Abstract
Background
Although cranioplasty (CP) is a frequently performed and simple procedure, complications are common, particularly bone flap resorption and infection. The timing of surgery is as an important contributory factor, but the optimal timing has not been clearly determined.
Objective
We retrospectively investigated bone flap resorption and surgical site infection after CP to determine the optimal timing of surgery for reduction of complications.
Methods
The study enrolled 126 patients who underwent decompressive craniectomy (DC) and subsequent CP. Patients with bone flap resorption or surgical site infection were analyzed as the “complication” group. Receiver operating characteristic curve analysis was performed and the Youden index was used to dichotomize “early CP” and “late CP” groups. Univariate and multivariate survival analyses were performed.
Results
The complication group included 42 patients. The Youden index was used to identify a cutoff value for the DC-CP interval of > 44 days, and this was used to define early (< 45 days) and late (≥ 45 days) CP. Late CP was a significant risk factor in univariate and multivariate Cox regression analyses.
Conclusion
This study showed that early CP before 45 days after DC is associated with a lower rate of bone flap resorption and surgical site infection than late CP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cranioplasty (CP) is required to restore the appearance of the skull, protect intracranial tissue, and reduce the psychological burden on patients who undergo preceding decompressive craniectomy (DC) [12]. CP is performed with an autologous bone flap or artificial material depending on the patient’s status. Autologous bone flap is usually the first choice in most institutions: it is readily available at no cost, is cosmetically acceptable, has growth potential, and is psychologically acceptable to the patients [1]. However, possible postoperative complications, including surgical site infection and bone flap resorption, occur [4]. The timing of CP is considered an important contributory factor. Recent studies identified “late CP” as a significant risk factor for bone flap resorption [21, 22]. Other studies found that surgical site infection was more common in patients who underwent either extremely early [16] or delayed CP [7, 19]. If early CP was associated with better neurological outcomes, as well as a lower risk of complications, it would be better to avoid delayed CP [3, 11, 14, 25]. However, there is no consensus on the optimal timing of CP, and the definition of “early” and “late” varies significantly among studies. Since the timing of CP is a factor that can be controlled by the surgeon, determining the optimal timing of CP may aid in decreasing the complication rate. We retrospectively reviewed our 13-year experience of CP to determine the optimal timing associated with fewer complications using statistical analyses.
Methods
Patients and operative technique
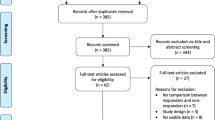
We retrospectively reviewed 156 patients who underwent standard DC and subsequent CP between January 2004 and December 2016. The study was approved by the Institutional Review Board of the Human Research Center of our hospital. Informed consent of the patient was not required because of the retrospective study design. The study excluded 28 patients who underwent CP with artificial materials, such as polymethyl methacrylate or titanium mesh plates. Two patients who had postoperative hematomas were also excluded. Thus, 126 patients were enrolled in the study (Fig. 1).
We performed DC when patients had refractory intracranial hypertension, despite administration of the best medical treatment. All patients received standard frontotemporoparietal craniectomy with durotomy and duroplasty. The bone flap was separated from the adherent tissue, packed in sterile towels, and stored at − 80 °C after surgery. Subsequent CP was scheduled after the resolution of brain swelling, based on the patient’s general medical status. The previous skin incision was reopened and the fibrous layer between the artificial dura and galea was prepared for reinsertion of the autologous bone flap. After the bone flap was washed several times with a povidone-iodine and normal saline solution, the flap was reimplanted with multiple epidural tack-up sutures and fixed in its original position, in close contact of the bone defect, using mini titanium plates and screws. The temporalis muscle was placed over the bone flap and fixed. Finally, the skin was closed with Vicryl and nylon sutures.
Clinical data collection and analysis
Data on the demographic characteristics and possible risk factors, including age, sex, reason for DC, unilateral or bilateral CP, bone dislodgement, multiplicity of bone flaps, preoperative skull fracture, operative time for CP, existence of shunting system (ventriculoperitoneal or lumboperitoneal shunt), the interval between DC and CP, and levels of inflammatory markers (erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP)) before and 1 week after CP were collected.
