Abstract
Background
The pineal region and dorsal midbrain are among the most challenging surgical targets. To approach lesions in this region that harbor a superior to inferior long axis, we describe the basic steps of the precuneal, interhemispheric, trans-tentorial approach and illustrate anatomical landmarks of this established, but not so popular, surgical trajectory.
Method
To study the anatomical landmarks and safety of this approach, the neurovascular anatomy was studied on 22 sides of 11 formalin-fixed latex-injected anatomical specimens. A step-by-step dissection of the precuneal interhemispheric trans-tentorial approach and study of the key anatomical landmarks was performed. An illustrative clinical case of a pontomesencephalic cavernous malformation (CM) resected through this approach is also detailed.
Results
The mean distance from the transverse sinus to the most posterior cortical vein draining into the superior sagittal sinus was 6.4 cm. The mean distance from the calcarine sulcus to the most posterior cortical vein was 5.3 cm. Key steps of the dissection are as follows: craniotomy exposing the posterior aspect of the superior sagittal sinus (SSS), durotomy and gentle retraction of the SSS edge, dissection of the interhemispheric fissure, linear incision of the tentorium that extends anteriorly to the incisura and lateral reflection of the tentorium, and arachnoidal dissection and exposure of the cerebellomesencephalic fissure.
Conclusion
The precuneal, interhemispheric, trans-tentorial approach affords excellent access to the falcotentorial junction, splenium, pineal region, quadrigeminal cistern, and dorsal pons once the cerebellomesencephalic fissure has been dissected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pineal region and dorsal midbrain (especially the area tucked within the cerebello-mesencephalic fissure) are among the most challenging surgical targets. Numerous approaches have been described to these regions including the supracerebellar infratentorial variants, occipital transtentorial approach (Poppen’s approach), and, to the dorsal pons, a telovelar or transvermian approach through the floor of the fourth ventricle [8, 12, 14, 15, 19, 21]. However, these approaches have restricted angles for resection of lesions with a craniocaudal long axis.
Recent publications have advocated for the use of a parietal, interhemispheric, transtentorial approach [10, 11, 21]. Notably, these reports only considered lesions of the superior vermis and falcotentorial junction. Despite rigid anatomical descriptions, several cavernous malformations (CMs) of the brainstem span more than one anatomical compartment. Specifically, some extend from the mid/inferior mesencephalon to the upper pons. In these cases, it is challenging to obtain an ideal view of the most inferior and medial portion of the CM. A posterior interparietal “precuneal” approach affords an unparalleled superior to inferior trajectory to the dorsal midbrain and upper pons. To study the efficacy of this approach to reach brainstem lesions and illustrate critical points, we provide a step-by-step anatomical dissection coupled with an illustrative case of a pontomesencephalic CM removed by the precuneal, interhemispheric, trans-tentorial approach (PCIT).
Method
All aspects of this study were approved by the Institutional Review Board and Biospecimens Committee (17–005898), as required by standard protocols.
The neurovascular anatomy was studied on 11 specimens and 22 hemispheres. With a flexible ruler, the distances from the top of the transverse sinus in the sagittal midline to the most posterior cortical vein and from the calcarine sulcus to the most posterior cortical vein were measured. Furthermore, a step-by-step dissection of the PCIT was performed in a formalin-fixed and latex-injected specimen under microscopic magnification to highlight important steps and identify anatomical limitations. To better detail the neurovascular anatomy, endoscopic pictures were taken with a 0-degree scope.
The anatomical study is supplemented by an illustrative clinical case to discuss some of the practical surgical pitfalls.
Results
Measurements
The mean distance from the transverse sinus to the most posterior cortical vein was 6.4 cm (3.5–9.2). The mean distance from the calcarine sulcus to the most posterior cortical vein was 5.3 cm (1.7–8.5). The mean distance from the torcula to the calcarine sulcus was 1.1 cm (0.3–2.1).
Step-by-step dissection
Patient positioning
The specimen head was positioned simulating the modified park bench position with the hypothetical side of the lesion down.
