, High WM Jr, Eisenberg HM. Disproportionately severe memory deficit in relation to normal intellectual functioning after closed head injury. J Neurol Neurosurg Psychiatry. 1988;51:1294–301.
Stein SC, Ross SE. Moderate head injury. A guide to initial management. J Neurosurg. 1992;77:562–4.
Stein SC. Minor head injury: 13 is unlucky number. J Trauma. 2001;50:759–60.
Fabbri A, Servadei F, Marchesini G, Stein SC, Vandelli A. Early predictors of unfavourable outcome in subjects with moderate head injury in the emergency department. J Neurol Neurosurg Psychiatry. 2008;79:567–73.
Compagnone C, d’Avella D, Servadei F, Angileri FF, Brambilla G, Conti C, et al. Patients with moderate head injury: a prospective multicenter study of 315 patients. Neurosurgery. 2009;64:690–6 discussion 696–7.
Teasdale G, Maas A, Lecky F, Manley G, Stocchetti N, Murray G. The Glasgow Coma Scale at 40 years: standing the test of time. Lancet Neurol. 2014;13:844–54.
Stocchetti N, Pagan F, Calappi E, Canavesi K, Beretta L, Citerio G, et al. Inaccurate early assessment of neurological severity in head injury. J Neurotrauma. 2004;21:1131–40.
Stuke L, Diaz-Arrastia R, Gentilello LM, Shafi S. Effect of alcohol on Glasgow. Coma Scale in head-injured patients. Ann Surg. 2007;245:651–5.
Rundhaug NP, Moen KG, Skandsen T, Schirmer-Mikalsen K, Lund SB, Hara S, Vik A. Moderate and severe traumatic brain injury: effect of blood alcohol concentration on Glasgow Coma Scale score and relation to computed tomography findings. J Neurosurg. 2015;122:211–8.
Wijdicks EF, Bamlet WR, Maramattom BV, Manno EM, McClelland RL. Validation of a new coma scale: the FOUR score. Ann Neurol. 2005;58:585–93.
McMahon CG, Yates DW, Campbell FM, Hollis S, Woodford M. Unexpected contribution of moderate traumatic brain injury to death after major trauma. J Trauma. 1999;47:891–5.
Gennarelli TA. Mechanisms of brain injury. J Emerg Med. 1993;11(Suppl 1):5–11.
Sahuquillo J, Poca MA. Diffuse axonal injury after head trauma. A review. In: Pickard J, Dolenc VV, Lobo-Antunes J, Reulen HJ, Sindou M, Strong AJ, et al., editors. Advances and technical standards in neurosurgery, vol. 27. Wien: Springer; 2002. p. 23–86.
Abdel-Dayem HM, Abu-Judeh H, Kumar M, Atay S, Naddaf S, El-Zeftawy H, Luo JQ. SPECT brain perfusion abnormalities in mild or moderate traumatic brain injury. Clin Nucl Med. 1998;23:309–17.
Chesnut RM, Marshall LF, Klauber MR, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34:216.
Manley G, Knudson MM, Morabito D, et al. Hypotension, hypoxia, and head injury: frequency, duration, and consequences. Arch Surg. 2001;136:1118.
Marmarou A, Anderson L, Ward J, et al. Impact of ICP instability and hypotension on outcome in patients with severe head trauma. J Neurosurg. 1991;75:159.
McHugh GS, Engel DC, Butcher I, et al. Prognostic value of secondary insults in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24:287.
Graham DI, Adams JH, Doyle D. Ischemic brain damage in fatal non-missile head injuries. J Neurol Sci. 1978;39:213–34.
Zauner A, Daugherty WP, Bullock MR, et al. Brain oxygenation and energy metabolism: part I-biological function and pathophysiology. Neurosurgery. 2002;51:289–301.
Poca MA, Sahuquillo J, Mena MP, Vilalta A, Rivero M. Actualizaciones en los métodos de monitorización cerebral regional en los pacientes neurocríticos: presión tisular de oxigeno, microdiálisis cerebral y técnica de espectroscopia por infrarrojos. Neurocirugia. 2005;16:385–410.
