Abstract
Background
Pressure reactivity index (PRx) has emerged as a means to continuously monitor cerebrovascular reactivity in traumatic brain injury (TBI). However, other intracranial pressure (ICP)-based continuous metrics exist, and may have advantages over PRx. The goal of this study was to perform a scoping overview of the literature on non-PRx ICP-based continuous cerebrovascular reactivity metrics in adult TBI.
Methods
We searched MEDLINE, BIOSIS, EMBASE, Global Health, SCOPUS, and Cochrane Library from inception to December 2019. Using a two-stage filtering of title/abstract, and then full manuscript, we identified pertinent articles. Data was abstracted to tables and each technique summarized, including pulse amplitude index (PAx), correlation between pulse amplitude of ICP and cerebral perfusion pressure (RAC), PRx55-15, and low-resolution metrics LAx and L-PRx.
Results
A total of 23 articles met the inclusion criteria, with the vast majority being retrospective in nature and based out of European centers. Sixteen articles focused on high-resolution metrics PAx, RAC, and PRx55-15, with 6 articles focusing on LAx and L-PRx. PAx may have a role in low ICP situations, where it appears to perform superior to PRx. RAC displays similar behavior to PRx, with a trend to stronger associations with favorable/unfavorable outcome at 6 months, and stronger parabolic relationship with CPP. PRx55-15 provides a focused assessment on the vasogenic frequency range associated with cerebral autoregulation, with preliminary data supporting a strong association with outcome in TBI. LAx and L-PRx display varying associations with 6-month outcome in TBI, depending on the window length of calculation, with shorter windows demonstrating stronger correlations with classical PRx.
Conclusions
Non-PRx continuous ICP-based cerebrovascular reactivity metrics can be split into high-resolution and low-resolution measures. High-resolution indices include PAx, RAC, and PRx55-15, while low-resolution indices include L-PRx and LAx. The true role for these metrics beyond classic PRx remains unclear. Each displays situations where it may prove superior over PRx, given limitations with this currently widely accepted measure. Much future investigation into each of these alternative metrics is required prior to adoption into the clinical monitoring armamentarium in adult TBI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multi-modal monitoring of cerebral physiology in traumatic brain injury (TBI) is increasingly becoming common place in advanced neurocritical care units worldwide [25]. However, not only do the raw physiology parameters returned by these devices carry potential importance in directing therapies and prognostication, but metrics derived through combination of various biomedical signal processing techniques can expand our understanding of cerebral physiology [10]. Continuous cerebrovascular reactivity monitoring in TBI is one such derived metric. Recently, data suggests that impaired cerebrovascular reactivity after TBI appears to remain unaffected by current intensive care unit (ICU)-based therapeutics [13, 54], with large portions of a patients ICU stay spent in an impaired state [54], and relatively unchanged rates of impairment over the past 25 years of Brain Trauma Foundation (BTF)-based changes in care [13]. Thus, impaired cerebrovascular reactivity could potentially account for the relatively unchanged mortality rates seen in moderate-to-severe TBI over the last few decades, despite overall advances in ICU and post-ICU care. Though, it should be acknowledged that such findings are from few studies, requiring further validation.
Over the previous two decades, literature has supported continuous cerebrovascular reactivity monitoring in adult TBI, with the most commonly quoted measures being those derived from intracranial pressure (ICP) [10, 45]. However, other metrics based on brain tissue oxygen (PbtO2) [1, 20, 22], near-infrared spectroscopy (NIRS) [28], thermal diffusion blood flow [29], and transcranial Doppler [8, 35] have been derived in TBI populations [45]. Such metrics are derived through evaluation of the correlation between slow-wave vasogenic fluctuations in a surrogate measure of pulsatile cerebral blood volume (CBV) or cerebral blood flow (CBF), and a driving pressure for flow, such as mean arterial pressure (MAP) or cerebral perfusion pressure (CPP). The ICP waveform is utilized as a surrogate for pulsatile CBV, for the most commonly described cerebrovascular reactivity metric, the pressure reactivity index (PRx) [10]. Specifically, PRx is derived through the moving Pearson correlation coefficient between slow-wave vasogenic fluctuations in ICP and MAP. Initially, raw full-waveform ICP and arterial blood pressure (ABP) data is decimated to 0.1 Hz (Hz) using a 10-s non-overlapping moving average filter, in order to focus on slow-wave frequency ranges associated with cerebral autoregulation. Then, Pearson correlation is conducted on thirty consecutive 10 s mean measures of ICP and MAP (i.e., 5 min worth of data). This is updated typically every minute, through sliding the calculation window. Classically, negative values are believed to represent “intact” cerebrovascular reactivity, and positive values represent “impaired” reactivity. This methodology focuses only on the time-domain relationship between signals. As an aside, there are alternatives that rely on frequency-domain assessments of the relationship between measured physiologies, which require more complex methodologies [15, 26]. Such measures are not widely employed in clinical monitoring for TBI at this time, and will not be discussed further here.
To date, PRx has displayed a strong association with global outcomes in adult TBI [10, 36, 51, 55], with an independent association with outcome beyond that accounted for by ICP [55]. PRx is one of the only measures receiving some validation in experimental animal models as a measure of the lower limit of autoregulation [6, 47, 53]. In addition, recent data has shown that impaired cerebrovascular reactivity metrics may predict lesion growth on follow-up computed tomography (CT) of the brain in TBI [30, 31]. Furthermore, there is a strong link between impaired cerebral metabolism and deranged PRx values [43]. Critical thresholds associated with 6-month outcomes have also been defined for PRx in adult TBI [36, 51]. Finally, PRx has been utilized in the exploration of individualized CPP and ICP targets in TBI care [2, 24, 38, 56, 57], sparking an ongoing Phase II study for individualized CPP targets [4].