Skin incision
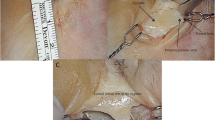
Different types of skin incisions can be used based on surgeon preference. However, for the purpose of this dissection and to adequately illustrate the anatomical structures involved, we chose a large, horse-shoe flap based on the transverse sinus. The incision extended medially just past the inion and laterally, 3 cm posterior to the posterior limit of the pinna. Superiorly, the incision was taken to the level of the lambda (Fig. 1a and 1b).
Macroscopic photos of positioning, skin incision, craniotomy, dural opening, and tentorial incision. a Positioning in the park bench position with the right side down. b Horseshoe skin incision based on the transverse sinus. c Inferior burr hole on the torcula and superior burr hole on the SSS. d Craniotomy extending lateral to the SSS. e Dural exposure after craniotomy. f View of the occipital lobe after cruciate durotomy. g The interhemispheric fissure with a view of the falcotentorial junction. The calcarine sulcus is marked with the green dashed line. h The tentorial incision inferior to the straight sinus extending anteriorly to the tentorial incisura
Craniotomy
Two burr holes were placed on the sinus: one on the inion to expose the torcula and one 7 cm above on the lambdoid suture. These burr holes allow for a rectangular bone flap, with its long axis on the superior sagittal sinus (SSS), to be lifted. Of note, the dura was separated from the inner table of the calvarium with a Penfield to avoid an incidental durotomy or injury to the SSS (Fig. 1c–1e).
Dural opening
To optimize illustration of the surgical anatomy in the cadaver, a cruciate dural opening is utilized in the specimen. A curvilinear flap based on the SSS provides protection for the SSS when the dura is reflected medially and facilitates its gentle retraction to expose the interhemispheric fissure. If the transverse sinus and the SSS are exposed, a cruciate durotomy allows for a dural leaflet to be reflected over the SSS and a dural leaflet to be reflected over the transverse sinus. The cruciate pattern of the dural opening is seen in Fig. 1f as both the transverse sinus and SSS were exposed. The sinus is covered with hemostatic material and during surgery, attention must be paid to keep the sinus moist with intermittent irrigation, and care should be taken to avoid prolonged retraction to prevent postoperative occlusion.
Interhemispheric dissection and tentorial incision
After the dura has been reflected medially, the arachnoid tethering the falcine surface of the precuneus to the SSS and falx is sharply dissected under microscopic magnification. Dissection of the interhemispheric fissure can begin approximately 6.3 cm above the torcula. Intraoperative neuronavigation is helpful at this stage to identify a preferential and ideal trajectory to the target. In order to allow for the brain to “fall down” and maximize the effect of gravitational “retraction,” arachnoid bands tethering the falcine surface of the precuneus should be widely and maximally dissected. Superior and anterior extension of the dissection may transgress bridging veins. Bridging veins on the cortical surface should be respected, and care should be made not to violate their integrity (Fig. 1g). This is often possible by “skeletonization” of the vein by freeing surrounding arachnoidal bands as the vein courses anteromedially to enter the SSS.
As the dissection proceeds, the falcotentorial junction comes into view and the posterior edge of the tentorial incisura can be easily identified. Once a safe location to incise the tentorium away from the straight sinus is found, the incision is made inferior and parallel to the straight sinus. Sharp dissection with microscissors should begin posteriorly and be carried anteriorly to the tentorial incisura. During surgery, this maneuver can be challenging due to the variable vascularization of the tentorium and its dural sinuses. Care must be exercised as to avoid transgression of the arachnoid lying on the undersurface of the tentorium. There are two methods to widen the tentorial incision: retraction using sutures or additional tentorial resection. Further dissection of the tentorium would be carried inferiorly and anteriorly as the straight sinus is superior to the incision (Fig. 1h).
