Abstract
Background
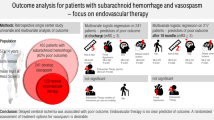
Intensive therapies of delayed cerebral ischemia (DCI) following aneurysmal subarachnoid hemorrhage (aSAH) have still controversial and unproven benefit. We aimed to compare the overall efficacy of two different center-driven strategies for the treatment of DCI respectively with and without vasospasm angioplasty.
Methods
Two hundred consecutive patients with aSAH were enrolled in each of two northern European centers. In an interventional center, vasospasm angioplasty was indicated as first line rather than rescue treatment of DCI using distal percutaneous balloon angioplasty technique combined with intravenous milrinone. In non-interventional center, induced hypertension was the only intensive therapy of DCI. Radiological DCI (new cerebral infarcts not visible on immediate post-treatment imaging), death at 1 month, and favorable outcome at 6 months (modified Rankin scale score ≤ 2) were retrospectively analyzed by independent observers and compared between two centers before and after propensity score (PS) matching for baseline characteristics.
Results
Baseline characteristics only differed between centers for age and rate of smokers and patients with chronic high blood pressure. In the interventional center, vasospasm angioplasty was performed in 38% of patients with median time from bleeding of 8 days (Q1 = 6.5;Q3 = 10). There was no significant difference of incidence of radiological DCI (9% vs.14%, P = 0.11), death (8% vs. 9%, P = 0.4), and favorable outcome 74% vs. 72% (P = 0.4) between interventional and non-interventional centers before and after PS matching.
Conclusions
Our results suggest either that there is no benefit, or might be minimal, of one between two different center-driven strategies for intensive treatment of DCI. Despite potential lack of power or unknown confounders in our study, these results question the use of such intensive therapies in daily practice without further optimization and validation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is a devastating condition that accounts for about 5% of all strokes. Delayed cerebral ischemia (DCI) is a common complication following aSAH that contributes to poor outcome [28]. Severe angiographic vasospasm is strongly associated with DCI [9, 12].
Many intensive therapies of DCI are currently performed in daily practice despite lack of evidence on their benefit. Percutaneous transluminal angioplasty of cerebral vasospasm has been the mainstay for prevention and treatment of DCI in many centers for years [4]. The conventional angioplasty typically combines a balloon angioplasty of proximal arteries with additional intra-arterial infusion of vasodilator for more distal stenosis [1]. It is recommended as rescue therapy for DCI refractory to hypertension therapy [11]. Its effectiveness is derived from meta-analyses of non-controlled studies [8]. Induced hypertension, which is still performed in many centers, has shown poor efficacy and safety in a recent controlled randomized trial [17].
Over the last decade, there was an increasing interest of new strategies for intensive treatment of DCI. Angioplasty performed in first line rather than rescue strategy—i.e., as soon as DCI is suspected—may improve the outcome [21]. Balloon angioplasty of distal arteries—where vasospasm predominates in 40% [9]—may improve the rate of radiological DCI with same safety compared to conventional angioplasty [10, 25, 31]. High dose of intravenous vasodilator in combination with angioplasty may improve vasospasm-related morbidity [2, 29, 30]. To date, the combination of such new strategies has not been compared to non-interventional strategy.
The aim of our study was to demonstrate the superiority of an interventional strategy including distal balloon angioplasty and high dose of intravenous milrinone in first-line strategy in patients with suspicion of DCI to the non-interventional strategy without angioplasty.
Methods
Study design
This was a retrospective, pragmatic, two-center-matched cohort study, comparing two driven strategies with superiority hypothesis of interventional strategy.
Ethic approval
The study was approved by the Ethics review board of our institutions (registration numbers: NHS = 5793, APH = 20,200,305,135,729) and was found to conform to generally accepted scientific principles and ethical standards. The informed consent from the patients or their relative was obtained within the written treatment contract signed on admission.
Population
A same number of consecutive adults admitted to two Northern Europe centers before the 01/11/19 with proven aneurysmal aSAH (modified Fisher scale ≥ 1) within 14 days of ictus were retrospectively included. Both centers have a well-defined catchment area for aSAH including between 2 and 3 million people. Patients who died within 4 days of ictus—i.e., before the vasospasm period—or those with prehospital DCI or lost-to-follow-up were excluded. In both centers, aneurysm, hydrocephalus, early brain injury, and extra neurological organ failure were assessed and managed according the current American Heart Association guidelines [11]. In particular, all the patients were hospitalized in intensive care unit (ICU). aSAH was assessed with non-enhanced computed tomography (CT) and CT angiography (CTA). Aneurysms were treated by endovascular treatment or clipping within 3 days of admission. Options of treatment were discussed in multidisciplinary meetings involving interventional neuroradiologists, vascular neurosurgeons, and ICU physicians. Non-enhanced CT at discharge and 3–6-month clinical and brain CT scan or MRI follow-up were arranged by interventional neuroradiologists and neurosurgeons.
