Abstract
Background
Cerebral hyperperfusion syndrome (CHS) is a common complication after direct bypass surgery in patients with Moyamoya disease (MMD). Since preventive measures may be inadequate, we assessed whether the blood flow difference between the superficial temporal artery (STA) and recipient vessels (△BF) and the direct perfusion range (DPR) are related to CHS.
Methods
We measured blood flow in the STA and recipient blood vessels before bypass surgery by transit-time probe to calculate △BF. Perfusion changes around the anastomosis before and after bypass were analyzed with FLOW800 to obtain DPR. Multiple factors, such as △BF, DPR, and postoperative CHS, were analyzed using binary logistic regression.
Results
Forty-one patients with MMD who underwent direct bypass surgery were included in the study. Postoperative CHS symptoms occurred in 13/41 patients. △BF and DPR significantly differed between the CHS and non-CHS groups. The optimal receiver operating characteristic (ROC) curve cut-off value was 31.4 ml/min for ΔBF, and the area under the ROC curve (AUC) was 0.695 (sensitivity 0.846, specificity 0.500). The optimal cut-off value was 3.5 cm for DPR, and the AUC was 0.702 (sensitivity 0.615, specificity 0.750).
Conclusion
Postoperative CHS is caused by multiple factors. △BF is a risk factor for CHS while DPR is a protective factor against CHS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Moyamoya disease (MMD) is characterized by a progressive intracranial arterial steno-occlusive disease of unknown etiology, especially at the terminal portion of the internal carotid artery, leading to an abnormal vascular network (moyamoya vessels) at the base of the brain, which causes severe hemodynamic impairment [9, 25]. The moyamoya vessels are very fragile and prone to bleeding or ischemia, so MMD typically presents clinically with intracranial hemorrhage and cerebral infarction or transient ischemic attack (TIA). To prevent brain ischemia and hemorrhage, direct revascularization adopts superficial temporal artery (STA)–middle cerebral artery (MCA) bypass surgery as a choice of treatment for MMD, which can establish a sufficient blood supply to immediately improve cerebral perfusion [8, 16]. Although direct bypass surgery is a very mature technique, there are still some postoperative complications that cannot be effectively resolved, such as postoperative ischemic/hemorrhagic stroke or cerebral hyperperfusion syndrome (CHS), during the acute phase after STA-MCA bypass surgery [4, 8]. CHS is a dangerous complication after bypass surgery, which can cause transient neurological deterioration [4, 10], such as severe headache, epilepsy, aphasia, focal neurologic deficits, and even delayed intracranial hematoma (ICH) [6, 20]. The incidence of CHS after direct bypass surgery for patients with MMD has been reported to be 5.9–47% [4, 8, 21, 27]. CHS may cause irreversible damage to nerve function. When CHS occurs, blood pressure needs to be controlled to reduce the incidence of complications. On the contrary, controlling blood pressure may lead to complications such as cerebral infarction. Our research may provide theoretical support for resolving this contradiction.
Intraoperative ICG video angiography (ICG-VA) combined with hemodynamic parameter analysis with the visually time-delayed color map obtained by FLOW800 software (Carl Zeiss Meditec AG) can monitor the regional cerebral blood flow (CBF) and evaluate the improvement of the cortex perfusion around the anastomotic site after direct bypass surgery in patients with MMD [2, 29]. An intraoperative transit-time ultrasonography blood flow probe can monitor the direction of blood flow and the pressure gradient between the donor artery and the recipient artery before and after direct revascularization surgery for MMD [17]. So far, no relevant literature has reported whether the relationship between the pressure gradient between the bypass vessels and the range of cerebral blood perfusion around the anastomotic site is related to CHS in patients with MMD after bypass surgery. In our study, we used an intraoperative transit-time ultrasonography blood flow probe to measure the blood flow of the cut STA [1] and MCA before direct bypass graft, and the FLOW800 software assessed the cerebral perfusion territory changes before and after direct bypass surgery in MMD. Then, we analyzed whether the blood flow difference between the STA and recipient vessels (△BF) and direct perfusion range (DPR) were related to CHS.