Marin-Caballos AJ, Murillo-Cabezas F, Dominguez-Roldan JM, Leal-Noval SR, Rincon-Ferrari MD, Muñoz-Sanchez MA. Monitorizacion de la presión tisular de oxigeno (PtiO2) en la hipoxia cerebral: aproximación diagnostica y terapeutica. Med Intensiva. 2008;32:81–90.
Coles JP, Fryer TD, Smielewski PS, et al. Incidence and mechanisms of cerebral ischemia in early clinical head injury. J Cereb Blood Flow Metab. 2004;24:202–11.
Menon DK, Coles JP, Gupta AK, Fryer TD, Smielewski P, Chatfield DA, Aigbirhio F, et al. Diffusion limited oxygen delivery following head injury. Crit Care Med. 2004;32:1384–90.
Cooper PR. Post-traumatic intracranial mass lesions. In: Cooper PR, editor. Head injury. 3rd ed. Baltimore: Williams & Wilkins; 1993. p. 275–331.
Houseman C, Belverud S, Narayan R. Closed head injury. In: Ellenbogen R, Abdulrauf S, editors. Principles of neurological surgery. Philadelphia: Saunders Elsevier; 2012. p. 325–47.
Alahmadi H, Vachhrajani S, Cusimano MD. The natural history of brain contusion: an analysis of radiological and clinical progression. J Neurosurg. 2010;112:1139–45.
Ragaisis V. Brain contusion: morphology, pathogenesis and treatment. Medicina 2002;38:243–9. (http//www.medicina.kmu.lt).
Kurland D, Hong C, Aarabi B, Gerzanich V, Simard M. Hemorrhagic progression of a contusion after traumatic brain injury: a review. J Neurorauma. 2012;29:19–31.
Wu HM, Huang SC, Vespa P, Hovda DA, Bergsneider M. Redefining the pericontusional penumbra following traumatic brain injury: evidence od deteriorating metabolic derangements based on positron emission tomography. J Neurotrauma. 2013;30:352–60.
Kawamata T, Mori T, Sato S, Katayama Y. Tissue hyperosmolality and brain edema in cerebral contusion. Neurosurg Focus. 2007;22(5):E5 1–7.
McLaughlin MR, Marion DW. Cerebral blood flow and vasoresponsivity within and around cerebral contusions. J Neurosurg. 1996;85:871–6.
Soustiel JF, Mahamid E, Goldsher D, Zaaroor M. Perfusion-CT for early assessment of traumatic cerebral contusions. Neuroradiology. 2008;50:189–96.
Andrews BT, Chiles BW, Olsen WL, Pitts LH. The effect of intracerebral hematoma location on the risk of brainstem compression and on clinical outcome. J Neurosurg. 1988;69:518–22.
Lee TT, Villanueva PA. Orbital-frontal delayed hemorrhagic contusions: clinical course and neurosurgical treatment protocol. Surg Neurol. 1997;48:333–7.
Saatman KE, Duhaime AC, Bullock R, Maas AIR, Valadka A, Manley GT, Workshop Scientific Team and Advisory Panel Members. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25:719–38.
Bullock RM, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, et al. Surgical management of traumatic parenchymal lesions. Neurosurgery. 2006;58:S2-25–46.
Maxwell WL, MacKinnon MA, Stewart JE, et al. Stereology of cerebral cortex after traumatic brain injury matched to the glasgow outcome score. Brain. 2010;133:139–60.
Sanjith S. Traumatic axonal injury in mild to moderate head injury—an illustrated review. Indian J Neurotrauma. 2011;8:71–6.
Omalu B, Bailes J, Hamilton RL, Kamboh MI, Hammers J, Case M, Fitzsimmons R. Emerging hystomorphologic phenotypes of chronic traumatic encephalopathy in American athletes. Neurosurgery. 2011;69:173–83.
Blatter DD, Gale SD, et al. Magnetic Resonance based brain and CSF measurement after traumatic brain injury: correlation with neuropsychological outcome. AJNR. 1997;18:1–10.