DCI prevention
In both centers, the primary prevention of DCI consisted in close neurological monitoring, oral nimodipine, early pharmacological thromboprophylaxis, and standard neuroprotective measures against secondary brain injury. According to the current state of the art of care, the patients were targeted to have normovolaemia, normotension, and normal hematocrit [11]. DCI was suspected in patients with increased headache, meningism, and body temperature, together with fluctuating decline in consciousness and clinical picture of focal neurological symptoms or infarction, not related to hydrocephalus, severe intracranial hypertension, seizure, complication of aneurysm treatment, or meningitis [18].
In center 1 (non-interventional strategy), patients with clinical or radiological signs of DCI were considered for pharmacologically induced hypertension with continuous intravenous noradrenalin. The systolic blood pressure (SBP) was targeted to achieve symptoms resolution, usually with values up to 180–210 mmHg [15]. In selected cases, unconscious patients underwent continuous EEG monitoring using the alpha-delta ratio to further optimize medical management aiming at increased values of cerebral blood flow through higher cerebral perfusion pressure. Neither vasospasm monitoring using transcranial doppler (TCD), CTA, digital subtracted angiography (DSA), nor angioplasty for vasospasm was routinely offered.
In center 2 (interventional strategy), the intensive therapy of DCI included emergent percutaneous balloon angioplasty—within 2 h from clinical or radiological suspicion of DCI, or within 6 h from severe angiographic vasospasm suspicion in unconscious patients without assessable signs of DCI—followed by high dose of intravenous milrinone(1.5 µg/kg/min) in first-line strategy. It was associated with euvolemia and respect of spontaneous high arterial pressure. Blood pressure targets were determined based on angiography data and neurological examination. Catecholamine support with norepinephrine was prescribed as needed for maintaining a minimal mean arterial pressure > 90 mmHg, especially under intravenous milrinone which may be associated with hypotension. A severe angiographic vasospasm defined as an arterial narrowing ≥ 50% from baseline or adjacent segments on DSA [9] and observed in at least one artery territory was required to consider angioplasty, in addition to clinical or radiological signs of DCI cited before. In unconscious patients, severe angiographic vasospasm was sufficient to consider that treatment. It is important to note that balloon angioplasty was indicated in first-line strategy whatever the response to medical treatment. This non-consensual approach over rescue therapy was based on the safety of this technique previously demonstrated in our center [25] and the aim to intervene earlier—in a prophylactic manner—in the pathological process that leads to DCI.
The technique of vasospasm angioplasty has been previously described [25]. It was exclusively performed using percutaneous balloon angioplasty technique in arteries with a baseline diameter larger than 1.5 mm and located proximal to the second branching of the cerebral arteries (i.e., up to the end of A2 and M2 branches of anterior and middle cerebral arteries) demonstrating narrowing ≥ 30%. A continuous intravenous infusion of a high dose of milrinone (up to 1.5 µg/kg/min then adapted to clinical tolerance) was systematically considered following angioplasty. This was continued up to 15 days from ictus. It was contraindicated when a cerebral infarction extended to involve a major part of a cerebral artery territory in order to limit reperfusion injury. Vasospasm was monitored within 3–14 days post ictus by means of twice-daily transcranial doppler (TCD) examination of the middle cerebral artery velocities [19]. CTA was performed in patients with positive TCD, or with any neurological deterioration, or systematically within 6–8 days from ictus in patients in whom TCD and neurological examination were not possible [32]. According to our local protocol, vasospasm < 30% on CTA ruled out severe angiographic vasospasm.