Materials and methods
Patient population and selection criteria
We retrospectively analyzed 41 patients with MMD who had undergone STA-MCA direct bypass surgery between October 2018 and October 2019. Patients received single anastomosis and blood flow measurements and FLOW800 during surgery. In general, the patients were selected for bypass surgery if the following criteria were met: (1) no contraindications for surgery; (2) all patients underwent digital subtractive angiography (DSA) and magnetic resonance angiography (MRA) before surgery to confirm the final diagnosis of MMD (Tokyo standard) [23]; (3) patients with MMD that affects the anterior circulation, with TIA or stroke, unaffected by drug therapy; (4) no history of iodine allergy and other dye allergies; (5) no unrecoverable sequelae; (6) no history of hypertension.
Surgical procedures
The general procedure is as follows: The superficial temporal artery is fully exposed and carefully separated, the dura mater is cut and the middle meningeal artery preserved, the appropriate MCA branch is then selected, and the blood flow of the STA and MCA branches (M4) are measured using a Doppler microprobe, and then analyzed by FLOW800 as follows. While temporarily obstructed, a single anastomosis of STA-MCA by end-to-side anastomosis, after anastomosis, encephalo-duro-arterio-synangiosis (EDAS) was also performed; then, the blood flow of the STA and recipient blood vessels was measured through the microprobe and analyzed using the FLOW800 software, and the patency of the anastomosis was confirmed.
Intraoperative measurement of cerebrovascular blood flow
In some studies, transit-time ultrasonography is used to measure the blood flow of the bypass graft, and the pressure gradient is expressed by the increase of blood flow velocity or blood flow to predict the occurrence of hyperperfusion or transient neurologic deficits (TNEs) [13, 17], and it has been shown that in patients after bypass surgery, the pressure gradient between STA and recipient vessels is well correlated with the difference in blood flow between the two [2, 24]. Therefore, we measured the blood flow of the STA and recipient blood vessels before bypass surgery using a transit-time probe and calculated the difference to represent the pressure gradient between the STA and recipient vessels, and it was clarified whether the anastomosis was unobstructed [17]. This study used a hook-shaped transit-time probe (Charbel Micro-Flowprobe; Transonic Systems, Inc., Ithaca, NY) to measure cerebral vascular blood flow, which is repeatable and non-invasive. The probe is stable and less susceptible to interference from other factors and has been demonstrated in in vitro and in vivo studies [14]. In our study, we measured the cut flow [1] of the STA before the anastomosis, i.e., the maximum potential flow of the vessel was measured without resistance at the distal end of the vessel. Blood flow should be measured in a similar range of blood pressure (we control the blood pressure difference within 10 mmHg by using monitoring) and PaCO2, both before and after anastomosis.
ICG angiography and FLOW800 software analysis
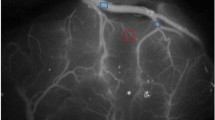
ICG fluorescein and FLOW800 software analyses were performed before and after bypass. At each angiography, ICG (25 mg, dissolved in 10 ml of normal saline) was injected into the cubital vein. In the same operation, fluoroscopy was performed in the same appropriate field before and after the anastomosis. The same parameters, such as focal length and magnification, multiples, distance from the field, and angle, were selected for angiography. After image acquisition, FLOW800 software was used for analysis. The FLOW800 provides a visually time-delayed color map of hemodynamics and perfusion changes by evaluating the fluorescent video sequences obtained by integrated ICG fluorescein angiography to compare the perfusion areas of the anastomotic regions before and after bypassing. The color map is color-coded according to the order of the ICG contrast agent flowing through the parts, and the color is color-coded according to the length of the ICG contrast agent. The color code is finally displayed in a static image. In the time-delayed color map, red indicates the earliest passage area. After the bypass surgery, the circulation time of the corresponding branch area becomes shorter and the cortical perfusion increases [29]. It has been pointed out that FLOW800 can be used to visualize hemodynamic changes after intracranial and extracranial bypass, but not for the quantitative analysis of blood perfusion [22], and the fluorescence intensity image we obtained after intraoperative ICG angiography is basically the same as the red area around the anastomosis on the delayed color map obtained by FLOW800 software analysis. Therefore, we define the area that becomes red in the delayed color image as the direct perfusion range (DPR) after bypass surgery (compared to before the anastomosis), and analyze the delayed color image using a semi-quantitative method. We place a ruler during the operation and measure the distance between the two furthest points in the DPR on the delayed color map. This distance represents the DPR (Fig. 1) and carry out statistical analysis. For the convenience of statistics, we calculate the distance of less than 1 cm as 1 cm; the distance of 1–2 cm is calculated as 2 cm; the distance of 2–3 cm is calculated as 3 cm; the distance of 3–4 cm is calculated as 4 cm; distance 4–5 cm is calculated as 5 cm (Fig. 2).
