Abstract
Background
Cranial reconstruction with autologous bone is still the gold standard although several biomaterials are available to re-establish the integrity of the cranial vault. Due to their biological and morphological characteristics, hydroxyapatite implants show promising results in small clinical cohort studies, especially within the paediatric population. Its biocompatibility and osteoconductivity should allow the formation of osseous bridging at the skull-prosthesis interface.
Objective
To examine the possible occurrence of osteointegration and to quantify it.
Methods
A retrospective study of patients with a hydroxyapatite implant from 2010 to 2014 at our neurosurgical department was conducted. Demographic, surgical and radiological data were studied. A senior neuroradiologist, a staff member neurosurgeon and a resident neurosurgeon independently performed the radiological evaluation. A new software analysis technique was developed to objectively quantify the degree of osteointegration.
Results
Seventeen implants were evaluated with an average patient age of 39 years and a mean follow-up of 155 weeks. Through radiologic evaluation, osseous bridging was deemed higher than 50% in six prostheses and higher than 75% in three. In five patients, no osteointegration could be seen. The remaining patients exhibited sparse signs of osteointegration, estimated between 10 and 50%. Software analysis showed an average osteointegration ratio of 37.4% with a 400-HU filter and 27.3% with a 700-HU filter.
Conclusion
In this small retrospective study of cranial hydroxyapatite implants, osteointegration did occur and to a degree of more than 50% in 1/3 of the patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Restoration of the cranial vault with autologous bone is still the first treatment of choice but the need for alternative implants for cranioplasty also exists, e.g. because of post-implant complications, multi-fragmented bone flaps and bone invading tumours. Autograft resorption is a well-known complication with an incidence ranging from 2 to 50% [14, 23]. Younger age, bone flap storage, cranioplasty timing and shunt-dependent hydrocephalus are considered to be risk factors for post-implant osteolysis [12, 16]. The autograft resorption rate is largely determined by these factors and estimated to be around 2.7%/year in a recent study [16]. Post-implant infection is also frequently reported. Some authors estimate the infection risk even up to 33% [2, 21]. Subsequent rapid and early restoration of the cranial vault is mandatory since it not only protects the brain and facilitates patient rehabilitation, it also re-establishes normal cerebral glucose metabolism and intracranial pressure dynamics [25]. This holds especially true in case of extensive (> 25 cm2) bone defects [5, 10, 19, 22]. For the past 100 years, very different materials were used for cranioplasty from synthetic or natural origin. The goals of any cranioplasty technique are to cover the skull defect, to give good cosmetic result and to protect the brain to avoid secondary neurological damage. This is preferably done with a substitute that is sterilisable, biocompatible, easy to design and non-thermal conducting and has a high impact of strength [5, 10, 20,21,22]. Porous hydroxyapatite is an interesting material because of its structural and chemical characteristics that mimic bone tissue. These features promote osteogenesis, which in turn can lead to partial or complete bony anchorage. Margin fusion at the bone-prosthesis edge has been described in both animal studies and clinical case reports [5, 10, 19]. This study reviews 17 cases of custom-made porous hydroxyapatite cranioplasty, both clinically and radiologically, and quantifies osteointegration.
Material and methods
Patient population
From October 2010 to October 2014, 21 patients underwent a custom-made hydroxyapatite cranioplasty procedure in our centre. At the time of the analysis, two patients were minors and did not receive post-operative CT or MR imaging. Due to radiation hygiene considerations, we did not want to scan them for study purposes so these two patients were excluded. Three other patients were also under age at the time of implantation but turned 18 several years before our assessment. One patient was lost to follow-up and two others refused cooperation. One trauma patient received two separate prostheses after bifrontal decompression but passed away 2 years post-implantation. Since follow-up scans were available up to 1 year, we decided to include this patient anyway. The implants were considered as two different prostheses within the same patient and evaluated separately. This brings the total to 17 implants in 16 patients.