Cisternal dissection
Once the tentorium is opened, the arachnoid membrane of the cerebellomesencephalic fissure comes into view. It is bounded anteriorly by the basal vein of Rosenthal and posteriorly by the cerebellar culmen. At this point, careful opening of the arachnoid drains cerebrospinal fluid (CSF), further relaxing the brain. This usually opens a wide corridor. Subsequently, the cisternal portion of the superior cerebellar artery (SCA) and its branches come into view. As the SCA and its branches supply the cerebellum, the arachnoid is dissected on their superior aspect so the vessels can be mobilized inferiorly along with the cerebellar surface. Maintaining an anteroinferior trajectory, dissection of the arachnoid tethering vessels along the cerebellomesencephalic fissure allows excellent visualization of the dorsal brainstem. Key anatomical structures in this region include the emergence and proximal cisternal segment of the trochlear nerve and branches of the SCA (Fig. 2a–2c). The lateral limit of this corridor is the perimesencephalic sulcus as seen in Fig. 2d. The surgical corridor, brainstem entry zone used in our illustrative case, and relationship to the basal vein of Rosenthal can be seen in Fig. 3.
Endoscopic photos of the precuneal trajectory to the quadrigeminal cistern and infratrochlear brainstem entry zone. a Supratentorial overview of the surgical trajectory after incision of the tentorium. The calcarine sulcus is marked with the green dashed line. b Overview of the cerebellomesencephalic fissure from an infratentorial perspective following the precuneal trajectory. c Infratrochlear brainstem which was used as the entry zone to the CM in the illustrative case. d Ipsilateral extension of the approach to the ambient cistern
Macroscopic pictures of dorsal brainstem from an approach specific and anatomical point of view. a Trajectory of the PCIT after cutting the tentorium inferior to the straight sinus and dissection of the cerebellomesencephalic fissure. b Anatomical dissection of the posterior brainstem and cerebellar peduncles. The red star indicates the infratrochlear brainstem entry zone used to remove the pontomesencephalic CM
The pineal region is also reached and exposed through this approach. The view of this region from a parieto-occipital trajectory is shown in Fig. 4.
Endoscope-assisted photos of the pineal region and quadrigeminal cistern from a parieto-occipital trajectory. The specimen is in a lateral position with the right side placed inferiorly. a An overview of the tectal plate and tributaries of the vein of Galen. b Detailed view of the pineal gland, pineal veins, and dorsal midbrain
Case illustration: pontomesencephalic cavernous malformation
Clinical presentation
A 24-year-old woman presented with multiple bleeds from a pontomesencephalic CM. Examination at the time of evaluation before surgery included a profound sensory deficit and ataxia. Surgical resection was pursued using the PCIT through an infratrochlear brainstem entry.
Surgical considerations
When deciding the best approach to a brainstem CMs, traditionally, the point where the CM comes closest to a parenchymal or ependymal surface is the main consideration. However, other equally important factors include the following: (1) the position of the most challenging portion of the CM (usually the epicenter of the first bleed and the most “established” part) as it is often adherent to the surrounding parenchyma; (2) patient symptoms which suggest how different areas of the brainstem are affected by the CM and associated bleed; (3) the need to obtain a trajectory which provides an ideal “view” and angle to the most challenging portion of the CM.
In the case herein illustrated, the patient had suffered multiple bleeds over time. Analysis of the original magnetic resonance imaging (MRI) identified the anatomical location where the CM originated (hence the most adherent to the surrounding parenchyma) which was at its inferomedial aspect. This can also be inferred by the presence of an established hemosiderin lining of this portion of the lesion on T2-weighted preoperative MRI (Fig. 5a) [3]. Although imaging may suggest that the lesion comes to the surface of the floor of the fourth ventricle (Fig. 5a), the patient did not have symptoms or signs specifically referrable to that anatomical location (i.e. abducens nerve palsy, facial nerve palsy, internuclear ophthalmoplegia). This indicated that, despite the radiological appearance, the structures packed along the floor of the fourth ventricle were intact. Thus, some normal but severely compressed parenchyma was likely to be present between the lesion and the floor of the fourth ventricle.