DPR measurement. a The image of the fluorescence intensity after intraoperative ICG angiography, before the anastomosis on the left, and after the anastomosis on the right. b FLOW800 software automatically generates time-delayed color maps. The left side is the image before the anastomosis, and the right side is the image after the anastomosis, all of which are independent images. c After FLOW800 automatically adjusted the parameters, the contrast image formed under the same parameters. The left side is the image before the anastomosis, and the right side is the image after the anastomosis. The five-pointed star is the anastomosis point, and the area around the anastomosis point that turns red is the direct perfusion area (DPR). In the DPR, the two points furthest away from each other were identified and represent DPR according to the distance between them
Postoperative management
Anti-epileptic drugs are routinely administered after surgery. Since the preoperative blood pressure was within normal range, we closely monitored the blood pressure after surgery and controlled the blood pressure to preoperative levels. Hyperperfusion symptoms usually appear within 1 week after surgery. When CHS appears, we continue to intravenously apply nimodipine while monitoring blood pressure to reduce systolic blood pressure by 10–30 mmHg, but not lower than 90 mmHg, to avoid symptoms of cerebral ischemia, and the symptoms of patients after treatment generally relieves within 2 weeks.
Definition of CHS
The diagnostic criteria for CHS include the following [4, 18, 28]: (1) CTP (CT perfusion) was used to define increase in CBF around the anastomosis; (2) obvious neurological symptoms, such as limb dysfunction, aphasia, persistent headache, vomiting; (3) MRA or DSA was used to clarify the patency of the bypass vessel. Brain MRI excluded any new cerebral infarction. In addition, other factors that may cause these symptoms, such as the temporal muscle compression of the brain surface and subdural hematoma, were excluded.
Statistical analysis
A paired sample t test was applied to data generated before and after bypassing. An independent sample t test was used for comparison between CHS and NON-CHS groups. Descriptive variables are shown as mean ± standard deviation (SD). Based on the Youden index of the receiver operating characteristic (ROC) curve to set the optimal cut-off value, binary logistic regression analysis was used to analyze the effects of multiple factors on CHS symptoms. Sex, type of disease, age, DPR, and △BF were considered as independent variables, and CHS symptoms were considered dependent variables. All statistical analyses were performed using IBM SPSS version 20. Differences with P < 0.05 were considered statistically significant.
Results
A total of 41 patients were included in the study, with an ischemic type of 28 and a hemorrhagic type of 13. The patient age range was 18 to 57 years, and the mean age was 39.76 ± 11.70 years. The profiles of the patients are detailed in Table 1. All patients were confirmed to have patency of the bypass by MRA or DSA within 1 week after surgery.
During the operation, all physiological parameters were kept within the normal range. There was no significant difference in systolic blood pressure, diastolic blood pressure, or PaCO2 before and after bypassing (Table 2).
There were 13 hemispheres with CHS after bypass and 28 hemispheres were NON-CHS. There were no significant differences in sex, age, disease type, and side between the two groups (Table 3). All patients with CHS were relieved of their symptoms within 2 weeks without irreversible damage.
The STA flow range was 18.6–74 ml/min, and the flow rate of the recipient blood vessel was 0.49–8.99 ml/min. The difference (ΔBF), ranging from 11.29 to 73.41 ml/min, was statistically significant between the CHS and non-CHS groups (Fig. 3).