Ethics committee approval and informed consent
Each patient signed an informed consent. Approval of the Ethics Committee of the hospital was obtained.
Surgical technique
A digital 3D prosthesis model was made based on a multiplanar CT scan with 1-mm slice thickness. After approval of the virtual 3D model by the neurosurgeon, the prosthesis was manufactured. All patients received the same type of custom-made hydroxyapatite prosthesis (Fin-Ceramic, Faenza, Italy). To generate maximum contact between the host bone and the prosthesis, the bone margins were freshened and cleaned from scar tissue either manually with raspatoria or with high-speed drills. The prosthesis was then carefully fixed to the skull by non-resorbing synthetic sutures. Dural tenting sutures were placed when deemed necessary. Neither cement nor titanium meshes were used. Correct position of the prosthesis was confirmed with a post-operative CT. In accordance with our post-operative protocol, follow-up visits were planned after 6 weeks. The patients were evaluated clinically and either with CT or MRI, depending on their underlying condition. The necessity of further follow-up visits was determined in function of the underlying pathology as well.
Radiological analysis
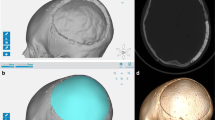
The last follow-up CT and MR images available in our database were used for analysis. Each multiplanar CT scan was performed with 1-mm slice thickness and a slide resolution of at least 512 × 512 pixels. These images were used to assess the bony anchorage of the prosthesis since CT is by far the superior imaging technique for bone structures. For the evaluation of osteointegration, we used the definition suggested by Staffa et al. [21]. They define osteointegration as a structural and functional connection between autologous bone and the prosthesis and state that this condition is achieved when the radiolucent line between the living bone and the implant is vanished on CT. A neurosurgeon, a neurosurgical resident with 2 years of experience in the field of radiology and a neuroradiologist independently carried out qualitative evaluation of the CT images. The patients were subsequently divided into six different groups based on the estimated percentage of the contour at which osteointegration has occurred (I, 0% = no osteointegration noticeable; II, 1–10%; III, 11–25%; IV, 26–50%; V, 51–75%; VI, 76–100%). Additional software was used to quantify the percentage of the contour of the prosthesis at which bony anchorage has occurred. This calculation was done by a new analysing method recently developed by the collaboration of our neurosurgical department and the software engineers of Materialise (Leuven, Belgium). The applied technique was based on the measurement of the Hounsfield units in every voxel at the bone-prosthesis interface and the application of two different segmentation filters of 400 and 700 HU by using MIMICS software (Mimics Innovation Suite, Materialise, Leuven, Belgium). The average HU density of human bone ranges from 400–700 HU (spongious bone) to 1800–2000 HU (cortical bone) [26]. The lower limit for bone density lies somewhere between 400 and 700 HU but there is no clear-cut threshold. While some authors state that voxel densities should exceed 700 HU to consider them as bone, others prefer a lower limit of 400 HU. Evidently, the selection of a proper threshold determines the results of the calculation of osseous bridging and osteointegration ratios will be lower if a more strict limit is chosen. Since there is no consensus about an exact threshold, we used both 400 and 700 HU as filters. The exact technique is described in Fig. 1a, b.