Preoperative and postoperative axial MRI of illustrative case. a Preoperative T2-weighted MRI showing typical appearance of a CM with hemorrhage of different ages. The lesion and the surrounding parenchyma are effacing the fourth ventricle. b Postoperative T2-weighted MRI showing the trajectory through which the brainstem was entered. This entry point is inferior to the trochlear nerve and lateral to the superior cerebellar peduncle
In devising an optimal approach, we were considering a trajectory which would provide an ideal superior to inferior view to the most challenging portion of the CM. Because of the considerations indicated above, a posterior approach through the floor of the fourth ventricle not favored; a supracerebellar/infratentorial approach would have provided an inferior to superior “bias” limiting the access to the most inferior portion of the lesion. Any approach through the superior cerebellar peduncle would have implied working at a greater depth into the brainstem before reaching the lesion.
Surgical procedure
The patient was placed in the modified park bench position with the right side down, and somatosensory and motor-evoked potentials were utilized. The side of the lesion was placed down to allow the ipsilateral brain to “fall down” after exposure of the interhemispheric fissure. The chin was flexed towards the chest, and the neck rotated 10–15 degrees inferiorly. Care was taken not to obstruct the ipsilateral jugular vein with flexion. Three-pin fixation for the head, a backboard, and a footboard were utilized to avoid inadvertent changes in patient positioning.
Stereotactic guidance was utilized to certify the trajectory and a craniotomy was centered over it. A right-sided parietal craniotomy that exposed the entire SSS was performed. Mannitol was administered to further relax the brain. After dural opening, the interhemispheric fissure was exposed fully inferiorly; the straight sinus was identified at the falcotentorial junction. Its location was confirmed using doppler ultrasonography. A window in the tentorium was fashioned by cutting the tentorium from posterior to anterior with microscissors parallel to the straight sinus. Bleeding from the severed tentorial edges was controlled with low-power bipolar cautery. The cerebellomesencephalic fissure was identified through the transparence of the arachnoid. Dissection of the arachnoid and corresponding cistern allowed for visualization of the trochlear nerve at its emergence. After confirming with intraoperative neuronavigation that we were indeed looking at the area where the hemorrhage came closest to the exposed surface, using an infratrochlear entry zone, the malformation was subsequently resected in a piecemeal fashion. The approach offered excellent visualization of the inferomedial portion of the lesion, which was the most adherent to the surrounding brainstem. The infratrochlear brainstem entry zone can be seen anatomically in Fig. 3b and radiologically in Fig. 5b.
Follow-up
The patient woke up with a transient left hemianopsia and hemibody neglect which cleared over a few days. Her sensory symptoms were unchanged and her balance improved. After a short inpatient stay in the physical medicine and rehabilitation unit, she was eventually discharged home. Postoperative MRI did not show DWI or FLAIR changes and suggested gross total resection of the CM (Fig. 6 and Fig. 7). Of note, the postoperative MRI showed a substantial amount of intact parenchyma between the floor of the fourth ventricle and the resection cavity (Fig. 6b).
Preoperative and postoperative sagittal MRI of illustrative case. a Preoperative T1-weighted MRI with contrast showing a CM of the dorsal pontomesencephalic junction effacing the floor of the fourth ventricle. b Postoperative T1-weighted MRI suggests gross total resection of the CM through the approach described. Although the preoperative MRI seems to show the lesion getting through the floor of the fourth ventricle, after resection, one can appreciate the presence of normal parenchyma between the surgical cavity and the floor of the fourth ventricle. As CMs often displace and compress the surrounding parenchyma, the preoperative MRI often underestimates the amount of normal brain present between the lesion and the surface
Postoperative FLAIR (a) and DWI MRI (b) through the primary visual cortex of the illustrative case showing no signal changes. She had transient visual field defect/neglect which resolved spontaneously in a few days. Despite the lack of signal changes on MRI, it is not uncommon for patients to have transient symptoms after posterior interhemispheric exposure. In absence of ischemic lesions or mechanical damage on MRI, these transient symptoms are most likely related to manipulation/displacement of mesial structures during surgery
Discussion
In this study, we illustrated the salient steps of the PCIT which affords visualization of the splenium down to the inferior-most portion of the cerebellomesencephalic fissure, providing a superior to inferior trajectory very useful for specific lesions of this region. As shown by our measurements, access to the interhemispheric corridor inferior to the lambdoid suture usually does not require the sacrifice of major bridging veins in the specimens studied. Starting the dissection 6.3 cm above the transverse sinus avoids direct retraction on the calcarine sulcus and bridging veins to the SSS. Furthermore, the efficacy of this approach was shown with the resection of a challenging CM of the dorsal pontomesencephalic junction.