After the bypass, the direct perfusion area around the anastomotic point was counted. As shown in Table 4, there was a significant difference in DPR between the CHS and non-CHS groups (P = 0.028) (Fig. 4).
Based on the Youden index (sensitivity + specificity-1) of the ROC curve, the optimal cut-off value for ΔBF was 31.4 ml/min, and the area under the ROC curve (AUC) was 0.695. When △BF > 31.4 ml/min, the occurrence of CHS could be predicted and the corresponding sensitivity was 0.846 and the specificity was 0.500. The optimal cut-off value for DPR was 3.5 cm, and the AUC was 0.702. When DPR < 3.5 cm, the occurrence of CHS could be predicted and the corresponding sensitivity was 0.615 and the specificity was 0.750 (Fig. 5).
We analyzed CHS, age, sex, side, disease type, △BF, and DPR, using binary logistic regression analysis, with CHS as the dependent variable (group: NON-CHS = 0, CHS = 1). The analysis showed that △BF and DPR were significant predictors of CHS symptoms. We then established a multi-factor logistic regression model, and the equation was as follows:
Logit(P) = 0.560 + 0.132△BF-1.993DPR.
Table 5 shows that the patient’s CHS symptoms were not caused by a single factor. △BF acts as a risk factor and DPR acts as a protection factor. In the case of a constant DPR, the greater △BF the greater the likelihood of CHS symptoms is. In the case of a constant △BF, the greater DPR, the less likely high perfusion will occur.
DPR, △BF are in the equation. According to the results, the model is established: Logit(P) = 0.560 + 0.132△BF-1.993DPR. △BF is a risk factor and DPR is a protection factor.
Discussion
This study is the first to predict the occurrence of CHS by DPR analyzed by FLOW800 software and the △BF measured by transit-time ultrasonography. We analyzed the parameters of △BF, perfusion range, age, gender, side, and disease type, and found that △BF and DPR were independent predictors of CHS. Binary logistic multi-factor regression analysis showed that △BF is a risk factor for CHS, and DPR is a protective factor. Using our model, we showed that the larger the △BF, the higher the risk of CHS. On the contrary, when △BF is equal, the larger the perfusion range, the smaller the probability of CHS appearing.
In our study, △BF has obvious difference between CHS group and non-CHS group. Based on the Youden index of ROC, when △BF is greater than 31.4 ml, the probability of CHS will increase significantly. And with the increase of BF, the risk of CHS will be higher, we believe that due to the vessels in the cerebral cortex suddenly inject large amounts of exogenous blood, leading to hyperperfusion symptoms. The mechanism of CHS after MMD direct bypass surgery has been reported as a sudden increase in blood flow in the cerebral arteries after bypass, and recovery of clinical symptoms due to impaired vascular regulation due to long-term ischemia [19]. Moreover, the loss of neurological function after bypass surgery is related to the high signal band of the fluid-attenuated inversion recovery (FLAIR) sequence in MRI. The high cortical signal indicates vasogenic edema, that is, excessive blood in the blood vessels causes impaired autoregulation of blood vessels and vascular permeability increases [7]. The above views are consistent with our study.
We believe that merely measuring the changes in intraoperative blood flow seems to be somewhat incomplete for predicting CHS. Different pressure gradients may have a certain effect on predicting CHS within the same perfusion range. When the same pressure gradient is within a different perfusion range, we believe that the effect is also different. In patients with MMD, the end of the internal carotid artery gradually occludes, the microvessels in the cortex gradually increase and expand, and the peripheral resistance is reduced by the expansion of the microvessels to compensate for CBF [3, 11]. Direct bypass surgery is an anastomosis of STA with recipient blood vessels, which can better increase cerebral cortex blood perfusion through the microvessels, and this phenomenon can be more intuitively revealed by the FLOW800’s delayed color map [22]. With FLOW800, we can more directly observe the direct perfusion range after bypass surgery. We believe that the larger the direct perfusion range of the same △BF, the more it can reduce the blood perfusion pressure of the recipient blood vessel, so it will reduce the damage to the autoregulatory function of the recipient blood vessel. Through our research, when DPR is greater than 3.5 cm, the chance of CHS will be reduced. Through multi-factor analysis, we have obtained a model of CHS occurrence probability: Logit(P) = 0.560 + 0.132△BF-1.993DPR. According to the model, we can find that △BF and DPR affect each other. If △BF is found to be large during the operation and DPR is small, then postoperative care must be taken to prevent the emergence of CHS.