a First, the prosthesis and skull were accurately segmented from each other. On the wrapped prosthesis, a surface was created corresponding to the contact area between the plate and the bone. This was then separated into a new part and given a thickness by applying a hollow operation in both directions. b Through application of the filters, Boolean subtraction and intersection, the volume of the contact area that has a Hounsfield unit greater than 400 HU could then be calculated. This corresponds to areas of osseous bridging between the implant and the skull. In areas with a density lower than the threshold of 400 HU, we concluded that no osseous bridging has occurred at that part of the interface and that these regions consisted out of fibrous tissue and fluids. Dividing the interface volume with HU > 400 HU by the total contact area enabled us to calculate the exact percentage of the contour of the prosthesis which was structurally imbedded in the surrounding host bone
If the hydroxyapatite scaffold becomes partially or fully integrated with the surrounding bone, one might expect to see internal signal alterations and signs of fibrovascular tissue formation inside the pores of the prosthesis. Reports in ophthalmologic literature discussing the imaging assessment of orbital hydroxyapatite implants have demonstrated that enhancement inside the implants is identical to histologically proven fibrovascular ingrowth and most likely reflects this process [6, 18]. To test this hypothesis in cranial allografts, we sought these features on post-implant MR imaging if available. The performed MR protocol consisted out of axial T2, FLAIR and DWI as well as three-plane T1 images with or without spectral fat saturation. Gadolinium was not routinely administered because its application was determined by the underlying pathology. The acquired MR images were then compared with the first post-operative MR imaging to detect small signal alternations inside the prosthesis.
Results
Patient characteristics (Table 1)
Sixteen patients were included in this retrospective analysis. The mean patient age at the time of implant was 39 years (range 14 to 71). There was significant male predominance with only two female patients. Four patients received their prosthesis after a previous decompression for traumatic brain injury and one after a poorly healed compression fracture. In two cases, a bone-invading meningioma was the underlying pathology and one patient received a pre-manufactured prosthesis for a partial congenital skull malformation. In nine cases, a hydroxyapatite implant was placed due to complications of the autograft: graft infection in three cases and partial resorption of the re-implanted autologous bone flap in six (Table 2).
The average prosthesis size was 130 cm2 (range 17.5–254 cm2). The mean duration of the cranial defect (this means the interval between the removal of the bone flap or the earlier re-implanted autograft and the implantation of the hydroxyapatite prosthesis) was 71 weeks, ranging from 0 days (immediate replacement of a bone flap or prosthesis into a HA implant) to an extreme of 17 years in one case. The median interval however was 4 weeks. The cosmetic results were excellent with no discontinuity detectable by touch. During the follow-up period, no signs of inflammation, toxicity or allergic reactions were noticed. Two patients had wound healing problems probably due to a suboptimal flap vascularisation, necessitating surgical re-intervention. Two other patients needed drainage of an epidural hematoma within 4 days after implantation. In one patient, a new hydroxyapatite prosthesis (“back-up implant”) was placed 22 months after first implantation because of a Y-shaped fracture and dislocation (Fig. 2). There was a minor head trauma 4 weeks before diagnosis but the patient was himself unaware of any disturbances. Another patient showed a mild dislocation of the anterior part of the prosthesis just below the hairline (probably due to detachment of one of the fixating sutures). Since the patient was oblivious of any problem and he was very reluctant towards surgical correction, conservative policy was maintained. Detailed information about the complications and comorbidities of our patients is shown in Table 3.
Computed tomography analysis
The mean interval between the allograft implantation and the CT scan used for analysis was 155 weeks (range 27–293 weeks) with a median of 126 weeks. The results of the radiological estimation of osteointegration are summarised in Fig. 3.
In five implants (19%), there were no signs of osseous bridging at all with an average interval between the implant and the time of analysis of 96 weeks. Not surprisingly, the patient with the broken implant after minor trauma belonged to this group. Osteointegration was deemed higher than 50% in six prostheses and in half of them, it was judged higher than 75% (group VI). The mean duration until the analysis was 134 weeks in group V and 202 weeks in group VI respectively. The remaining patients exhibited sparse signs of osteointegration, estimated lower than 25%. The average age of patients was lowest in the 75–100% group (21 years) while in all other groups, mean age was higher than 40. Additional software analysis enabled us to quantify the degree of osteointegration (expressed as percentage). When we used 400 HU as a threshold (which means that every voxel with a density of 400 HU or more is considered to consist out of bone), we found an average osteointegration percentage of 37.39%. The 700-HU threshold gave an average ratio of 27.32%. The calculated osteointegration percentage of each patient is shown in detail in Table 2.