MacKenzie JD, Siddiqui F, Babb JS, et al. Brain atrophy in mild or moderate traumatic brain injury: a longitudinal quantitative analysis. AJNR. 2002;23:1509–15.
Chen H, Guo Y, Chen SW, et al. progressive epidural hematoma in patients with head trauma: incidence, outcome, and risk factors. Emerg Med Int., 2012. doi:10.1155/2012/134905.
Jamjoom A, Cummins B, Jamjoom ZA. Clinical characteristics of traumatic extradural hematoma: a comparison between children and adults. Neurosurg Rev. 1994;17:277–81.
Cheung PS, Lam JM, Yeung JH, Graham CA, Rainer TH. Outcome of traumatic extradural haematoma in Hong Kong. Injury. 2007;38:76–80.
Yilmazlar S, Kocaeli H, Dogan S, Abas F, Aksoy K, Korfali E, Doygun M. Traumatic epidural haematomas of nonarterial origin: analysis of 30 consecutive cases. Acta Neurochir (Wien). 2005;147:1241–8.
Walcott BP, Khanna A, Kwon CS, Phillips HW, Nahed BV, Coumans JV. Time interval to surgery and outcomes following the surgical treatment of acute traumatic subdural hematoma. J Clin Neurosci. 2014;21:2107–11.
Ryan CG, Thompson RE, Temkin NR, Crane PK, Ellenbogen RG, Elmore JG. Acute traumatic subdural hematoma: current mortality and functional outcomes in adult patients at a Level I trauma center. J Trauma Acute Care Surg. 2012;73:1348–54.
Wilberger JE Jr, Harris M, Diamond DL. Acute subdural hematoma: morbidity, mortality, and operative timing. J Neurosurg. 1991;74:212–8.
Grandhi R, Bonfield CM, Newman WC, Okonkwo DO. Surgical management of traumatic brain injury: a review of guidelines, pathophysiology, neurophysiology, outcomes, and controversies. J Neurosurg Sci. 2014;58:249–59.
Mathew P, Oluoch-Olunya DL, Condon BR, Bullock R. Management of the conscious patient with acute subdural hematoma: outcome with initial conservative treatment. Acta Neurochir (Wien). 1993;121:100–8.
Eisenberg HM, Gary HE Jr, Aldrich EF, Saydjari C, Turner B, Foulkes MA, Jane JA, Marmarou A, Marshall LF, Young HF. Initial CT findings in 753 patients with severe head injury: a report from the NIH traumatic coma data bank. J Neurosurg. 1990;73:688–98.
Marshall LF, Marshall SB, Klauber MR, van Berkum Clark M. A new classification of head injury based on computerized tomography. J Neurosurg. 1991;75(Suppl):S14–20.
Narayan RK, Greenberg RP, Miller JD, Enas GG, Choi SC, Kishore PR, Selhorst JB, Lutz HA III, Becker DP. Improved confidence of outcome prediction in severe head injury: a comparative analysis of the clinical examination, multimodality evoked potentials, CT scanning, and intracranial pressure. J Neurosurg. 1981;54:751–62.
Servadei FD, Murray GD, Key P, Teasdale GM, Dearden M, Iannotti F, The European Brain Injury Consortium, et al. The value of the “worst” computed tomographic scan in clinical studies of moderate and severe head injury. Neurosurgery. 2000;46:70–5.
Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57:1173–82 discussion 1173–82.
Chieragato A, Fainardi E, Morselli-Labate AM, Antonelli V, Compagnone C, Targa L, Kraus J, Servadei F. Factors associated with neurological outcome and lesion progression in traumatic subarachnoid hemorrhage patients. Neurosurgery. 2005;56:671–80.
Quílez ME, López-Aguilar J, Blanch L. Organ crosstalk during acute lung injury, acute respiratory distress syndrome, and mechanical ventilation. Curr Opin Crit Care. 2012;18:23–8.
Mazzeo AT, Fanelli V, Mascia L. Brain-lung crosstalk in critical care: how protective mechanical ventilation can affect the brain homeostasis. Minerva Anesthesiol. 2013;79:299–309.