Data collection
Vascular risk factors, World Federation of Neurological Surgeons Grade at baseline, and functional outcome were retrospectively extracted from patients’ chart. Modified-Rankin scale (mRS) was computed from 6-month ± 1 month follow-up to take into consideration delayed improvement following poor-grade aSAH [35]. If missing, it was interpolated from mRS at discharge, 3-month mRS, or 1-year mRS when ≤ 2. It was then dichotomized in ≤ 2 (favorable outcome) and > 2. In cases where mRS 2 and 3 were difficult to distinguish, mRS = 2 was determined when the patient could be considered capable to work at least half time in his previous job or to live independently at home. Modified Fisher grade at baseline and in-hospital delayed cerebral infarction (radiological DCI) were retrospectively extracted from all available acute and follow-up CT and MRI scans. Radiological DCI was determined following a previously reported method [9]. It was defined as any in-hospital cerebral infarction occurring within 3–21 days after ictus, not related to other specific cause such as iatrogenic infarction, low-flow infarction, infarction because of mechanical effect of brain shift and herniation, or cardio embolism. Time from ictus to angioplasty was also assessed.
Bias limitation
We included all the patients addressed for aSAH rather than only those eligible for intervention in order to avoid sampling biases. Actually, selection criteria for intervention strongly differed between centers. In particular, it is likely that more patients with better prognosis were selected for intervention in center 2 (i.e., not only patients with medically refractory vasospasm but also patients with severe vasospasm without evidence of DCI).
Consequently, sampling only patients with intervention would have confoundingly improved the outcome in center 2 compared to center 1. We also considered a pragmatic approach in order to include in the results the effect of the selection process that involved all the patients.
Endpoints were analyzed by 2 independent observers, including a 10-year experienced interventional neuroradiologist (MAL) (both centers), a 5-year experienced neuroradiologist in center 1 (DS) (center 1), a clinical research associated (Center 2), and/or an ICU physician (center 2). Conflicts were resolved with open data consensus or if no consensus by considering the best hypothesis (no radiological DCI or mRS ≤ 2). One observer was independent from the center and treatment to ensure impartiality.
A propensity score matching between centers was used in order to adjust for the group effect of observed and potentially hidden confounders [7]. The set of variables for PS matching were selected among variables that are associated with DCI and outcome, including age [13], WFNS grade, mFisher scale, size of aneurysm (dichotomized in < or ≥ 10 mm) and cerebrospinal fluid shunting [28]. It also included variables which could potentially be associated with unknown or unobserved confounders such as sex and vascular risk factors [33].
Power of the study
In order to improve the power of the study, we compared two very opposite strategies: one strategy without any specific therapy targeting vasospasm and one strategy which likely includes one of the most intensive protocol of therapy targeting vasospasm reported in literature. There strategies were driven by a well-defined protocol in each center, and therefore were not diluted by difference between physicians. We tested the superiority hypothesis of interventional strategy. The number of patients to include was based on the following hypothesis of radiological DCI exposition: exposed control = 16% [23] and exposed case = 8% obtained in previous cohort of center 2 treated with same strategy as in the present study [25].
Statistical analysis and study plan
Continuous variables were expressed as median and quartiles (Q1–Q3). Nominal variables were expressed as numbers and percentages and estimated with 95% confidence interval (CI95%). The interventional strategy was compared to the non-interventional strategy with a hypothesis of superiority. The primary outcome was in-hospital radiological DCI. Secondary outcomes were 1-month mortality, 6-month mRS ≤ 2, and 6-month mRS ≤ 3.The number of patients to include was computed using a two-group continuity-corrected Chi-square test with approximately 80% power to reject the null hypothesis (1-sided, error rate of 2.5%). It was estimated to 400 (200 in each group), resulting in an odds ratio = 0.45, and 15% exclusion after propensity score matching. The group effect was adjusted from the observed data using a propensity score(PS) 1:1 matching [7]. The PS was estimated from the observed data using a logistic-regression model, including a set of variables selected among variables that are associated with DCI and outcome, including age [13], sex, vascular risk factors [33], WFNS grade, mFisher scale, size of aneurysm (dichotomized in < and ≥ 10 mm), and CSF shunting [28]. Each patient of center 1 was matched to one control of center 2 with similar PS using the nearest neighbor approach, with no replacement and a caliper size of 0.2. Covariate balance between the two groups after PS matching was assessed using the standardized mean difference (SMDs). An absolute SMD < 10% was considered to support the assumption of balance between the groups. Outcomes were compared in the propensity matched population. All P values were two-tailed, and P < 0.05 was considered significant. Statistical analyses were conducted with R.4.0.2 (R Computing, Vienna, Austria).