Magnetic resonance imaging analysis
We could evaluate appropriate MR images of 13 patients but only in 7 patients, gadolinium was administered. In five of them, contrast enhancement was observed both at the centre and at the periphery of the prosthesis. Interestingly, all these patients showed obvious signs of osteointegration on CT and three of them more than 50% (see Table 2). No signs of bone formation were observed in the two patients without contrast enhancement. Figure 4 gives an example of the correlation between the findings on MR (Fig. 4a, b) and CT (Fig. 4c) imaging.
a–c Axial T1 before (a) and after (b) IV gadolinium contrast administration. Axial CT in bone window (c). After administration of contrast, there is a clear contrast enhancement within the prosthesis. CT images demonstrate a complete osteointegration of the prosthesis with a complete disappearance of the radiolucent line between the implant and the host bone
Discussion
Since the introduction of hydroxyapatite grafts in reconstructive surgery, approximately 2900 implants were placed between 1997 and 2013 [17, 28]. The manufacturers claim that its specific chemical composition triggers the formation of new bone and ultimately results in a stable anchorage [4]. Martini et al. demonstrated this osteointegration capacity in a preclinical animal model [10]. Histological, histomorphometric and 3D micro-computed tomographic analysis revealed evidence of rapid osteogenesis at the bone-hydroxyapatite interface and inside the prosthesis itself. They observed an average bone-to-implant contact of 49.8% after 6 months, increasing to 74.5% the following half year. Several clinical reports describe newly formed bone at the margins of explanted prostheses [1, 4, 5, 13, 15, 19]. Staffa et al. reported good osteointegration results with an integration of less than 75% of the contour in only 7 of the 60 patients after 6 months post-implantation [21]. Hardy et al. used an artificial classification consisting of five groups to prospectively evaluate the osteointegration ratio in eight patients [7]. The authors noticed variable (dural) ossification below the prostheses but failed to analyse the degree of bone formation within the prosthesis. In both studies, a neuroradiologist visually evaluated the degree of osteointegration on CT imaging. Zaccaria et al. reported an osteointegration of nearly 100% in five children and incomplete osteointegration of 69% in one infant on CT scans carried out between 9 and 40 months post-operative [26]. They mention the application of MIMICS software for the calculation of the osteointegration percentage but do not clearly specify the used measuring method.
By the combined approach of visual evaluation and software analysis, we could calculate the degree of osteointegration in the different implanted hydroxyapatite prostheses. With an average interval between the implantation of the allograft and the timing of the scanning of almost 3 years, 37% of the contour was osteointegrated with a 400-HU threshold and 27.32% with a 700-HU threshold. Furthermore, the calculated results showed that either no osteointegration occurs at all or the osteointegration ratio lies between 25 and 75%. Of notice, with software analysis complete, 100% osteointegration was never obtained. The highest ossification ratio (82%) was observed in a 17-year-old patient with a 1-year post-implant follow-up. Visual assessment tended to underestimate the osteointegration percentage in patients with sparse signs of osseous bridging when compared to calculated integration percentage. The opposite holds true in patients with generous signs of osteointegration.
Although the follow-up period in this study is significantly longer compared to that of other reports, lower osteointegration ratios and larger variability between different patients were seen. Several factors could explain this discrepancy. First, patient age is an important factor. While Zaccaria et al. conducted their analysis in a paediatric population (mean age 9.6 years), our patient group consisted out of adult patients (mean age 41 years) [26]. It has been well documented that with increasing age, bone formation ability decreases [24]. So it is no surprise to find that the average age of the patients in group VI (75–100%) was almost half of the other groups. Other assumed non-surgical factors that might play a role include metabolic factors, smoking, the size of the bone defect, previous infections and aetiology of the cranial defect [3, 19]. Several surgical factors also influence the capacity of osteogenesis. Careful placement and tight allograft fixation are important to reduce micro-movements and freshening of the edges with the removal of any scar tissue is necessary to maximise margin adherence [11, 27, 29]. Preservation of a viable dura mater and adequate blood irrigation of the overlying musculocutaneous flap enables vascular ingrowth which provides nutrients necessary for bone formation [8, 10, 21, 22]. Post-gadolinium T1 MR images make it possible to visualise the presence of these small vessels inside the prosthesis [6, 18]. In five patients, we could indeed detect clear punctiform enhancement inside the implant. Interestingly, we only observed the presence of fibrovascular tissue on MRI in patients with more than 50% osteointegration on the CT images.