However, despite the promise of PRx, it remains experimental still in the world of advanced monitoring in TBI, with alternative ICP-derived continuous cerebrovascular reactivity measures emerging in the literature [3, 11, 19, 49]. These alternative ICP-based metrics have been overshadowed by the large, and ever growing, body of literature on PRx [45]. With that said, these other ICP-based metrics do deserve some attention and discussion around when they should be considered for monitoring. The goal of this scoping review is to provide a comprehensive overview of the available literature on these alternative (i.e., non-PRx) ICP-based continuous metrics of cerebrovascular reactivity in adult TBI.
Methods
In order to be comprehensive in this scoping overview of alternative ICP-based continuous cerebrovascular reactivity metrics, a systematic search of the literature was conducted, using the methodology outlined in the Cochrane Handbook for Systematic Reviewers [18], with reporting in keeping with the Preferred Reporting In Systematic Reviews and Meta-Analysis (PRISMA) [32] and PRISMA Scoping Review (PRISMA-ScR) guidelines [44].
Search question, population, inclusion and exclusion criteria
The question posed for this systematic review was what literature is available on time-domain non-PRx ICP-based continuous cerebrovascular reactivity measures in adult TBI? The following time domain continuous pressure techniques were searched: pulse amplitude index (PAx), correlation between pulse amplitude of ICP and CPP (RAC), bandpass filtered PRx55-15, and low-resolution-based PRx alternatives (long-PRx (L-PRx) and LAx). Low resolution is defined as using minute-by-minute mean physiologic measures in the derivation of the index. We purposefully did not evaluate frequency-domain-based alternative measures, such as continuously updating wavelet phase-shift methodologies [26], given the current focus clinically is on time-domain-based metrics, such as PRx and its alternatives, given their current greater ease of derivation and clinical application.
Inclusion/exclusion criteria
Inclusion criteria were all full manuscript studies including human subjects with TBI (any severity), studies with 5 or more patients, adults only (age 18 or older), and the use of a continuous non-PRx ICP-based cerebrovascular reactivity metric (as listed above). Exclusion criteria were non-English studies, animal studies, exclusively pediatric patient populations, studies of less than 5 patients, and studies on non-PRx ICP-based continuous measures of cerebrovascular reactivity not based in the time domain. We chose a limit of 5 patients or more, so as to avoid small incidental case reports on the topic of ICP-derived cerebrovascular reactivity measures.
Search strategy
MEDLINE, BIOSIS, EMBASE, Global Health, SCOPUS, and Cochrane Library from inception to December 2019 were searched using individualized search strategies. The search strategy for MEDLINE can be seen in Appendix A of the supplementary materials. Finally, reference lists of any review articles on autoregulation/cerebrovascular reactivity techniques were reviewed for relevant studies on continuous techniques in adult TBI.
Study selection
A two-step review of all articles returned by our search strategies was performed. First, the reviewer independently screened titles and abstracts of the returned articles to decide if they met the inclusion criteria. Second, full text of the chosen articles was then assessed to confirm if they met the inclusion criteria. Any meeting abstracts identified within the database search results were crosschecked with MEDLINE in order to determine if any full manuscripts were subsequently published based on these abstract results.
Data collection
Data were extracted from the selected articles and stored in an electronic database. Data fields included patient demographics, type of study, article country of origin, number of patients, technique described, and study outcomes described. We split the techniques into high resolution (i.e., PAx, RAC, PRx55-15) and low resolution (i.e., L-PRx and LAx), with Table 1 displaying the high-resolution studies, and Table 2 displaying the low-resolution studies.
Results
Search results
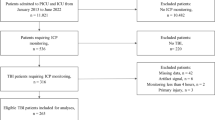
A total of 111 results were obtained from the search strategy highlighted in Appendix A of the Supplementary Materials. Appendix B provides the PRISMA flow diagram for the search results. Through the first filter and second filter, a total of 23 articles met the inclusion criteria to be included in the review. A total of 16 studies were pertaining to high-resolution techniques, while 7 pertained to low-resolution techniques. All studies, except one [41], focused on entirely European and United Kingdom data. Tables 1 and 2 provide the details of the high-resolution and low-resolution studies, respectively.
“The basics”
The reader will find the following sub-sections describing some more details regarding the specific ICP-derived continuous metrics. In general, the derivation of these time-domain-based measures follows similar principals, evaluating the relationship between pulsatile CBV and a driving pressure (MAP or CPP). First, both the ICP and MAP/CPP signals are processed to focus on a frequency range associated with cerebrovascular reactivity. This is typically in the range of 0.05 to 0.005 Hz, and involves the application of either non-overlapping smoothing average filters, low-frequency bandpass filters, or both, depending on the metric of interest. Second, with the signal data processed, the update frequency for the data is either every 10 s, or minute, depending on the measure. Third, a window of data for the processed ICP and MAP/CPP data is then utilized to derive a Pearson correlation coefficient. This data window varies depending on the measure, with most using a 5-min window of data for the high-resolution measures. Longer window lengths are used for low-resolution metrics such as LAx and L-PRx. Fourth, this calculation is then updated every minute. In general, positive index values are believe to represent “impaired” cerebrovascular reactivity, while negative values denote “intact” cerebrovascular reactivity.
High-resolution techniques
Table 1 provides an overview of the available literature body for high-resolution non-PRx ICP-derived continuous cerebrovascular reactivity metrics in adult TBI. The following sub-sections will highlight each of PAx, RAC, and PRx55-15, their derivation method, and potential benefit over PRx in TBI monitoring. For details regarding individual studies, we refer the reader to Table 1, and the referenced literature.
-
1.
PAx
Pulse amplitude index (PAx) is derived using the relationship between slow-wave fluctuations in the fundamental amplitude of ICP (AMP) and MAP. AMP is derived using the fundamental Fourier amplitude of full-waveform ICP data over a 10-s window, updated every 10 s. A moving Pearson correlation coefficient is then derived between AMP and MAP, in keeping with the methodology described previously for PRx. The rationale behind its derivation focuses on the potential for AMP to provide a better surrogate metric for pulsatile CBV. Statistically, PAx appears to behave similarly to PRx, RAC, near-infrared-based indices, and transcranial Dopple-based systolic flow index [46, 48].