Nuances of the craniotomy for this approach are the epicenter of the craniotomy and the extent to which the SSS is exposed. The epicenter of the craniotomy in the sagittal plane should be based on where the stereotactically determined trajectory to the lesion meets the calvarium. If the lesion has several long axes and, thus, requires more working angles, a craniotomy that extends more superiorly and/or inferiorly may be performed. If the lesion extends laterally on the ipsilateral side, a craniotomy that exposes the entire SSS and a small portion of dura of the contralateral hemisphere may allow for an optimal ipsilateral working angle as the falx can be retracted contralaterally. Alvernia et al. demonstrated superior viewing angles off midline when the SSS is fully exposed as the falx can be maximally retracted [2]. Careful surgical technique minimizes the risk of any iatrogenic injury to the SSS [9]. To prevent SSS thrombosis, it is important to keep the exposed sinus moist during surgery and relax retraction on the SSS after access to the interhemispheric fissure has been gained.
A potential limitation of the interhemispheric approach is the presence of large, cortical draining veins into the SSS. Confirming prior clinical observations, in our study, we found that the mean distance from the torcula to the posterior most bridging vein was 6.4 cm [16]. These distances allow for a safe precuneal, interhemispheric working corridor that usually does not require the sacrifice of bridging veins. Moreover, if needed, bridging veins limiting the most anterior aspect of this exposure can be often mobilized by “skeletonizing” the vein and freeing surrounding arachnoidal bands as the vein courses anteromedially to join the SSS. Although study of the venous anatomy in preoperative studies is important for planning, bridging veins, as also confirmed by our anatomical study, are rarely an issue for this approach.
An important consideration of any interhemispheric approach is the amount of brain retraction necessary to provide adequate visualization of the operative corridor. With regard to the occipital and parietal regions, special considerations should be taken as to where retraction is appropriate. Specifically, the calcarine sulcus should not be mobilized with static retractors as there is a risk of visual field deficit. Proper patient position with the ipsilateral (to the pathology) side down to maximize the effects of gravity, wide opening of the interhemispheric fissure and release of arachnoidal bands tethering the brain for an adequate anterior to posterior extension, and CSF drainage after opening the quadrigeminal cistern are all critical maneuvers to afford maximal brain relaxation. In spite of the depth of the operative corridor, static mechanical retractors are not necessary and, indeed, in the case herein illustrated, the CM was removed without the use of mechanical retractors. However, despite all possible precautions and a pristine postoperative MRI sequence (including FLAIR and DWI), transient postoperative neurological changes are not uncommon. These changes may manifest as visual field deficit/neglect on the nondominant hemisphere and confusion and aphasia on the dominant side. These clinical changes are transient provided postoperative MRI does not show mechanical or ischemic damage. In the authors experience, these changes always resolve after 24–72 hours and are probably related to the mobilization and prolonged displacement of midline structures.
Incision of the tentorium can be challenging because of the variable consistency of the tentorial sinus. Crucial considerations in the tentorial incision are avoidance of the straight sinus to prevent potentially life-threatening venous infarct and management of venous bleeding from the highly vascular tentorium itself. While in some patients, the straight sinus is easily visible, in others, its boundaries are not easily demarcated. Usually, the location of the straight sinus can be inferred by surrounding anatomical landmarks. When in doubt, its course can be confirmed and marked with intraoperative micro-doppler. To minimize bleeding during the tentorial incision, it is important to make the first “nick” across the entire thickness of the tentorial dura while avoiding damaging the arachnoid underneath. Subsequently, prophylactic bipolar coagulation across both dural leaflets of the tentorium prior to sharply cutting it with microscissors minimizes tedious bleeding during this maneuver. Some studies suggest the use of indocyanine green to localize the straight sinus, but doppler ultrasonography is more cost-effective, efficient, and safe [7, 18].