There was one patient with a clinically unnoticed fracture of the implant, 2 years after surgery (Fig. 2). A minor trauma was still sufficient to result in a fracture. The high porosity of the ceramic implants is crucial for bone formation but gives it the mechanical strength of spongious bone, especially within the first weeks post-implantation. Some authors state that the biomechanical resistance is more equal to that of normal bone tissue, once the first 12 weeks post-implantation are passed. In our opinion, this remains controversial and is largely dependent on the patients’ individual rate of bone formation [21]. Even though there are several publications describing spontaneous healing of fractured hydroxyapatite implants, conservative measures in a non-dislocated fracture are only justified in selected cases [9, 20,21,22]. Because of the intrinsic fragility of the implant, synthetic alternatives (like PEEK) may be the better option in patients with a high tendency to fall.
There are several limitations to this study. Most important objections are its retrospective nature and limited number of patients. The radiological data were not acquired through a standardised study protocol, but via daily practice. On the other hand, the strength of the study is the fact that we tried to find an objective method for osteointegration evaluation and that we used the concept of MR imaging to demonstrate fibrovascular ingrowth. Our calculation technique is based on CT imaging which is the cornerstone for evaluation of bony fusion. Nevertheless, we acknowledge that we do not have histologic confirmation of our calculated results. In humans, this would require redo surgery which is of course not possible but future animal studies with histologic analysis of the explanted prosthesis could be used to validate the software technique. Additionally, prospective multicentre research with fixed timing for CT imaging and MRI is necessary to provide a temporal course of the changes in osteointegration measures over time.
Conclusion
Cranial hydroxyapatite implants can lead to osteointegration, especially in younger patients. In this small retrospective study, osteointegration of more than 50% of the fusion surface of cranial hydroxyapatite implants occurred in one third of patients. Software analysis of CT imaging has the potential to provide an objective measurement of osteointegration. Well-designed prospective studies are needed to validate these results.
References
Brie J, Chartier T, Chaput C, Delage C, Pradeau B, Caire F, Boncoeur M-P, Moreau J-J (2013) A new custom made bioceramic implant for the repair of large and complex craniofacial bone defects. J Cranio-Maxillofac Surg 41:403–407. https://doi.org/10.1016/j.jcms.2012.11.005
Cheng YK, Weng HH, Yang JT, Lee MH, Wang TC, Chang CN (2008) Factors affecting graft infection after cranioplasty. J Clin Neurosci 15:1115–1119. https://doi.org/10.1016/j.jocn.2007.09.022
Fini M, Giavaresi G, Salamanna F, Veronesi F, Martini L, De Mattei M, Tschon M (2011) Harmful lifestyles on orthopedic implantation surgery: a descriptive review on alcohol and tobacco use. J Bone Miner Metab 29:633–644. https://doi.org/10.1007/s00774-011-0309-1
Frassanito P, De Bonis P, Mattogno PP, Mangiola A, Novello M, Brinchi D, Pompucci A, Anile C (2013) The fate of a macroporous hydroxyapatite cranioplasty four years after implantation: macroscopical and microscopical findings in a case of recurrent atypical meningioma. Clin Neurol Neurosurg 115:1496–1498. https://doi.org/10.1016/j.clineuro.2012.11.032