Clinical literature on the use of PAx in TBI patients is limited to date. Initial retrospective studies suggested a potential benefit of PAx over PRx in 6-month outcome prediction, but only for those with low mean ICP values (i.e., less than 15 mmHg) over their ICU stay. In this study, PAx demonstrated a superior ability to predict mortality over PRx, in those with mean ICP values below 15 mmHg (− 0.04 ± 0.16 vs. − 0.14 ± 0.16, χ2 = 6, p = 0.01) [3]. Similarly, PAx was shown in one study to correlate with transcranial Doppler-based mean flow index (R2 = 0.46, p < 0.0002) [33]. Furthermore, critical thresholds for PAx in association with 6-month outcomes have been defined as 0, for favorable/unfavorable outcome, and + 0.25 for mortality [51]. This was conducted in non-craniectomized patients, so as to avoid any potential influence of decompressive hemicraniectomy on cerebrovascular reactivity measures [42]. Finally, impaired cerebrovascular reactivity, as defined by PAx, appears to be associated with admission CT imaging markers of diffuse intracranial injury, and advanced age [50]. Recent neuroimaging work has demonstrated that worse PAx values (r = 0.64, p = 0.0006) may be associated with CT-based lesion progression, particularly peri-contusional edema, during the acute phase of ICU care [30].
However, recent larger studies, both retrospective and prospective, have demonstrate inferiority of PAx in outcome prediction over PRx and RAC [51, 57, 58]. Furthermore, optimal CPP (CPPopt) based on PAx appear to have limited association with 6-month outcomes, with most PAx-CPPopt measures failing to reach any statistically significant associations with mortality or favorable/unfavorable outcomes at 6 months in the multi-center Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) high-resolution cohort [57]. Thus, the role of PAx and its derived physiology targets remains in question in adult TBI monitoring.
-
2.
RAC
RAC, defined as the correlation (R) between AMP (A) and CPP (C), is derived from the moving Pearson correlation between AMP and CPP. A relatively newly described index of cerebrovascular reactivity in TBI, its accurate interpretation is complex and beyond the scope of the review. Detailed description of this index can be found in the referenced articles [49]. In general, using CPP as the driving metric for flow, and AMP as the surrogate measure for pulsatile CBV, RAC provides information regarding both cerebrovascular reactivity [3] and cerebral compensatory reserve [9, 52]. Continuously measured cerebral compensatory reserve had been described in various sources in both the hydrocephalus literature and adult TBI literature through the index RAP—the correlation (R) between vasogenic slow-waves in AMP (A) and ICP (P) [21, 52]. Thus, with RAC being derived from the correlation between AMP and CPP, it provides information both from PAx (described in the previous section) and RAC indices, or so it is believed [49]. Its main limitation currently is complexity of interpretation. RAC appears to behave similarly to PRx, PAx, near-infrared-based indices, and transcranial Doppler-based systolic flow index, statistically [46, 48].
RAC and its evaluation in adult TBI patients is still considered exploratory and experimental in nature, as highlighted by the initial study fully describing this index [49]. Compared to PRx, RAC appears to perform similarly in its association with both mortality and favorable/unfavorable outcome at 6 months, in both retrospective [51] and multi-center prospective data sets [58]. In fact, RAC trends to higher AUC for favorable/unfavorable outcome association, compared to PRx, suggesting it may prove superior in this area of outcome prognostication. Thresholds for 6-month mortality and favorable/unfavorable outcome have also been described as − 0.10 and − 0.05, respectively [51]. Furthermore, RAC appears to display a more uniform parabolic relationship with CPP, suggesting it may carry a role in CPPopt determination [49, 58]. Some preliminary work in RAC-based CPPopt determination has demonstrated comparable performance versus PRx [57], though this work is quite exploratory in nature at this time. Finally, recent neuroimaging work has demonstrated that worse RAC values may be associated with CT-based lesion progression, particularly peri-contusional edema, during the acute phase of ICU care [30].
-
3.
PRx55-15
In an attempt to focus on purely the vasogenic frequency range associated with cerebral autoregulation (i.e., 0.02 to 0.07 Hz) [14, 19, 37, 59], PRx55-15 was developed to remove very-low-frequency bands (i.e., 0.01 to 0.005 Hz) of ICP and MAP outside of this range typically found in classic PRx calculations, and provide a “pure” metric [19]. PRx classically contains the slow-wave vasogenic frequency band of 0.05 to 0.005 Hz [7, 10, 14]. In order to derive PRx55-15, raw full-waveform ICP and MAP data require processing using a frequency bandpass filter for 0.018 to 0.067 Hz (i.e., oscillations with a period of 55 to 15 s in duration) [19]. Then, 5-min sliding data windows are used to derive the cerebrovascular reactivity metric, similar to classic PRx [10].
Given the relatively new nature of this index, there exists very limited literature on its application in TBI. Small retrospective studies have found that PRx55-15 is independently associated with global 6-month outcome, and displays reduced variability compared to PRx [19, 39]. Furthermore, there appears to be a positive correlation between microdialysis-based lactate:pyruvate ratio and impaired cerebrovascular reactivity, as measured through PRx55-15 [40]. However, much further validation of this metric is required prior to widespread adoption.