CHS is one of the serious complications after revascularization of moyamoya disease, especially in adults, its incidence can be as high as 50% [12]. It has been reported in the literature that single-photon emission tomography (SPECT) measures the changes of CBF before and after operation to diagnose CHS and then manage the clinical symptoms [5]. However, SPECT examinations can only be performed at specific times. It is difficult to examine in an emergency, and the results of SPECT may be affected by sedation or emotional agitation. Recently, some articles have shown that there is no significant correlation between TNEs and CBF after revascularization [7, 15, 26]. It can be seen that the quantitative measurement of CBF to predict postoperative neurological deficit is still controversial. Therefore, in the diagnostic criteria of CHS, we take a qualitative observation of the increase of CBF around the anastomosis. In the study, the patients in the CHS group alleviated their symptoms after treatment such as lowering blood pressure. Moreover, we measure blood flow by transit-time ultrasonography to obtain △BF, and obtain DPR by FLOW800 time-lapse color map. Both methods are relatively simple, will not cause invasive trauma, and during surgery, it is possible to predict whether CHS occurs after surgery, which is conducive to early measures and can better take perioperative management to make the patient get the best prognosis. According to our model, if the risk of CHS is high, we suggest control the postoperative blood pressure to a lower level, which is about 10–20 mmHg lower than before. Generally, if the blood pressure drops too low, it may cause the contralateral ischemia of bilateral moyamoya disease. Of course, the appropriate blood pressure reduction level will be further studied.
This study has certain limitations. Our research uses a hand-held transit-time probe, which may have certain errors in measurement. In addition, in actual operation, the injection speed of ICG and blood pressure cannot be completely controlled. Heart rate also affects the intensity of fluorescence. It is impossible to measure blood flow when there is not enough space around the anastomosis or the tiny recipient blood vessels. The choice of surgery field cannot be completely controlled before and after bypassing. ICG fluorescence cannot be displayed outside the skull window. Therefore, when evaluating the time delay color map of FLOW800, attention should be paid to the limitations of these parameters. Being a retrospective research is another limitation of this study, with a limited sample size and a single-center study, which may also affect the universality of the results. Due to the small sample size, this conclusion may have certain errors. We believe that there will be more advanced tools in the future and a large sample of multi-center tests to further validate or correct the results of this study.
Conclusions
The results of this study indicate that CHS is not caused by a single factor but by a combination of multiple factors. By monitoring △BF and DPR after bypass, we found that △BF is a risk factor for CHS, and DPR is a protective factor. △BF is positively correlated with CHS when DPR is unchanged. In the case of unchanged △BF, DPR is negatively correlated with CHS. These results may provide useful information for perioperative management of MMD.