In the literature, the precuneal interhemispheric approach has traditionally been utilized to approach lesions that involve the supratentorial space, the falcotentorial junction, and the atrium of the lateral ventricles [1, 5, 13]. We propose that this approach, with its transtentorial adjunct, provides a good working window to the dorsal midbrain and pons, as well as the ambient cistern. Furthermore, it may provide a safer trajectory to regions generally associated with high surgical morbidity, such as lesions which extend from the dorsal midbrain inferiorly to abut the floor of the fourth ventricle [4, 20]. The workhorse approach to the dorsal midbrain is the supracerebellar/infratentorial approach and its variants, namely, the lateral, paramedian, and midline. While these approaches do provide a good anterior to posterior trajectory and are likely better to reach lesions of the pineal region that extend superiorly and anteriorly, lesions that extend inferior to the cerebellomesencephalic fissure are hard to reach, even after fully dissecting the arachnoid, as the caudal angle may be insufficient [17]. Supracerebellar transtentorial modifications of these established posterior and posterolateral approaches, provide a preferentially inferior to superior operative trajectory and are well versed for lesions of the pulvinar and thalamomesencephalic junction. [6] The PCIT, when tailored to the right lesions, may make up for the limitations of these more common approaches. Further studies should be done to compare the utility of these approaches.
Conclusion
The PCIT provides excellent access from the splenium down to the cerebellomesencephalic fissure and can be used in selected patients with pontomesencephalic lesions. Compared to other surgical routes, it provides an unparalleled superior to inferior view and angle to this region. Careful presurgical planning, knowledge of the pertinent surgical anatomy and its variations, as well as respect for basic important surgical steps, are key to the success of the operation.
Data availability
All data analyzed during this study are included in this published article.
References
Aftahy AK, Kaywan Aftahy A, Barz M, Wagner A, Liesche-Starnecker F, Negwer C, Meyer B, Gempt J (2021) The interhemispheric fissure—surgical outcome of interhemispheric approaches. Neurosurg Rev 44(4):2099–2110
Alvernia JE, Lanzino G, Melgar M, Sindou MP, Mertens P (2009) Is exposure of the superior sagittal sinus necessary in the interhemispheric approach? Neurosurgery 65(5):962–4; discussion 964–5
Dammann P, Wrede K, Jabbarli R, Müller O, Mönninghoff C, Forsting M, Sure U (2017) Of bubbles and layers: which cerebral cavernous malformations are most difficult to dissect from surrounding eloquent brain tissue? Neurosurgery 81(3):498–503
Ferguson SD, Levine NB, Suki D, Tsung AJ, Lang FF, Sawaya R, Weinberg JS, McCutcheon IE (2018) The surgical treatment of tumors of the fourth ventricle: a single-institution experience. J Neurosurg 128(2):339–351
Geyik M, Erkutlu I, Alptekin M, Gezgin I, Mizrak A, Pusat S, Gok A (2017) Parieto-occipital interhemispheric precuneal approach to the lesions of the atrium: experience with 66 patients. Turk Neurosurg 27(3):325–332
Giammattei L, Starnoni D, Benes V et al (2021) Extreme lateral supracerebellar infratentorial approach: surgical anatomy and review of the literature. World Neurosurg 147:89–104
Hope-Ross M, Yannuzzi LA, Gragoudas ES, Guyer DR, Slakter JS, Sorenson JA, Krupsky S, Orlock DA, Puliafito CA (1994) Adverse reactions due to indocyanine green. Ophthalmology 101(3):529–533
Huang C, Bertalanffy H, Kar S, Tsuji Y (2021) Microsurgical management of midbrain cavernous malformations: does lesion depth influence the outcome? Acta Neurochir 163(10):2739–2754