Low-resolution techniques
Table 2 provides an overview of the literature for low-resolution ICP-derived continuous cerebrovascular reactivity metrics in adult TBI. Low-frequency autoregulation index (LAx) and long-PRx (L-PRx) variants exist, with nomenclature depending on the center and study. In general, these measures are derived using minute-by-minute mean ICP and MAP data. Moving Pearson correlation coefficients are then calculated between these minute-by-minute measures, using various described window lengths of 5 min up to and including 120 min. The most commonly quoted window lengths for calculation are on the order of 20 to 30 min in length. These metrics were developed for situations where high-resolution full-waveform ICP and MAP data was not available. Many commercially available ICU monitors have data output frequencies limited to 1 Hz or less, leaving classic PRx, or other high-resolution techniques not feasible. Similarly, many centers do not have the biomedical engineering expertise to extract, store and process full-waveform ICP and MAP data, if the ability to export such data exists. As such, in an attempt to expand the accessibility of cerebrovascular reactivity monitoring outside of a limited set of expert academic centers, these metrics were developed. The main limitation is that they evaluate the ultra-low-frequency band (i.e., below 0.005 Hz) for ICP and MAP, and uncertainty regarding the appropriate window length for calculation. It remains unknown how much, if any, autoregulation information is contained within this frequency range [19, 34]. Though, recent data suggests that shorter window lengths demonstrate strong statistical associations with classic PRx [41].
Various clinical studies exist correlating L-PRx/LAx metrics to high-resolution PRx, or other non-ICU-based continuous measures of cerebrovascular reactivity. Using a 20-min calculation window, L-PRx has been shown to be moderately correlated with ICP (r = 0.467, p = 0.011), and elevated lactate:pyruvate ratio on microdialysis [34], similar to larger microdidalysis studies with high-resolution PRx [43]. Similarly, LAx, studied using different window lengths, demonstrated strong outcome association at 6 months [16, 17]. Further, impaired cerebrovascular reactivity, defined by LAx, was shown to reduce the CPP threshold for insults tolerated in this patient cohort [17]. Furthermore, it has been demonstrated that LAx may be utilized to determine CPPopt, with strong outcome associations [11, 12]. However, some studies have questioned the strength of outcome association for these low-resolution metrics, displaying superior performance of PRx over L-PRx [23]. Thus, given the current limited and conflicting literature body, these metrics should still remain exploratory in nature.
Discussion
We aimed to produce a comprehensive scoping overview of the literature regarding ICP-based non-PRx continuous cerebrovascular reactivity metrics in adult TBI. The literature remains scare to date regarding the applicability of these metrics. However, it must be acknowledged that many of these measures display promise above and beyond classic PRx monitoring. The main drawback with PRx monitoring and management strategies is its dependency on a few selected ICU monitoring solutions; thus, if other indices show potential, it could offer solutions for more widespread implementation. Some important aspects deserve highlighting.
First, the high-resolution ICP-based alternative metrics appear to display some promise. PAx may have a role in those patients with low mean ICP for cerebrovascular reactivity assessments [3, 33], and potentially even CPPopt determination [57]. RAC, though complex in interpretation, carries information regarding both cerebrovascular reactivity and compensatory reserve [49], with trends toward stronger favorable/unfavorable outcome association at 6 months compared to PRx [51, 58]. Further, RAC may have an emerging role in CPPopt determination, given the stronger parabolic relationship seen between RAC and CPP, compared to PRx and CPP, on preliminary analysis [49, 57]. Finally, PRx55-15 provides a cerebrovascular reactivity index with more focus on the true autoregulation slow-wave frequency range [19], with strong outcome association [19, 39]. However, overall the literature on these three metrics is limited, leaving their application currently as experimental. Furthermore, all three indices are much more complex to derive, requiring comfort with either Fourier analysis [3, 49], or bandpass filtering techniques [19]. This may limit this adoption to non-expert clinical centers. Much further investigation is required to validate their associations with outcome, assess their role in prognostication, and determine applicability in the derivation of individualized physiologic targets in TBI.
Second, the low-resolution metrics, L-PRx and LAx, have very limited literature to date [11, 34]. They show promise in that they can be derived using minute-by-minute data, opening the doors for widespread adoption into centers without biomedical engineering expertise, or the ability to obtain high-frequency full-waveform physiologic data. Preliminary data indicates that L-PRx and LAx have some moderate association with high-resolution PRx [23], and demonstrate strong associations with outcome [11, 16]. In addition, they may even be applicable to the derivation of individualized CPPopt [12], potentially expanding this type of personalized medicine to non-specialized centers. However, L-PRx and LAx should still be considered very experimental, given it is unclear what aspect of the autoregulation they are truly measuring [14, 19, 23], and the optimal window length for calculation remains unclear [41].
Third, the literature body surrounding ICP-derived PRx alternatives is complex. Over the years, various measures of cerebrovascular reactivity have been derived based on ICP signals. Yet, none have gained the traction of the original metric in this field, PRx. As new measures are derived, they tend to be compared to PRx. This yields a situation where newer metrics tend to take a back-seat to PRx, regardless of their comparative performance. Indices which perform poorly in comparison to PRx tend to be “shelved,” rarely emerging in future studies on advanced neuromonitoring. Similarly, those metrics which perform similar to PRx also tend to be overlooked, as PRx is easier to derive and has a larger captive audience based on it being the historical first measure. This produces a situation where it is difficult for newer measures to gain traction and acceptance in the literature and amongst end-users, despite their nuanced potential. As multi-center collaborative groups and data sets emerge, it is imperative that these ICP-derived alternatives be re-explored.
Fourth, the strength of relationships and conclusions in specific studies must be considered preliminary and exploratory. As mentioned above, many studies compare newer ICP-derived metrics to PRx. Thus, we are comparing ICP to ICP-derived measures. This brings up the issue of collinearity in comparison between measures of similar origin. As such, the strength of statistically significant results requires some degree of skepticism in their interpretation given most have not accounted for such collinearity. One way around this would be to assess the relationship between two, or more, such measures while evaluating the variance inflation factor analysis. Another would be to explore the higher-resolution behavior of such measures over time using time-series methodologies, which would also account for autocorrelation within a given metric over time [41]. To date, very little work in this area has been conducted, and will be the focus of future multi-center collaborative data sets [5].