References
Alaraj A, Ashley WW Jr, Charbel FT, Amin-Hanjani S (2008) The superficial temporal artery trunk as a donor vessel in cerebral revascularization: benefits and pitfalls. Neurosurg Focus 24:E7
Awano T, Sakatani K, Yokose N, Kondo Y, Igarashi T, Hoshino T, Nakamura S, Fujiwara N, Murata Y, Katayama Y, Shikayama T, Miwa M (2010) Intraoperative EC-IC bypass blood flow assessment with indocyanine green angiography in moyamoya and non-moyamoya ischemic stroke. World Neurosurg 73:668–674
Czabanka M, Pena-Tapia P, Schubert GA, Woitzik J, Horn P, Schmiedek P, Vajkoczy P (2009) Clinical implications of cortical microvasculature in adult Moyamoya disease. J Cereb Blood Flow Metab 29:1383–1387
Fujimura M, Mugikura S, Kaneta T, Shimizu H, Tominaga T (2009) Incidence and risk factors for symptomatic cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with moyamoya disease. Surg Neurol 71:442–447
Fujimura M, Niizuma K, Endo H, Sato K, Inoue T, Shimizu H, Tominaga T (2015) Quantitative analysis of early postoperative cerebral blood flow contributes to the prediction and diagnosis of cerebral hyperperfusion syndrome after revascularization surgery for moyamoya disease. Neurol Res 37:131–138
Fujimura M, Shimizu H, Mugikura S, Tominaga T (2009) Delayed intracerebral hemorrhage after superficial temporal artery-middle cerebral artery anastomosis in a patient with moyamoya disease: possible involvement of cerebral hyperperfusion and increased vascular permeability. Surg Neurol 71:223–227 discussion 227
Hamano E, Kataoka H, Morita N, Maruyama D, Satow T, Iihara K, Takahashi JC (2017) Clinical implications of the cortical hyperintensity belt sign in fluid-attenuated inversion recovery images after bypass surgery for moyamoya disease. J Neurosurg 126:1–7
Hwang JW, Yang HM, Lee H, Lee HK, Jeon YT, Kim JE, Lim YJ, Park HP (2013) Predictive factors of symptomatic cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in adult patients with moyamoya disease. Br J Anaesth 110:773–779
Kim JE, Jeon JS (2014) An update on the diagnosis and treatment of adult Moyamoya disease taking into consideration controversial issues. Neurol Res 36:407–416
Kim JE, Oh CW, Kwon OK, Park SQ, Kim SE, Kim YK (2008) Transient hyperperfusion after superficial temporal artery/middle cerebral artery bypass surgery as a possible cause of postoperative transient neurological deterioration. Cerebrovasc Dis 25:580–586
Kim SJ, Son TO, Kim KH, Jeon P, Hyun SH, Lee KH, Yeon JY, Kim JS, Hong SC, Shin HJ, Bang OY (2014) Neovascularization precedes occlusion in moyamoya disease: angiographic findings in 172 pediatric patients. Eur Neurol 72:299–305
Kim T, Oh CW, Bang JS, Kim JE, Cho WS (2016) Moyamoya disease: treatment and outcomes. J Stroke 18:21–30
Lee M, Guzman R, Bell-Stephens T, Steinberg GK (2011) Intraoperative blood flow analysis of direct revascularization procedures in patients with moyamoya disease. J Cereb Blood Flow Metab 31:262–274
Lundell A, Bergqvist D, Mattsson E, Nilsson B (1993) Volume blood flow measurements with a transit time flowmeter: an in vivo and in vitro variability and validation study. Clin Physiol 13:547–557
Machida T, Nakano S, Ishige S, Ono J, Fujikawa A (2017) Subcortical low-intensity lesions on fluid-attenuated inversion recovery images after revascularization surgery for Moyamoya disease. World Neurosurg 98:512–519
Miyamoto S, Yoshimoto T, Hashimoto N, Okada Y, Tsuji I, Tominaga T, Nakagawara J, Takahashi JC (2014) Investigators JAMT: effects of extracranial-intracranial bypass for patients with hemorrhagic moyamoya disease: results of the Japan Adult Moyamoya trial. Stroke 45:1415–1421
Morisawa H, Kawamata T, Kawashima A, Hayashi M, Yamaguchi K, Yoneyama T, Okada Y (2013) Hemodynamics and changes after STA-MCA anastomosis in moyamoya disease and atherosclerotic cerebrovascular disease measured by micro-Doppler ultrasonography. Neurosurg Rev 36:411–419
Nakagawa A, Fujimura M, Arafune T, Sakuma I, Tominaga T (2009) Clinical implications of intraoperative infrared brain surface monitoring during superficial temporal artery-middle cerebral artery anastomosis in patients with moyamoya disease. J Neurosurg 111:1158–1164