We classified the patients into “complication” and “no complication” groups, based on whether surgical site infection or bone flap resorption occurred. Other complications, such as postoperative hematoma, subdural hygroma, and wound dehiscence, were not considered. Surgical site infection was diagnosed clinically by any evidence of infection, including fever, erythema, swelling, elevated white blood cell count or inflammatory marker levels, results of blood or tissue cultures, and/or ring-enhancing fluid collection on contrast-enhanced cranial images (Fig. 2). Bone flap resorption was diagnosed with serial brain computed tomography (CT). We defined bone flap resorption as more than 50% thinning of the bone flap compared to the thickness of the contralateral region (Fig. 3).
Patients were also classified into “early CP” and “late CP” groups. To statistically define early and late periods, we used a receiver operating characteristic (ROC) curve and the Youden index. This method was used to obtain a cutoff value for the DC-CP interval to dichotomize continuous variables (DC-CP interval) into categorical variables (early and late CP).
Statistical analysis was performed using standard software (SPSS version 22.0, IBM, Chicago, IL, USA). Continuous variables are reported as median values ± standard deviation, while categorical data are reported as frequencies and percentages. Statistical significance was defined as a p value < 0.05. Univariate and multivariate Cox regression analysis were used to identify the risk factors for complications and to elucidate the differences between the groups, respectively.
Results
The area under the ROC curve (AUC) was 0.60, and the associated criterion of Youden index was > 44 days (Fig. 4 and Table 1). Early (< 45 days) and late (≥ 45 days) CP were defined based on these reference values. Figure5 shows the distribution of the patients.
Among the 126 enrolled patients, 42 were in the “complication” group (Table 2). The CP complication rate was 33.3% in our study; 11 patients were diagnosed with surgical site infection and 31 were diagnosed with bone flap resorption.
The results of univariate and multivariate Cox regression analyses are shown in Table 3. The parameters of late CP and postoperative ESR and CRP were statistically significant in the univariate analysis. Multivariate Cox regression analysis was performed with these parameters, and only late CP was statistically significant. Postoperative inflammatory marker levels were relatively high in the “complication” group; however, these were not significant factors in the multivariate analysis.
Discussion
This study used a statistical method to define “early cranioplasty”, and the result showed that early cranioplasty is “cranioplasty within 45 days.” Although CP is considered a simple procedure, the incidence of complications after CP is relatively high, ranging from 12 to 50%, and numerous risk factors for CP-associated complications have been identified in previous studies [7, 19, 21, 22]. Our study focused on the timing of CP as a risk factor associated with surgical site infection and bone flap resorption. The timing of surgery is important because it can be modified by the clinician.
Prior to investigating the optimal timing for surgery, we should define the complications of CP. In the current study, surgical site infection and bone flap resorption are regarded as complications of CP because these are the main causes of bone flap implantation failure, which is a unique procedure of CP [4, 9, 10, 15, 22]. Several studies reported that late CP was a significant risk factor for bone flap resorption [21, 22]. It is presumed that a long interval between DC and CP leads to decreased bone flap viability, which, in turn, leads to failure of bone remodeling [21, 22]. On the other hand, there are some argues about the association between the timing of CP and surgical site infection. A recent study revealed that the rate of infection was high if CP was performed extremely early (≤ 14 days) [16]. This may be associated with a patient’s medical condition or systemic infection. Recent studies found no significant differences in the incidence of infection between early and late CP, and some reported a higher incidence of infection in the late CP groups [7, 14, 19, 20].
The optimal timing for CP has not been clearly determined. The definition of early and late varies among reports [5, 16, 19]. Most studies defined early and late periods based on a standard period of approximately 90 days between DC and CP. These studies choose the 90-day time point due to the following reasons: (1) in the authors’ experience, the CP procedures are often performed approximately 90 days after the initial craniectomy [6, 8, 17]; (2) in their data, the median time to CP was approximately 90 days, which served as a cutoff for defining early/late time points [3, 24]; and (3) subsequent studies followed the majority of the studies that used the 90-day time point [13].
We used ROC curve analysis and the Youden index to define the early and late periods. The ROC curve analysis can be used to evaluate the diagnostic ability of continuous variables for distinguishing between a diseased and non-diseased population. It is frequently used to define the optimal cutoff value for classifying the continuous variables into dichotomous categories. In our series, the continuous variables would be the DC-CP interval and a diseased population means the complication group. Marked sensitivity and specificity values were noted in a graphical plot (Fig. 4) which generated for the continuous variable, DC-CP interval. Using the ROC curve analysis, Youden’s J index (also called the Youden index) was adopted, which signifies the maximum potential effectiveness (Table 1). Highest sensitivity and specificity were shown for 44 days of surgery, and this value was used to identify the early and late CP groups. Most of the complications occurred in the late period with high sensitivity (90.48%). Univariate and multivariate Cox regression analyses were performed after defining early and late CP. Subsequent analyses verified that late CP was a statistically significant risk factor for complications.