Keogh AJ, Sharma RR, Vanner GK (1993) The anterior interhemispheric trephine approach to anterior midline aneurysms: results of treatment in 72 consecutive patients. Br J Neurosurg 7(1):5–12
La Rocca G, Sabatino G, Altieri R, Mazzucchi E, Rapisarda A, Ius T, Garbossa D, Cofano F, Olivi A, Della Pepa GM (2021) Parietal interhemispheric transfalcine transtentorial approach for posterior incisural space lesions: from the lab to the operative room. Neurosurg Rev 44(3):1779–1788
Lopez-Gonzalez MA, Jaeger A, Kaplan B, Eastin TM, Kore L, Gospodarev V, Patel PD, Sharafeddin F (2019) Retractorless interhemispheric transtentorial approach for large lesions in the posterior incisural space. Surg Neurol Int 10:130
Ono M, Ono M, Rhoton AL Jr, Barry M (1984) Microsurgical anatomy of the region of the tentorial incisura. J Neurosurg 60(2):365–399
Patra DP, Savardekar AR, Dossani RH, Mohammed N, Narayan V, Georgescu M-M, Nanda A (2019) Posterior interhemispheric precuneal approach: fundamental principles and case illustration: 3-dimensional operative video. Oper Neurosurg (Hagerstown) 17(2):E58–E58
Poppen JL (1966) The right occipital approach to a pinealoma. J Neurosurg 25(6):706–710
Simon E, Afif A, M’Baye M, Mertens P (2015) Anatomy of the pineal region applied to its surgical approach. Neurochirurgie 61(2–3):70–76
Sonabend AM, Bowden S, Bruce JN (2016) Microsurgical resection of pineal region tumors. J Neurooncol 130(2):351–366
Spazzapan P, Velnar T, Bosnjak R (2020) Endoscopic supracerebellar infratentorial approach to pineal and posterior third ventricle lesions in prone position with head extension: a technical note. Neurol Res 42(12):1070–1073
Takami H, Tanaka S, Takayanagi S, Nakatomi H, Saito N (2021) Indocyanine green illuminates the way to cut the tentorium in occipital transtentorial approach: technical note. Br J Neurosurg 1–3
Tsuji Y, Kar S, Bertalanffy H (2019) Microsurgical management of midbrain cavernous malformations: predictors of outcome and lesion classification in 72 patients. Operative Neurosurgery 17(6):562–572
Winkler EA, Birk H, Safaee M et al (2017) Erratum to: Surgical resection of fourth ventricular ependymomas: case series and technical nuances. J Neurooncol 131(2):423
Yamamoto I (2001) Pineal region tumor: surgical anatomy and approach. J Neurooncol 54(3):263–275
Zhao K, Quillin J, Liu JK (2021) Endoscopic-assisted parieto-occipital interhemispheric precuneal transtentorial approach for microsurgical resection of vermian arteriovenous malformation: operative video and technical nuances. Neurosurgical Focus: Video 4(1):V9
Acknowledgements
The authors thank Joseph I. and Barbara Ashkins Professorship in surgery.
Funding
This work was supported in part by funds from the Joseph and Barbara Ashkins Endowed Professorship in Surgery.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by A. Yohan Alexander, Luciano Leonel, and Edoardo Agosti. The manuscript was written by A. Yohan Alexander and reviewed by all authors. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of the Mayo Clinic approved this study.
Consent to participate
Informed consent was obtained from all individual participants included in this study.
Consent to publish
Informed consent was obtained from all individual participants for publication of data included in this study.
Informed consent
Informed consent was obtained from all participants included in this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurosurgical Anatomy
Rights and permissions
About this article
Cite this article
Alexander, A.Y., Leonel, L.C.P.C., Agosti, E. et al. The precuneal interhemispheric, trans-tentorial corridor to the pineal region and brainstem, surgical anatomy, and case illustration. Acta Neurochir 164, 1095–1103 (2022). https://doi.org/10.1007/s00701-022-05167-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-022-05167-0