Noguchi T, Kawashima M, Nishihara M, Egashira Y, Azama S, Irie H (2015) Noninvasive method for mapping CVR in moyamoya disease using ASL-MRI. Eur J Radiol 84:1137–1143
Ogasawara K, Komoribayashi N, Kobayashi M, Fukuda T, Inoue T, Yamadate K, Ogawa A (2005) Neural damage caused by cerebral hyperperfusion after arterial bypass surgery in a patient with moyamoya disease: case report. Neurosurgery 56:E1380 discussion E1380
Ohue S, Kumon Y, Kohno K, Watanabe H, Iwata S, Ohnishi T (2008) Postoperative temporary neurological deficits in adults with moyamoya disease. Surg Neurol 69:281–286 discussion 286-287
Prinz V, Hecht N, Kato N, Vajkoczy P (2014) FLOW 800 allows visualization of hemodynamic changes after extracranial-to-intracranial bypass surgery but not assessment of quantitative perfusion or flow. Neurosurgery 10(Suppl 2):231–238 discussion 238-239
Research Committee on the Pathology, Treatment of Spontaneous Occlusion of the Circle of Willis, Health Labour Sciences Research Grant for Research on Measures for Infractable Diseases (2012) Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir (Tokyo) 52:245–266
Saito M, Saga T, Hayashi H, Noro S, Wada H, Kamada K (2018) Quantitative blood flow assessment by multiparameter analysis of indocyanine green video angiography. World Neurosurg 116:e187–e193
Suzuki J, Kodama N (1983) Moyamoya disease--a review. Stroke 14:104–109
Uda K, Araki Y, Muraoka S, Ota S, Wada K, Yokoyama K, Nishihori M, Izumi T, Okamoto S, Wakabayashi T (2018) Intraoperative evaluation of local cerebral hemodynamic change by indocyanine green videoangiography: prediction of incidence and duration of postoperative transient neurological events in patients with moyamoya disease. J Neurosurg 1:1–9
Yamaguchi K, Kawamata T, Kawashima A, Hori T, Okada Y (2010) Incidence and predictive factors of cerebral hyperperfusion after extracranial-intracranial bypass for occlusive cerebrovascular diseases. Neurosurgery 67:1548–1554 discussion 1554
Yang T, Higashino Y, Kataoka H, Hamano E, Maruyama D, Iihara K, Takahashi JC (2018) Correlation between reduction in microvascular transit time after superficial temporal artery-middle cerebral artery bypass surgery for moyamoya disease and the development of postoperative hyperperfusion syndrome. J Neurosurg 128:1304–1310
Ye X, Liu XJ, Ma L, Liu LT, Wang WL, Wang S, Cao Y, Zhang D, Wang R, Zhao JZ, Zhao YL (2013) Clinical values of intraoperative indocyanine green fluorescence video angiography with Flow 800 software in cerebrovascular surgery. Chin Med J 126:4232–4237
Author information
Authors and Affiliations
Contributions
Rong Wang and Dongxu Yang conceived and designed the research; Dongxu Yang, Rong Wang, Xiaohong Zhang, Cunxin Tan, Zhiguang Han, Yutao Su, Ran Duan, Guangchao Shi, Junshi Shao, Penghui Cao, and Shihao He performed the research, analyzed the data, and carried out the statistics. Dongxu Yang and Xiaohong Zhang wrote the manuscript, and Rong Wang revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This is a retrospective study and for this type of study formal consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Comments
The authors perform a retrospective review of 41 patients with MMD who underwent STA-MCA bypass to evaluate for a relationship between changes in pre and post anastomosis blood flow in the donor and recipient vessels as well as FLOW800 are associated with cerebral hyperperfusion syndrome.
The authors do a great job of quantifying a difficult to predict problem.
Fady Charbel
Illinois, USA
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Vascular Neurosurgery
Rights and permissions
About this article
Cite this article
Yang, D., Zhang, X., Tan, C. et al. Intraoperative transit-time ultrasonography combined with FLOW800 predicts the occurrence of cerebral hyperperfusion syndrome after direct revascularization of Moyamoya disease: a preliminary study. Acta Neurochir 163, 563–571 (2021). https://doi.org/10.1007/s00701-020-04599-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-020-04599-w