The early period defined in the present study was earlier than that defined in the previous reports. This raises concern regarding the remaining brain swelling or possibility of infection after CP [25]. A retrospective multicenter study reviewed the resolution period of brain swelling with serial brain CT and identified that CP can be performed approximately 34 days after DC [26]. Recent reports found that early CP is not associated with infection and provides a clear dissection plane during the operative procedure, thereby reducing complications, such as sinking skin flap syndrome, subdural hygromas, and bone graft resorption [2, 18]. Early CP allows for a shorter duration of hospital stay and lower costs [3, 11, 19]. Moreover, a recent systematic review and meta-analysis revealed that early CP is associated with greater neurological improvement [14].
There are several limitations in our study. The incidence of complications was relatively high (33.3%). This may be due to the use of a definition of bone flap resorption that was stricter than that used in other studies. We defined bone flap resorption based on the clear findings of serial brain CT; however, other studies defined resorption based on the need for surgical revision [21, 22] or on physical examination [23]. Extremely early CP (within 2 weeks after DC) cases were not included in our series because many patients of traumatic brain injury still showed some swelling in that period. A sample size of 126 enrolled patients may be not adequate to determine the optimal timing for surgery using statistical methods. A multicenter study with a larger cohort may show different results. Finally, the AUC of our series was 0.60, with high sensitivity but relatively low specificity.
Conclusion
There is no consensus on the optimal timing of CP and the definition of “early” and “late” varies significantly among studies. To our knowledge, no study has identified the optimal timing of CP using statistical methods to date. Based on our results, early CP may be defined as CP performed within 45 days after DC. Late CP was a significant risk factor in the univariate and multivariate Cox regression analyses. This study showed that early CP, within 45 days after DC, is associated with a lower rate of bone flap resorption and surgical site infection compared to late CP.
References
Artico M, Ferrante L, Pastore FS, Ramundo EO, Cantarelli D, Scopelliti D, Iannetti G (2003) Bone autografting of the calvaria and craniofacial skeleton: historical background, surgical results in a series of 15 patients, and review of the literature. Surg Neurol 60:71–79
Ban SP, Son YJ, Yang HJ, Chung YS, Lee SH, Han DH (2010) Analysis of complications following decompressive craniectomy for traumatic brain injury. J Korean Neurosurg Soc 48:244–250
Bender A, Heulin S, Rohrer S, Mehrkens JH, Heidecke V, Straube A, Pfefferkorn T (2013) Early cranioplasty may improve outcome in neurological patients with decompressive craniectomy. Brain Inj 27:1073–1079
Brommeland T, Rydning PN, Pripp AH, Helseth E (2015) Cranioplasty complications and risk factors associated with bone flap resorption. Scand J Trauma Resusc Emerg Med 23:75
Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, Servadei F, Walters BC, Wilberger JE, Surgical Management of Traumatic Brain Injury Author G (2006) Surgical management of acute subdural hematomas. Neurosurgery 58:S16–S24 discussion Si-iv
Chang V, Hartzfeld P, Langlois M, Mahmood A, Seyfried D (2010) Outcomes of cranial repair after craniectomy. J Neurosurg 112:1120–1124
Fan MC, Wang QL, Sun P, Zhan SH, Guo P, Deng WS, Dong Q (2018) Cryopreservation of autologous cranial bone flaps for cranioplasty: a large sample retrospective study. World Neurosurg 109:e853–e859
Hng D, Bhaskar I, Khan M, Budgeon C, Damodaran O, Knuckey N, Lee G (2015) Delayed cranioplasty: outcomes using frozen autologous bone flaps. Craniomaxillofac Trauma Reconstr 8:190–197
Honeybul S (2010) Complications of decompressive craniectomy for head injury. J Clin Neurosci 17:430–435