In general, we are still a ways away from adoption of either the high-resolution or low-resolution ICP-based alternative measures. All should remain experimental at this time. Future work in the area will take coordination between multiple international centers of excellent in cerebrovascular reactivity monitoring, pooled data, and ongoing prospective data collection strategies, in order to validate current findings and truly understand the role of these measures in adult TBI care.
Limitations
Despite a systematically conducted scoping overview of the literature being conducted, there are some significant limitations of this review and the outlined literature body, which deserve mentioning. First, despite an all-inclusive search strategy, it appears that there remains limited literature for ICP-derived continuous cerebrovascular reactivity monitoring, outside of PRx. Thus, even though some interesting trends regarding specific alternative indices were mentioned in this review, these metrics remain exploratory at this time. They should not be adapted to widespread clinical monitoring of cerebrovascular reactivity without proper validation and exploration in multi-center datasets. Furthermore, most studies were generated from a select few centers in Europe with specialized expertise in biomedical signal processing and its application to advanced monitoring in TBI patients. As such, many studies have overlapping patient populations from these few centers of excellence, given such data is both cumbersome to collect, analyze, and interpret. Given many studies in the area of biomedical engineering techniques in TBI monitoring are exploratory, the same datasets are utilized to try to answer different questions/aspects of cerebral physiology post-injury. It is impossible to explore and answer all questions related to different measures of cerebral physiology or cerebrovascular reactivity in one or two papers, given the complexity of analysis and interpretation, as well as the myriad of very different questions being answered. The archived data sets from the few centers of excellence who lead this area are optimally positioned to be able to answer such preliminary questions, and have thus generated many papers from a relatively small data source. Of course, validation of such findings is another story, and highlights the need for future multi-center data collection strategies in TBI, which may be able to both validate and provide clarity regarding the utility of such measures.
Second, most of the studies included were retrospective in nature. The patient populations were heterogeneous in nature, with varied periods of high-frequency digital physiologic monitoring described. Many of these studies came from the same centers, with overlapping patient populations between articles. Thus, it is unknown how many unique patients were studied overall. Furthermore, these patients were adult TBI populations receiving active treatment for ICP and CPP during their ICU care. Meaning, their described behavior does not represent the true untreated behavior, or nature behavior, in adult TBI. As such, lack of association between these alternative metrics and classical PRx must be taken with some degree of skepticism. No strong conclusions regarding these alternative measures can be made at this time, as they all suffer from significant and varied biases.
Third, given the heterogenous nature of these studies, formal meta-analysis is not possible, nor would it have led to interpretable results. As such, we are left with a simple scoping overview of the literature. This emphasizes the preliminary and exploratory nature of the described studies, necessitating much further investigation in larger multi-center cohorts.
Fourth, at this time, we are unable to make definitive comments on the utility of longer window versions of PRx, or weighted window approaches. One of the included studies documented various window lengths in the determination of CPPopt based on LAx [11, 12]. It is possible that LAx or L-PRx provide different pieces of information regarding cerebral autoregulatory status, by variations in window length and/or weighting used in the derivation of such indices. Shorter versus longer windows may provide information on dynamic or static aspects of cerebral vasomotion. However, it must be acknowledged that increasing window lengths does run the risk of including information beyond the vasogenic frequency range associated with autoregulation, further muddying the picture. Thus, at this time, much further work is required to both validate such measures and investigate their ability to measure aspects of the Lassen curve in experimental models, and their application in TBI monitoring. Such work will be the ongoing efforts of the CENTER-TBI high-resolution sub-study [27] and the CAnadian High Resolution Traumatic Brain Injury (CAHR-TBI) Research Collaborative [5].
Fifth, given the small number of studies, from a few centers in Europe, with occasional overlapping data sets, there runs the risk that the identified studies in this area suffer from publication bias, with only positive findings being described. This is true of many emerging and niche areas in medicine, where the focus is only on positive findings. As such, this is another factor which limits the ability to extrapolate the findings of the studies included in this scoping review. However, one must acknowledge that recent multi-center data sets have begun to provide evidence in support of the previous single center retrospective findings [57, 58]. This again highlights the need for ongoing multi-center initiatives in advanced neuromonitoring [5, 27].
Finally, we limited our search to continuous metrics, based in the time domain. This was purposefully conducted, as current application of continuous cerebrovascular reactivity monitoring in adult TBI utilizes time-domain-based approaches. Such approaches are convenient and have a high ease of application for clinicians at the bedside. With that said, there are some frequency-domain ICP-based continuous measures that were not described in this review, such was wavelet-PRx [26]. These metrics may prove useful in the future, with improved background processes for derivation and better understanding of their interpretation. However, at this time, they have limited applicability in the clinical monitoring of the TBI patient.
Future directions
Based on this scoping review, it is clear that the literature on ICP-derived continuous cerebrovascular reactivity indices is limited. As alluded to above, there exists a role for future evaluation of such measures. Future work necessitates multi-center prospectively acquired high-frequency physiologic data sets in order to provide validation to these previous exploratory works. This work will focus not only on the derived cerebrovascular reactivity metrics themselves, but also on individualized physiology thresholds, such as CPPopt or individual ICP thresholds, in order to assess which measure may be optimally positioned to derive such personalized targets. This will require large patient numbers, in addition to quality control in the data collection/archival processes. Such initiatives are underway with the CENTER-TBI HR cohort [27] and the recently developed CAHR-TBI initiative in Canada [5]. Furthermore, evaluation of lower-resolution metrics, such as LAx and L-PRx, is a focus of our collaborative research group between Canada, Sweden, and Finland [41]. Such work will focus on evaluating the role of these alternative measures in the clinical monitoring of cerebrovascular reactivity, and will employ more complex statistical methodologies, accounting for various patient factors, injury patterns, and collinearity. This work will also employ time-series methodologies to account for autocorrelation, and hopefully better describe the higher-frequency relationship between such measures in vivo in TBI. Finally, we plan to explore machine learning methodologies in the assessment of co-variance and behavior of these various indices over time. Such initiatives will be multi-disciplinary endeavors, including computer science, engineering, neuroscience, physiology, and population health experts.