Results
Population
The inclusion process is detailed in Table 1. Four hundred patients were analyzed (200 in each group). More patients were excluded in center 2 (interventional strategy) compared to center 1 (non-interventional strategy) due to death before vasospasm period (P = 0.026). The comparison of baseline characteristics between both centers is shown in Table 2. Compared to center 1, patients in center 2 were older (P = 0.040), more likely to smoke (P = 0.001), and had less chronic high blood pressure (P = 0.034).
Intensive treatment of DCI
The comparison of intensive treatment of DCI between both centers is shown in Table 3. Compared to center 1, patients in center 2 were also more likely to receive intensive treatment of DCI (P < 0.001) that was initiated in 43% of the cases vs. 22% in center 1. The median (Q1–Q3) time from onset to angioplasty was 8 days (6.5–10). No patient in center 1 had angioplasty or intravenous milrinone. No patient in center 2 had induced hypertension as the only treatment of DCI, but 14% had norepinephrine support together with intravenous vasodilator.
Outcome
The outcome and comparison between both centers are shown in Table 4. No significant difference of rate of radiological DCI, 1-month mortality, and 6-month favorable outcome (both with mRS ≤ 2 and ≤ 3) was observed before PS matching (P ≥ 0.09). After PS matching, 159 patients remained in each group. The characteristics of patients and DSM are shown in Tables 2, 3, and 4. No significant difference of rate of radiological DCI, 1-month mortality, and 6-month favorable outcome was neither observed after PSM matching (P ≥ 0.15).
Discussion
Our study did not demonstrate superiority of an interventional strategy, including distal percutaneous balloon angioplasty and high dose of intravenous milrinone, compared to non-interventional strategy with induced-hypertension, as first-line treatment for prevention of radiological DCI and improvement of functional outcome.
We compared two opposite strategies that have both received limited attention in literature. Actually, most previous studies included rescue therapy with conventional vasospasm angioplasty [8]. Only one randomized clinical trial has managed DCI with purely non-interventional strategy. In Kirkpatrick et al.’s trial comparing simvastatin vs. placebo in 803 patients, only 3% of the patients had conventional rescue angioplasty. The population of this study has baseline characteristics and outcome within same range as in center 1 (expect for highest rate of clipping and younger patients): 16% vs. 15% for radiological DCI, 9% vs. 10% for death, and 70% vs. 71% for favorable outcome respectively [23]. To date, we previously published the only cohort series of patients managed with the same interventional strategy as in center 2 [25]. In that study of 232 patients previously treated in the same center, baseline characteristic and outcome did not show any significant difference with our present study (P ≥ 0.2). Two observational retrospective studies have reported the use of distal percutaneous balloon angioplasty in rescue strategy and without adjunctive intravenous vasodilator: Santillan et al. in a series of 32 patients with symptomatic vasospasm and Chen et al. in a series of 394 patients with aSAH with a favorable functional outcome (mRS ≤ 2) at 3 months of 82% in both [10, 31]. In Boulouis et al.’s meta-analysis, rescue therapy including conventional angioplasty was associated with favorable outcome at last follow-up in about 66% patients with aSAH [8]. Therefore, the baseline characteristics and outcomes in center 2 appear in line with ones reported in literature on aSAH.
Despite promising feasibility studies [10, 25, 31], our results do not support the effectiveness of first-line distal balloon angioplasty with intravenous milrinone for prevention of DCI and improvement of outcome compared to non-interventional strategy. Accordingly, all the studies to date with intravenous vasodilators or conventional angioplasty in rescue therapy have failed to demonstrate their effectiveness vs. non-interventional treatment [8, 29].
Our study that evaluates the combination of one of the most intensive strategy targeting vasospasm reported in literature questions the “more is more” strategy advanced by Jabbarli et al. stating that the more intensive is the endovascular treatment of vasospasm, the better is the outcome. Differently from Jabbarli’s retrospective study, we analyzed a cohort of patient following homogeneous center-driven protocol and treated after 2012 with updated guidelines about the ICU management. In particular, the “triple H” therapy is no more recommended for prevention of DCI [11]. Furthermore, intra-arterial infusion of nimodipine was the mainstay of therapy in Jabbarli et al. rather than balloon angioplasty.