Kim JS, Cheong JH, Ryu JI, Kim JM, Kim CH (2015) Bone flap resorption following cranioplasty after decompressive craniectomy: preliminary report. Korean J Neurotrauma 11:1–5
Liang W, Xiaofeng Y, Weiguo L, Gang S, Xuesheng Z, Fei C, Gu L (2007) Cranioplasty of large cranial defect at an early stage after decompressive craniectomy performed for severe head trauma. J Craniofac Surg 18:526–532
Lu Y, Hui G, Liu F, Wang Z, Tang Y, Gao S (2012) Survival and regeneration of deep-freeze preserved autologous cranial bones after cranioplasty. British J Neurosurg 26:216–221
Malcolm JG, Rindler RS, Chu JK, Grossberg JA, Pradilla G, Ahmad FU (2016) Complications following cranioplasty and relationship to timing: a systematic review and meta-analysis. J Clin Neurosci 33:39–51
Malcolm JG, Rindler RS, Chu JK, Chokshi F, Grossberg JA, Pradilla G, Ahmad FU (2017) Early cranioplasty is associated with greater neurological improvement: a systematic review and meta-analysis. Neurosurgery 82:278–288
Martin KD, Franz B, Kirsch M, Polanski W, von der Hagen M, Schackert G, Sobottka SB (2014) Autologous bone flap cranioplasty following decompressive craniectomy is combined with a high complication rate in pediatric traumatic brain injury patients. Acta Neurochir 156:813–824
Morton RP, Abecassis IJ, Hanson JF, Barber JK, Chen M, Kelly CM, Nerva JD, Emerson SN, Ene CI, Levitt MR, Chowdhary MM, Ko AL, Chesnut RM (2017) Timing of cranioplasty: a 10.75-year single-center analysis of 754 patients. J Neurosurg 128:1648–1652
Paredes I, Castano-Leon AM, Munarriz PM, Martinez-Perez R, Cepeda S, Sanz R, Alen JF, Lagares A (2015) Cranioplasty after decompressive craniectomy. A prospective series analyzing complications and clinical improvement. Neurocirugia 26:115–125
Piedra MP, Thompson EM, Selden NR, Ragel BT, Guillaume DJ (2012) Optimal timing of autologous cranioplasty after decompressive craniectomy in children. J Neurosurg Pediatr 10:268–272
Piedra MP, Nemecek AN, Ragel BT (2014) Timing of cranioplasty after decompressive craniectomy for trauma. Surg Neurol Int 5:25
Quah BL, Low HL, Wilson MH, Bimpis A, Nga VDW, Lwin S, Zainuddin NH, Wahab NA, Salek MAA (2016) Is there an optimal time for performing cranioplasties? Results from a prospective multinational study. World Neurosurg 94:13–17
Ruopp MD, Perkins NJ, Whitcomb BW, Schisterman EF (2008) Youden index and optimal cut-point estimated from observations affected by a lower limit of detection. Biom J 50:419–430
Schoekler B, Trummer M (2014) Prediction parameters of bone flap resorption following cranioplasty with autologous bone. Clin Neurol Neurosurg 120:64–67
Schuss P, Vatter H, Oszvald A, Marquardt G, Imohl L, Seifert V, Guresir E (2013) Bone flap resorption: risk factors for the development of a long-term complication following cranioplasty after decompressive craniectomy. J Neurotrauma 30:91–95
Stieglitz LH, Fung C, Murek M, Fichtner J, Raabe A, Beck J (2015) What happens to the bone flap? Long-term outcome after reimplantation of cryoconserved bone flaps in a consecutive series of 92 patients. Acta Neurochir 157:275–280
Tsang AC, Hui VK, Lui WM, Leung GK (2015) Complications of post-craniectomy cranioplasty: risk factor analysis and implications for treatment planning. J Clin Neurosci 22:834–837
Yang NR, Song J, Yoon KW, Seo EK (2018) How early can we perform cranioplasty for traumatic brain injury after decompressive craniectomy? A retrospective multicenter study. World Neurosurg 110:e160–e167
Acknowledgements
This was supported by Korea University Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee [2017GR0098] and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Additional information
This article is part of the Topical Collection on Neurosurgery general
Rights and permissions
About this article
Cite this article
Kim, J.H., Hwang, SY., Kwon, TH. et al. Defining “early” cranioplasty to achieve lower complication rates of bone flap failure: resorption and infection. Acta Neurochir 161, 25–31 (2019). https://doi.org/10.1007/s00701-018-3749-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-018-3749-8