However, future work cannot only rely on clinical data sets, as every cerebrovascular reactivity measure has yet to be validated as a reliable measure of the Lassen curve. As such, future work necessitates experimental large animal studies to evaluate the ability of such metrics to detect both the lower limit and upper limit of autoregulation, during extremes of both MAP and ICP. Such large animal model work is expensive, and will require multi-center collaborative approaches in order to accomplish such goals.
Conclusions
Non-PRx continuous ICP-based cerebrovascular reactivity metrics can be split into high-resolution and low-resolution measures. High-resolution indices include PAx, RAC, and PRx55-15, while low-resolution indices include L-PRx and LAx. It remains unclear the true role for this metrics beyond classic PRx. Each displays situations where it may prove superior over PRx, given limitations with this currently widely accept measure. Much future investigation into each of these alternative metrics is required prior to adoption into the clinical monitoring armamentarium in adult TBI.
References
Andresen M, Donnelly J, Aries M, Juhler M, Menon D, Hutchinson P, Smielewski P (2018) Further controversies about brain tissue oxygenation pressure-reactivity after traumatic brain injury. Neurocrit Care 28(2):162–168
Aries MJH, Czosnyka M, Budohoski KP et al (2012) Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med 40(8):2456–2463
Aries MJH, Czosnyka M, Budohoski KP, Kolias AG, Radolovich DK, Lavinio A, Pickard JD, Smielewski P (2012) Continuous monitoring of cerebrovascular reactivity using pulse waveform of intracranial pressure. Neurocrit Care 17(1):67–76
Beqiri E, Smielewski P, Robba C et al (2019) Feasibility of individualised severe traumatic brain injury management using an automated assessment of optimal cerebral perfusion pressure: the COGiTATE phase II study protocol. BMJ Open 9(9):e030727
Bernard F, Gallagher C, Griesdale D, Kramer A, Sekhon M, Zeiler FA (2020) The Canadian High-Resolution Traumatic Brain Injury (CAHR-TBI) research collaborative. Can J Neurol Sci J Can Sci Neurol:1–20
Brady KM, Lee JK, Kibler KK, Easley RB, Koehler RC, Shaffner DH (2008) Continuous measurement of autoregulation by spontaneous fluctuations in cerebral perfusion pressure: comparison of 3 methods. Stroke 39(9):2531–2537
Brady KM, Easley RB, Kibler K et al (2012) Positive end-expiratory pressure oscillation facilitates brain vascular reactivity monitoring. J Appl Physiol Bethesda Md 1985 113(9):1362–1368
Budohoski KP, Reinhard M, Aries MJH, Czosnyka Z, Smielewski P, Pickard JD, Kirkpatrick PJ, Czosnyka M (2012) Monitoring cerebral autoregulation after head injury. Which component of transcranial Doppler flow velocity is optimal? Neurocrit Care 17(2):211–218
Calviello L, Donnelly J, Cardim D, Robba C, Zeiler FA, Smielewski P, Czosnyka M (2018) Compensatory-reserve-weighted intracranial pressure and its association with outcome after traumatic brain injury. Neurocrit Care 28(2):212–220
Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD (1997) Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery 41(1):11–17 discussion 17-19
Depreitere B, Güiza F, Van den Berghe G, Schuhmann MU, Maier G, Piper I, Meyfroidt G (2014) Pressure autoregulation monitoring and cerebral perfusion pressure target recommendation in patients with severe traumatic brain injury based on minute-by-minute monitoring data. J Neurosurg 120(6):1451–1457
Depreitere B, Güiza F, Van den Berghe G, Schuhmann MU, Maier G, Piper I, Meyfroidt G (2016) Can optimal cerebral perfusion pressure in patients with severe traumatic brain injury be calculated based on minute-by-minute data monitoring? Acta Neurochir Suppl 122:245–248
Donnelly J, Czosnyka M, Adams H et al (2019) Twenty-five years of intracranial pressure monitoring after severe traumatic brain injury: a retrospective, single-center analysis. Neurosurgery 85(1):E75–E82
Fraser CD, Brady KM, Rhee CJ, Easley RB, Kibler K, Smielewski P, Czosnyka M, Kaczka DW, Andropoulos DB, Rusin C (2013) The frequency response of cerebral autoregulation. J Appl Physiol Bethesda Md 1985 115(1):52–56
Govindan RB, Brady KM, Massaro AN, Perin J, Jennings JM, DuPlessis AJ, Koehler RC, Lee JK (2019) Comparison of frequency- and time-domain autoregulation and vasoreactivity indices in a piglet model of hypoxia-ischemia and hypothermia. Dev Neurosci:1–13
Güiza F, Depreitere B, Piper I et al (2015) Visualizing the pressure and time burden of intracranial hypertension in adult and paediatric traumatic brain injury. Intensive Care Med 41(6):1067–1076
Güiza F, Meyfroidt G, Piper I et al (2017) Cerebral perfusion pressure insults and associations with outcome in adult traumatic brain injury. J Neurotrauma 34(16):2425–2431
Higgins J, Green S (2008) Cochrane handbook for systematic review of interventions, 1st edn. Wiley-Blackwell
Howells T, Johnson U, McKelvey T, Enblad P (2015) An optimal frequency range for assessing the pressure reactivity index in patients with traumatic brain injury. J Clin Monit Comput 29(1):97–105
Jaeger M, Lang EW (2013) Cerebrovascular pressure reactivity and cerebral oxygen regulation after severe head injury. Neurocrit Care 19(1):69–73
Kim D-J, Czosnyka Z, Keong N, Radolovich DK, Smielewski P, Sutcliffe MPF, Pickard JD, Czosnyka M (2009) Index of cerebrospinal compensatory reserve in hydrocephalus. Neurosurgery 64(3):494–501 discussion 501-502
Lang EW, Czosnyka M, Mehdorn HM (2003) Tissue oxygen reactivity and cerebral autoregulation after severe traumatic brain injury. Crit Care Med 31(1):267–271