Fricia M, Passanisi M, Salamanna F, Parrilli A, Giavaresi G, Fini M (2015) Osteointegration in custom-made porous hydroxyapatite cranial implants: from reconstructive surgery to regenerative medicine. World Neurosurg 84:591.e511–591.e596. https://doi.org/10.1016/j.wneu.2015.03.027
Galluzzi P, De Francesco S, Giacalone G, Cerase A, Monti L, Vallone IM, Lazzeretti L, Venturi C, Hadjistilianou T (2011) Contrast-enhanced magnetic resonance imaging of fibrovascular tissue ingrowth within synthetic hydroxyapatite orbital implants in children. Eur J Ophthalmol 21:521–528. https://doi.org/10.5301/ejo.2011.6298
Hardy H, Tollard E, Derrey S, Delcampe P, Peron JM, Freger P, Proust F (2012) Clinical and ossification outcome of custom-made hydroxyapatite prothese for large skull defect. Neuro-Chirurgie 58:25–29. https://doi.org/10.1016/j.neuchi.2011.09.006
Kiyokawa K, Hayakawa K, Tanabe HY, Inoue Y, Tai Y, Shigemori M, Tokutomi T (1998) Cranioplasty with split lateral skull plate segments for reconstruction of skull defects. J Craniomaxillofac Surg 26:379–385
Lopez Gonzalez A, Perez Borreda P, Conde Sardon R (2015) Fracture of a HTR-PMI cranioplastic implant after severe TBI. Childs Nerv Syst 31:333–336. https://doi.org/10.1007/s00381-014-2493-5
Martini L, Staffa G, Giavaresi G, Salamanna F, Parrilli A, Serchi E, Pressato D, Arcangeli E, Fini M (2012) Long-term results following cranial hydroxyapatite prosthesis implantation in a large skull defect model. Plast Reconstr Surg 129:625e–635e. https://doi.org/10.1097/PRS.0b013e318244220d
Mastrogiacomo M, Scaglione S, Martinetti R, Dolcini L, Beltrame F, Cancedda R, Quarto R (2006) Role of scaffold internal structure on in vivo bone formation in macroporous calcium phosphate bioceramics. Biomaterials 27:3230–3237. https://doi.org/10.1016/j.biomaterials.2006.01.031
Matsuno A, Tanaka H, Iwamuro H, Takanashi S, Miyawaki S, Nakashima M, Nakaguchi H, Nagashima T (2006) Analyses of the factors influencing bone graft infection after delayed cranioplasty. Acta Neurochir 148:535–540; discussion 540. https://doi.org/10.1007/s00701-006-0740-6
Messina G, Dones I, Nataloni A, Franzini A (2011) Histologically demonstrated skull bone integration in a hydroxyapatite prosthesis in a human. Acta Neurochir 153:1717–1718. https://doi.org/10.1007/s00701-011-1014-5
Movassaghi K, Ver Halen J, Ganchi P, Amin-Hanjani S, Mesa J, Yaremchuk MJ (2006) Cranioplasty with subcutaneously preserved autologous bone grafts. Plast Reconstr Surg 117:202–206
Ono H, Sase T, Tanaka Y, Takasuna H (2017) Histological assessment of porous custom-made hydroxyapatite implants 6 months and 2.5 years after cranioplasty. Surg Neurol Int 8:8. https://doi.org/10.4103/2152-7806.198735
Park SP, Kim JH, Kang HI, Kim DR, Moon BG, Kim JS (2017) Bone flap resorption following cranioplasty with autologous bone: quantitative measurement of bone flap resorption and predictive factors. J Korean Neurosurg Soc 60:749–754. https://doi.org/10.3340/jkns.2017.0203.002
Rienzo AD, Iacoangeli M, di Somma LGM, Alvaro L, Nocchi N, Scerrati M (2012) Shape modifications of porous hydroxyapatite prostheses to improve rigid implant fixation: experience in 12 cases. Surg Neurol Int 3:161
Spirnak JP, Nieves N, Hollsten DA, White WC, Betz TA Gadolinium-enhanced magnetic resonance imaging assessment of hydroxyapatite orbital implants. Am J Ophthalmol 119:431–440. https://doi.org/10.1016/S0002-9394(14)71228-0