Lauerman MH, Stein DM. Multicompartment management of patients with severe traumatic brain injury. Curr Opin Anaesthesiol. 2014;27:219–24.
Reilly PL, Graham DI, Adams JH, Jennett B. Patients with head injury who talk and die. Lancet. 1975;2:375–7.
Goldschlager T, Rosenfeld JV, Winter CD. ‘Talk and die’ patients presenting to a major trauma centre over a 10 year period: a critical review. J Clin Neurosci. 2007;14:618–23.
Marshall LF, Toole BM, Bowers SA. The national traumatic coma data bank. Part 2: patients who talk and deteriorate: implications for treatment. J Neurosurg. 1983;59:285–8.
Rockswold GL, Leonard PR, Nagib MG. Analysis of management in thirty-three closed head injury patients who ‘‘talked and deteriorated’’. Neurosurgery. 1987;21:51–5.
Davis DP, Kene M, Vilke GM, et al. Head-injured patients who ‘‘talk and die’’: the San Diego perspective. J Trauma. 2007;62:277–81.
ATLS®. Advanced Trauma Life Support. American College of Surgeons. 8th edition. Chicago. 2008.
Godoy DA, Piñero GR, Videtta W. Injuria cerebral aguda. Abordaje diagnostico-terapéutico inicial. En Protocolos en Emergencias y Urgencias. Lizardi Pedro. Editorial Manual Moderno. México, 2010, capitulo 3, pp. 15–20.
Harris T, Davenport R, Hurst T, Jones J. Improving outcome in severe trauma: trauma systems and initial management: intubation, ventilation and resuscitation. Postgrad Med J. 2012;88:588–94.
Bouzat P, Francony G, Declety P, Genty C, Kaddour A, Bessou P, et al. Transcranial Doppler to screen on admission patients with mild to moderate traumatic brain injury. Neurosurgery. 2011;68:1603–10.
Jaffres P, Brun J, Declety P, Bosson JL, Fauvage B, Schleiermacher A, et al. Transcranial Doppler to detect on admission patients at risk for neurological deterioration following mild and moderate brain trauma. Intensive Care Med. 2005;31:785–90.
Roozenbeek B, Lingsma HF, Lecky FE, Lu J, Weir J, Butcher I, McHugh GS, Murray GD, Perel P, Maas AI, Steyerberg EW, International Mission on Prognosis Analysis of Clinical Trials in Traumatic Brain Injury (IMPACT) Study Group, Corticosteroid Randomisation After Significant Head Injury (CRASH) Trial Collaborators, Trauma Audit and Research Network (TARN). Prediction of outcome after moderate and severe traumatic brain injury: external validation of the International Mission on Prognosis and Analysis of Clinical Trials (IMPACT) and corticoid randomisation after significant head injury (CRASH) prognostic models. Crit Care Med. 2012;40:1609–17.
Lingsma H, Andriessen TM, Haitsema I, Horn J, van der Naalt J, Franschman G, Maas AI, Vos PE, Steyerberg EW. Prognosis in moderate and severe traumatic brain injury: external validation of the IMPACT models and the role of extracranial injuries. J Trauma Acute Care Surg. 2013;74:639–46.
Mercier E, Boutin A, Lauzier F, Fergusson DA, Simard JF, Zarychanski R, et al. Predictive value of S-100β protein for prognosis in patients with moderate and severe traumatic brain injury: systematic review and meta-analysis. BMJ. 2013;4(346):f1757.
Poca MA, Sauquillo J, Baguena M, et al. Incidence of intracranial hypertension after severe head injury: a prospective study using the Traumatic Coma Data Bank classification. Acta Neurochir Suppl (Wien). 1998;71:27–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None for all authors.
Rights and permissions
About this article
Cite this article
Godoy, D.A., Rubiano, A., Rabinstein, A.A. et al. Moderate Traumatic Brain Injury: The Grey Zone of Neurotrauma. Neurocrit Care 25, 306–319 (2016). https://doi.org/10.1007/s12028-016-0253-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-016-0253-y