Lang EW, Kasprowicz M, Smielewski P, Santos E, Pickard J, Czosnyka M (2015) Short pressure reactivity index versus long pressure reactivity index in the management of traumatic brain injury. J Neurosurg 122(3):588–594
Lazaridis C, DeSantis SM, Smielewski P, Menon DK, Hutchinson P, Pickard JD, Czosnyka M (2014) Patient-specific thresholds of intracranial pressure in severe traumatic brain injury. J Neurosurg 120(4):893–900
Le Roux P, Menon DK, Citerio G et al (2014) The International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: a list of recommendations and additional conclusions: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit Care 21(Suppl 2):S282–S296
Liu X, Donnelly J, Czosnyka M et al (2017) Cerebrovascular pressure reactivity monitoring using wavelet analysis in traumatic brain injury patients: a retrospective study. PLoS Med 14(7):e1002348
Maas AIR, Menon DK, Steyerberg EW, Citerio G, Lecky F, Manley GT, Hill S, Legrand V, Sorgner A, CENTER-TBI Participants and Investigators (2015) Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery 76(1):67–80
Mathieu F, Khellaf A, Ku JC, Donnelly J, Thelin EP, Zeiler FA (2019) Continuous near-infrared spectroscopy monitoring in adult traumatic brain injury: a systematic review. J Neurosurg Anesthesiol. https://doi.org/10.1097/ANA.0000000000000620
Mathieu F, Khellaf A, Thelin EP, Zeiler FA (2019) Continuous thermal diffusion-based cerebral blood flow monitoring in adult traumatic brain injury: a scoping systematic review. J Neurotrauma 36(11):1707–1723
Mathieu F, Zeiler FA, Whitehouse DP, Das T, Ercole A, Smielewski P, Hutchinson PJ, Czosnyka M, Newcombe VFJ, Menon DK (2019) Relationship between measures of cerebrovascular reactivity and intracranial lesion progression in acute TBI patients: an exploratory analysis. Neurocrit Care. https://doi.org/10.1007/s12028-019-00885-3
Mathieu F, Zeiler FA, Ercole A et al (2020) Relationship between measures of cerebrovascular reactivity and intracranial lesion progression in acute TBI patients: a CENTER-TBI study. J Neurotrauma. https://doi.org/10.1089/neu.2019.6814
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4):264–269 W64
Radolovich DK, Aries MJH, Castellani G, Corona A, Lavinio A, Smielewski P, Pickard JD, Czosnyka M (2011) Pulsatile intracranial pressure and cerebral autoregulation after traumatic brain injury. Neurocrit Care 15(3):379–386
Sánchez-Porras R, Santos E, Czosnyka M, Zheng Z, Unterberg AW, Sakowitz OW (2012) “Long” pressure reactivity index (L-PRx) as a measure of autoregulation correlates with outcome in traumatic brain injury patients. Acta Neurochir 154(9):1575–1581
Sorrentino E, Budohoski KP, Kasprowicz M, Smielewski P, Matta B, Pickard JD, Czosnyka M (2011) Critical thresholds for transcranial Doppler indices of cerebral autoregulation in traumatic brain injury. Neurocrit Care 14(2):188–193
Sorrentino E, Diedler J, Kasprowicz M et al (2012) Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit Care 16(2):258–266
Stauss HM (2007) Identification of blood pressure control mechanisms by power spectral analysis. Clin Exp Pharmacol Physiol 34(4):362–368
Steiner LA, Czosnyka M, Piechnik SK, Smielewski P, Chatfield D, Menon DK, Pickard JD (2002) Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med 30(4):733–738
Svedung Wettervik T, Howells T, Enblad P, Lewén A (2019) Temporal neurophysiological dynamics in traumatic brain injury: role of pressure reactivity and optimal cerebral perfusion pressure for predicting outcome. J Neurotrauma. https://doi.org/10.1089/neu.2018.6157
Svedung Wettervik T, Howells T, Ronne-Engström E, Hillered L, Lewén A, Enblad P, Rostami E (2019) High arterial glucose is associated with poor pressure autoregulation, high cerebral lactate/pyruvate ratio and poor outcome following traumatic brain injury. Neurocrit Care 31(3):526–533
Thelin EP, Raj R, Bellander B-M et al (2019) Comparison of high versus low frequency cerebral physiology for cerebrovascular reactivity assessment in traumatic brain injury: a multi-center pilot study. J Clin Monit Comput. https://doi.org/10.1007/s10877-019-00392-y
Timofeev I, Czosnyka M, Nortje J, Smielewski P, Kirkpatrick P, Gupta A, Hutchinson P (2008) Effect of decompressive craniectomy on intracranial pressure and cerebrospinal compensation following traumatic brain injury. J Neurosurg 108(1):66–73
Timofeev I, Carpenter KLH, Nortje J et al (2011) Cerebral extracellular chemistry and outcome following traumatic brain injury: a microdialysis study of 223 patients. Brain J Neurol 134(Pt 2):484–494
Tricco AC, Lillie E, Zarin W et al (2018) PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 169(7):467–473
Zeiler FA, Donnelly J, Calviello L, Smielewski P, Menon DK, Czosnyka M (2017) Pressure autoregulation measurement techniques in adult traumatic brain injury, part II: a scoping review of continuous methods. J Neurotrauma 34(23):3224–3237
Zeiler FA, Donnelly J, Menon DK, Smielewski P, Zweifel C, Brady K, Czosnyka M (2017) Continuous autoregulatory indices derived from multi-modal monitoring: each one is not like the other. J Neurotrauma 34(22):3070–3080
Zeiler FA, Donnelly J, Calviello L, Lee JK, Smielewski P, Brady K, Kim D-J, Czosnyka M (2018) Validation of pressure reactivity and pulse amplitude indices against the lower limit of autoregulation, part I: experimental intracranial hypertension. J Neurotrauma 35(23):2803–2811
Zeiler FA, Donnelly J, Cardim D, Menon DK, Smielewski P, Czosnyka M (2018) ICP versus laser Doppler cerebrovascular reactivity indices to assess brain autoregulatory capacity. Neurocrit Care 28(2):194–202