Our results also add to the argument that DCI would be mediated by other mechanisms than large vessel vasospasm, such as microthromboembolism, microcirculatory dysfunction, cortical spreading ischemia, and delayed neuronal injury that might not be prevented by therapy targeting vasospasm [11, 28, 34]. Adverse events with therapy targeting vasospasm could also contribute to poor outcome in the interventional group, such as ischemic stroke related to angioplasty in 6 to 8% of the cases [3, 25]. Intravenous milrinone is currently presumed to be effective in the treatment of cerebral vasospasm following only retrospective cases series. Moreover, reviews of literature or small randomized trial suggest this treatment as other intravenous vasodilators might be detrimental, being associated with higher rate of hypotension requiring vasopressor, and pneumonia [26, 27, 29]. However, the hypothesis of the futility of strategies targeting vasospasm does not match with our local experience, where distal balloon angioplasty has been demonstrated to be effective for preventing radiological DCI [25]. We discuss below further hypotheses.
First, the interventional strategy in our study might have been not optimized. This hypothesis is in line with our previous results that most of the radiological DCI (77% in territory-based analysis) occurred before any angioplasty [25]. The diagnosis of severe angiographic vasospasm might have been done too late in many cases due to the low sensitivity of our protocol of vasospasm monitoring [16, 24]. A better optimization of diagnostic algorithms using TCD, CTA, and CT-perfusion, as well as EEG monitoring are likely needed to improve the effectiveness of our interventional strategy. Finally, performing the vasospasm angioplasties earlier (i.e. at the 6th day from ictus rather than the 8th in our study) might also contribute to better outcome [21].
Second, many unassessed or unknown confounding determinants of outcomes may have been unequally present in each center. According to that hypothesis, the clinical outcomes after aSAH have been previously shown to differ between centers independently from the baseline characteristics [14]. In particular, the daily ICU management of the blood volume and pressure that probably plays the main role for prevention of DCI has not been assessed and compared between our study centers. Different drugs used in ICU such anesthetics could also be confounding [6]. Although controversial, a more systematic and earlier use of induced hypertension and hypervolemia in center 2 might have reduced the risk of DCI [17]. Less selective criteria of admission in center 2 suggested by the higher rate of patient with WFNS > 1 or dying within the first days, lower socioeconomic status in center 2 suggested by the higher rate of smokers and lower rate of successfully managed chronic high blood pressure, as well as probable race disparities between centers [20] could also has been confounding in our study.
Limitations
Several methodological limitations may also have contributed to underestimation of the effectiveness of the interventional strategy. First, the cohort size Might be too small to address the study aim. In particular, the full sample of patients adressed for aSAH included a majority of patients who had no intervention (neither angioplasty nor induced hypertension). However, based on our results, the number of patient to be included to demonstrate a significant decrease of DCI with interventional strategy would be 500 patients by group (more than 10 000 patients for mRS ≤ 3), suggesting if not effect at least a minimal benefit of interventional strategy. The judgements criteria used in our study may also have insufficient power. The radiological DCI does not explore all the DCI phenomena that could be reversible or associated with brain injury not visible on conventional imaging [28]. The mRS does not assess properly certain cognitive dysfunction that is frequent in multiple territory brain injuries [5, 28, 35].
Intravenous infusion of milrinone and distal balloon angioplasty used in the interventional group have low level of evidence and are likely performed only in selected centers throughout the world. The treatment of DCI was applied in the non-interventional group nor can it be regarded as the standard-of-care treatment, since the use of induced hypertension therapy has been recently questioned in HIMALAIA-study [15]. Thus, these center-specific approaches to management of cerebral vasospasm do not warrant the generalizability of our study results. Although the judgment criteria have been analyzed by independent observers, the open labelled retrospective analysis may have introduced systematic information biases which could substantially impact the study results.
Differences in admittance policies between 2 centers may have introduced sampling bias. However, both centers had a same referral process for aSAH patients based on a single center, exclusive, and regional catchment area. Even if less patients with a very poor presentation may have been contraindicated for curative approach to care in center 2, they were excluded from analysis since most of them died within 4 days from bleeding. In fact, we found the same distribution of WFNS grade in each center. Moreover, the propensity score matching might paradoxically increase imbalance of hidden unobserved confounders [22] and be not appropriate for this kind of inter center comparison. However, given the few differences of baseline characteristics in our sample before PS matching, it is unlikely that others methods of matching would have changed our results.
Conclusion
Our results do not add evidence to support intensive therapies targeting vasospasm such as first-line distal balloon angioplasty. Despite potential lack of power of our study, unknown confounders between centers, or center-specific approach in terms of interventional and medical management avoiding any generalization, our results suggest either that there is no benefit, or might be minimal, of such intensive therapies targeting vasospasm. Hence, our study questions the use of such expansive therapies in daily practice without further optimization and validation. A randomized trial is strongly warranted.