Zeiler FA, Donnelly J, Menon DK, Smielewski P, Hutchinson PJA, Czosnyka M (2018) A description of a new continuous physiological index in traumatic brain injury using the correlation between pulse amplitude of intracranial pressure and cerebral perfusion pressure. J Neurotrauma. https://doi.org/10.1089/neu.2017.5241
Zeiler FA, Donnelly J, Nourallah B, Thelin EP, Calviello L, Smielewski P, Czosnyka M, Ercole A, Menon DK (2018) Intracranial and extracranial injury burden as drivers of impaired cerebrovascular reactivity in traumatic brain injury. J Neurotrauma 35(14):1569–1577
Zeiler FA, Donnelly J, Smieleweski P, Menon D, Hutchinson PJ, Czosnyka M (2018) Critical thresholds of ICP derived continuous cerebrovascular reactivity indices for outcome prediction in non-craniectomized TBI patients: PRx, PAx and RAC. J Neurotrauma 35(10):1107–1115
Zeiler FA, Kim D-J, Cabeleira M, Calviello L, Smielewski P, Czosnyka M (2018) Impaired cerebral compensatory reserve is associated with admission imaging characteristics of diffuse insult in traumatic brain injury. Acta Neurochir 160(12):2277–2287
Zeiler FA, Lee JK, Smielewski P, Czosnyka M, Brady K (2018) Validation of intracranial pressure-derived cerebrovascular reactivity indices against the lower limit of autoregulation, part II: experimental model of arterial hypotension. J Neurotrauma 35(23):2812–2819
Zeiler FA, Ercole A, Beqiri E, et al (2019) Cerebrovascular reactivity is not associated with therapeutic intensity in adult traumatic brain injury: a CENTER-TBI analysis. Acta Neurochir. Wien
Zeiler FA, Ercole A, Beqiri E, et al (2019) Association between cerebrovascular reactivity monitoring and mortality is preserved when adjusting for baseline admission characteristics in adult TBI: A CENTER-TBI Study. J Neurotrauma. https://doi.org/10.1089/neu.2019.6808
Zeiler FA, Ercole A, Cabeleira M, et al (2019) Patient-specific ICP epidemiologic thresholds in adult traumatic brain injury: a CENTER-TBI validation study. J Neurosurg Anesth. In Press
Zeiler FA, Ercole A, Cabeleira M, Carbonara M, Stocchetti N, Menon DK, Smielewski P, Czosnyka M, CENTER-TBI High Resolution (HR ICU) Sub-Study Participants and Investigators (2019) Comparison of performance of different optimal cerebral perfusion pressure parameters for outcome prediction in adult traumatic brain injury: a collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) study. J Neurotrauma 36(10):1505–1517
Zeiler FA, Ercole A, Cabeleira M, Zoerle T, Stocchetti N, Menon DK, Smielewski P, Czosnyka M, CENTER-TBI High Resolution Sub-Study Participants and Investigators (2019) Univariate comparison of performance of different cerebrovascular reactivity indices for outcome association in adult TBI: a CENTER-TBI study. Acta Neurochir 161(6):1217–1227
Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, Levine BD (2002) Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation 106(14):1814–1820
Funding
FAZ receives research support from the Manitoba Public Insurance (MPI) Neurscience/TBI Research Endowment, the United States National Institutes of Health (NIH) through the National Institute of Neurological Disorders and Stroke (NINDS), the Canadian Institutes of Health Research (CIHR), the Canadian Foundation for Innovation (CFI), the University of Manitoba VPRI Research Investment Fund (RIF), the University of Manitoba Rudy Falk Clinician-Scientist Professorship, and the Health Sciences Centre Foundation Winnipeg. EPT receives research support from Svenska Sällskapet för Medicinsk Forskning (SSMF), Hjärnfonden (Mattsons Stiftelse) and Region Stockholm Funding (ALF). RR receives research support from Finska Läkaresällskapet and Medicinska Understödsföreningen Liv & Hälsa. AG is support by the Clinician Investigator program at the University of Manitoba. LF is supported by a Department of Surgery GFT Surgeons Research Grant from clinical earnings of GFT surgeons at the University of Manitoba, Winnipeg, Manitoba, Canada.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable, given this is a review.
Additional information
Comments
Traumatic brain injury (TBI) affects millions of people each year and the most severe cases are treated in highly specialized neuro-intensive care units using multimodal neuromonitoring. Various invasive/non-invasive measurements are integrated to aid real-time assessment of brain physiology and guide therapeutic interventions to prevent secondary brain injury. The pressure reactivity index (PRx) has been proposed as a metric to continuously monitor cerebrovascular reactivity, but other similar indices with different advantages have been put forward. In this scoping review, the authors summarize the scientific evidence of non-PRx (ICP based) indices of cerebrovascular reactivity in adult TBI. As concluded in the paper, the literature is limited and primarily consists of retrospective investigations from a few centers. Currently, PRx-based optimization of cerebral perfusion pressure is investigated in a clinical feasibility trial (COGiTATE), but much further research is needed (including investigations in animal models) before any of the other indices can be put into clinical testing.
Alexander Lilja-Cyron
Copenhagen, Denmark
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Brain trauma
Rights and permissions
About this article
Cite this article
Hasen, M., Gomez, A., Froese, L. et al. Alternative continuous intracranial pressure-derived cerebrovascular reactivity metrics in traumatic brain injury: a scoping overview. Acta Neurochir 162, 1647–1662 (2020). https://doi.org/10.1007/s00701-020-04378-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-020-04378-7