Abbreviations
- aSAH:
-
Aneurysmal subarachnoid hemorrhage
- DCI:
-
Delayed cerebral ischemia
- ICU:
-
Intensive care unit
- TCD:
-
Transcranial doppler
- CTA:
-
Computed tomography angiography
- DSA:
-
Digital subtracted angiography
- mRS:
-
Modified Rankin scale
- WFNS:
-
World federation of neurological surgeon’s scale
- PS:
-
Propensity score
- SBP:
-
Systolic blood pressure
References
Abruzzo T, Moran C, Blackham KA, Eskey CJ, Lev R, Meyers P, Narayanan S, Prestigiacomo CJ (2012) Invasive interventional management of post-hemorrhagic cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. J Neurointerv Surg 4:169–177. https://doi.org/10.1136/neurintsurg-2011-010248neurintsurg-2011-010248
Abulhasan YB, Ortiz Jimenez J, Teitelbaum J, Simoneau G, Angle MR (2020) Milrinone for refractory cerebral vasospasm with delayed cerebral ischemia. J Neurosurg 1–12. https://doi.org/10.3171/2020.1.JNS1931072020.1.JNS193107
Adami D, Berkefeld J, Platz J, Konczalla J, Pfeilschifter W, Weidauer S, Wagner M (2019) Complication rate of intraarterial treatment of severe cerebral vasospasm after subarachnoid hemorrhage with nimodipine and percutaneous transluminal balloon angioplasty: Worth the risk? J Neuroradiol 46:15–24. https://doi.org/10.1016/j.neurad.2018.04.001
Al-Mufti F, Amuluru K, Damodara N, El-Ghanem M, Nuoman R, Kamal N, Al-Marsoummi S, Morris NA, Dangayach NS, Mayer SA (2018) Novel management strategies for medically-refractory vasospasm following aneurysmal subarachnoid hemorrhage. J Neurol Sci 390:44–51. https://doi.org/10.1016/j.jns.2018.02.039
Andersen CR, Fitzgerald E, Delaney A, Finfer S (2019) A systematic review of outcome measures employed in aneurysmal subarachnoid hemorrhage (aSAH) Clinical Research. Neurocrit Care 30:534–541. https://doi.org/10.1007/s12028-018-0566-010.1007/s12028-018-0566-0
Athiraman U, Dhar R, Jayaraman K, Karanikolas M, Helsten D, Yuan J, Lele AV, Rath GP, Tempelhoff R, Roth S, Zipfel GJ (2020) Conditioning effect of inhalational anesthetics on delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Neurosurgery. https://doi.org/10.1093/neuros/nyaa3565898777
Austin PC (2011) An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46:399–424. https://doi.org/10.1080/00273171.2011.568786
Boulouis G, Labeyrie MA, Raymond J, Rodriguez-Regent C, Lukaszewicz AC, Bresson D, Ben Hassen W, Trystram D, Meder JF, Oppenheim C, Naggara O (2017) Treatment of cerebral vasospasm following aneurysmal subarachnoid haemorrhage: a systematic review and meta-analysis. Eur Radiol 27:3333–3342. https://doi.org/10.1007/s00330-016-4702-y
Brami J, Chousterman B, Boulouis G, Dorze ML, Majlath M, Saint-Maurice JP, Civelli V, Froelich S, Houdart E, Labeyrie MA (2020) Delayed cerebral infarction is systematically associated with a cerebral vasospasm of large intracranial arteries. Neurosurgery 86:E175–E183. https://doi.org/10.1093/neuros/nyz3405566476
Chen CT, Chen CC, Wang AY, Wu YM, Chin SC, Hsieh PC, Yeap MC, Hsu SY, Lin YJ (2020) Early strategy of scepter XC balloon angioplasty and simultaneous Nimodipine infusion for vasospasm following ruptured aneurysm. BMC Neurol 20:271. https://doi.org/10.1186/s12883-020-01856-4
Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, Patel AB, Thompson BG, Vespa P (2012) Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 43:1711–1737. https://doi.org/10.1161/STR.0b013e3182587839
Crowley RW, Medel R, Dumont AS, Ilodigwe D, Kassell NF, Mayer SA, Ruefenacht D, Schmiedek P, Weidauer S, Pasqualin A, Macdonald RL (2011) Angiographic vasospasm is strongly correlated with cerebral infarction after subarachnoid hemorrhage. Stroke 42:919–923. https://doi.org/10.1161/STROKEAHA.110.597005
Darkwah Oppong M, Iannaccone A, Gembruch O, Pierscianek D, Chihi M, Dammann P, Koninger A, Muller O, Forsting M, Sure U, Jabbarli R (2018) Vasospasm-related complications after subarachnoid hemorrhage: the role of patients’ age and sex. Acta Neurochir (Wien) 160:1393–1400. https://doi.org/10.1007/s00701-018-3549-1
Dijkland SA, Jaja BNR, van der Jagt M, Roozenbeek B, Vergouwen MDI, Suarez JI, Torner JC, Todd MM, van den Bergh WM, Saposnik G, Zumofen DW, Cusimano MD, Mayer SA, Lo BWY, Steyerberg EW, Dippel DWJ, Schweizer TA, Macdonald RL, Lingsma HF (2019) Between-center and between-country differences in outcome after aneurysmal subarachnoid hemorrhage in the Subarachnoid Hemorrhage International Trialists (SAHIT) repository. J Neurosurg 1–9. https://doi.org/10.3171/2019.5.JNS19483
Gathier CS, van den Bergh WM, van der Jagt M, Verweij BH, Dankbaar JW, Muller MC, Oldenbeuving AW, Rinkel GJE, Slooter AJC (2018) Induced hypertension for delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: a randomized clinical trial. Stroke 49:76–83. https://doi.org/10.1161/STROKEAHA.117.017956
Greenberg ED, Gold R, Reichman M, John M, Ivanidze J, Edwards AM, Johnson CE, Comunale JP, Sanelli P (2010) Diagnostic accuracy of CT angiography and CT perfusion for cerebral vasospasm: a meta-analysis. AJNR Am J Neuroradiol 31:1853–1860. https://doi.org/10.3174/ajnr.A2246
Haegens NM, Gathier CS, Horn J, Coert BA, Verbaan D, van den Bergh WM (2018) Induced hypertension in preventing cerebral infarction in delayed cerebral ischemia after subarachnoid hemorrhage. Stroke 49:2630–2636. https://doi.org/10.1161/STROKEAHA.118.022310
Hijdra A, Van Gijn J, Stefanko S, Van Dongen KJ, Vermeulen M, Van Crevel H (1986) Delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: clinicoanatomic correlations. Neurology 36:329–333. https://doi.org/10.1212/wnl.36.3.329
Hollingworth M, Jamjoom AAB, Bulters D, Patel HC (2019) How is vasospasm screening using transcranial Doppler associated with delayed cerebral ischemia and outcomes in aneurysmal subarachnoid hemorrhage? Acta Neurochir (Wien) 161:385–392. https://doi.org/10.1007/s00701-018-3765-8
Howard VJ, Madsen TE, Kleindorfer DO, Judd SE, Rhodes JD, Soliman EZ, Kissela BM, Safford MM, Moy CS, McClure LA, Howard G, Cushman M (2019) Sex and race differences in the association of incident ischemic stroke with risk factors. JAMA Neurol 76:179–186. https://doi.org/10.1001/jamaneurol.2018.38622716974
Jabbarli R, Pierscianek D, Rolz R, Darkwah Oppong M, Kaier K, Shah M, Taschner C, Monninghoff C, Urbach H, Beck J, Sure U, Forsting M (2019) Endovascular treatment of cerebral vasospasm after subarachnoid hemorrhage: more is more. Neurology 93:e458–e466. https://doi.org/10.1212/WNL.0000000000007862
King G, Nielsen R (2019) Why propensity scores should not be used for matching. Polit Anal 27(4):435–454. https://doi.org/10.1017/pan.2019.11
Kirkpatrick PJ, Turner CL, Smith C, Hutchinson PJ, Murray GD (2014) Simvastatin in aneurysmal subarachnoid haemorrhage (STASH): a multicentre randomised phase 3 trial. Lancet Neurol 13:666–675. https://doi.org/10.1016/S1474-4422(14)70084-5
Kumar G, Shahripour RB, Harrigan MR (2016) Vasospasm on transcranial Doppler is predictive of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Neurosurg 124:1257–1264. https://doi.org/10.3171/2015.4.JNS15428
Labeyrie MA, Gaugain S, Boulouis G, Zetchi A, Brami J, Saint-Maurice JP, Civelli V, Froelich S, Houdart E (2019) Distal balloon angioplasty of cerebral vasospasm decreases the risk of delayed cerebral infarction. AJNR Am J Neuroradiol 40:1342–1348. https://doi.org/10.3174/ajnr.A6124
Lannes M, Zeiler F, Guichon C, Teitelbaum J (2017) The use of milrinone in patients with delayed cerebral ischemia following subarachnoid hemorrhage: a systematic review. Can J Neurol Sci 44:152–160. https://doi.org/10.1017/cjn.2016.316
Lunkiewicz J, Brandi G, Willms J, Strassle C, Narula G, Keller E, Muroi C (2021) The effect of nimodipine on pulmonary function in artificially ventilated patients with aneurysmal subarachnoid hemorrhage. Acta Neurochir (Wien). https://doi.org/10.1007/s00701-021-04837-9
Macdonald RL (2014) Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol 10:44–58. https://doi.org/10.1038/nrneurol.2013.246
Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, Vajkoczy P, Wanke I, Bach D, Frey A, Nowbakht P, Roux S, Kassell N (2012) Randomized trial of clazosentan in patients with aneurysmal subarachnoid hemorrhage undergoing endovascular coiling. Stroke 43:1463–1469. https://doi.org/10.1161/STROKEAHA.111.648980
Saber H, Desai A, Palla M, Mohamed W, Seraji-Bozorgzad N, Ibrahim M (2018) Efficacy of cilostazol in prevention of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: a meta-analysis. J Stroke Cerebrovasc Dis 27:2979–2985. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.06.027
Santillan A, Knopman J, Zink W, Patsalides A, Gobin YP (2011) Transluminal balloon angioplasty for symptomatic distal vasospasm refractory to medical therapy in patients with aneurysmal subarachnoid hemorrhage. Neurosurgery 69:95–101; discussion 102. https://doi.org/10.1227/NEU.0b013e31821424f9
Shankar JJ, Tan IY, Krings T, Terbrugge K, Agid R (2012) CT angiography for evaluation of cerebral vasospasm following acute subarachnoid haemorrhage. Neuroradiology 54:197–203. https://doi.org/10.1007/s00234-011-0876-9
Slettebo H, Karic T, Sorteberg A (2020) Impact of smoking on course and outcome of aneurysmal subarachnoid hemorrhage. Acta Neurochir (Wien) 162:3117–3128. https://doi.org/10.1007/s00701-020-04506-3
Vergouwen MD, Ilodigwe D, Macdonald RL (2011) Cerebral infarction after subarachnoid hemorrhage contributes to poor outcome by vasospasm-dependent and -independent effects. Stroke 42:924–929. https://doi.org/10.1161/STROKEAHA.110.597914
Wilson DA, Nakaji P, Albuquerque FC, McDougall CG, Zabramski JM, Spetzler RF (2013) Time course of recovery following poor-grade SAH: the incidence of delayed improvement and implications for SAH outcome study design. J Neurosurg 119:606–612. https://doi.org/10.3171/2013.4.JNS121287
Acknowledgements
We would like to acknowledge the following contributors for their assistance in data assessment and proofreading the manuscript: Wilhelm Kueker, Jash Patel, Vicky Young, and Rufus Corkill from the neuroradiology and neurosurgery departments of John Radcliffe Hospital (Oxford, UK); Clément Jourdaine, Jonathan Brami, Vittorio Civelli, Armand Aymard, Jean-Pierre Saint-Maurice, and Alexis Guedon from the interventional neuroradiology department of Lariboisière Hospital (Paris, France); Anne-Laure Bernat, Matthieu Le Dorze, Etienne Gayat, Fabrice Vallee, and Joaquim Matteo from the ICU and neurosurgery departments of Lariboisière Hospital (Paris, France); and Olivier Chassany from APHP/INSERM (Paris, France).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standard. For this type of study, formal consent is not required.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Vascular Neurosurgery—Aneurysm
Rights and permissions
About this article
Cite this article
Labeyrie, MA., Simonato, D., Gargalas, S. et al. Intensive therapies of delayed cerebral ischemia after subarachnoid hemorrhage: a propensity-matched comparison of different center-driven strategies. Acta Neurochir 163, 2723–2731 (2021). https://doi.org/10.1007/s00701-021-04935-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-021-04935-8