Sprio S, Fricia M, Maddalena GF, Nataloni A, Tampieri A (2016) Osteointegration in cranial bone reconstruction: a goal to achieve. J Appl Biomater Funct Mater 14:e470–e476. https://doi.org/10.5301/jabfm.5000293
Staffa G, Nataloni A, Compagnone C, Servadei F (2007) Custom made cranioplasty prostheses in porous hydroxy-apatite using 3D design techniques: 7 years experience in 25 patients. Acta Neurochir 149:161–170; discussion 170. https://doi.org/10.1007/s00701-006-1078-9
Staffa G, Barbanera A, Faiola A, Fricia M, Limoni P, Mottaran R, Zanotti B, Stefini R (2012) Custom made bioceramic implants in complex and large cranial reconstruction: a two-year follow-up. J Craniomaxillofac Surg 40:e65–e70. https://doi.org/10.1016/j.jcms.2011.04.014
Stefini R, Esposito G, Zanotti B, Iaccarino C, Fontanella MM, Servadei F (2013) Use of “custom made” porous hydroxyapatite implants for cranioplasty: postoperative analysis of complications in 1549 patients. Surg Neurol Int 4:12. https://doi.org/10.4103/2152-7806.106290
Stefini R, Zanotti B, Nataloni A, Martinetti R, Scafuto M, Colasurdo M, Tampieri A (2015) The efficacy of custom-made porous hydroxyapatite prostheses for cranioplasty: evaluation of postmarketing data on 2697 patients. J Appl Biomater Funct Mater 13:e136–e144. https://doi.org/10.5301/jabfm.5000211
Stenderup K, Rosada C, Justesen J, Al-Soubky T, Dagnaes-Hansen F, Kassem M (2004) Aged human bone marrow stromal cells maintaining bone forming capacity in vivo evaluated using an improved method of visualization. Biogerontology 5:107–118. https://doi.org/10.1023/B:BGEN.0000025074.88476.e2
Winkler PA, Stummer W, Linke R, Krishnan KG, Tatsch K (2000) Influence of cranioplasty on postural blood flow regulation, cerebrovascular reserve capacity, and cerebral glucose metabolism. J Neurosurg 93:53–61. https://doi.org/10.3171/jns.2000.93.1.0053
Zaccaria L, Tharakan SJ, Altermatt S (2016) Hydroxyapatite ceramic implants for cranioplasty in children: a single-center experience. Childs Nerv Syst 33:343–348
Zanotti B, Zingaretti N, Almesberger D, Verlicchi A, Stefini R, Ragonese M, Guarneri GF, Parodi PC (2014) Enhancing dermal and bone regeneration in calvarial defect surgery. Indian J Plast Surg 47:325–332. https://doi.org/10.4103/0970-0358.146581
Zanotti B, Verlicchi A, Indiani S, Scarparo SA, Zingaretti N, Parodi PC (2015) Spontaneous fractures in custom-made porous hydroxyapatite cranioplasty implants: is fragility the only culprit? Acta Neurochir 157:517–523. https://doi.org/10.1007/s00701-014-2319-y
Zanotti B, Verlicchi A, Stefini R, Salgarelli AC, Zingaretti N, Parodi PC, Matteo C, Robiony M (2015) Surgical pitfalls with custom-made porous hydroxyapatite cranial implants. Plastic and Aesthetic Research 2:7–11
Acknowledgements
Software analysis technique was developed together with Materialise (Leuven, Belgium).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Each patient signed an informed consent. Approval of the Ethics Committee of the hospital was obtained.
Competing interests
The authors declare that they have no competing interests.
Additional information
Comments
Even if there are a number of papers dealing with HA cranioplasty implants, very few papers have really examined the possibility of osteointegration. Since HA cranioplasty is expensive, the plus offered by this method of cranial reconstruction is bone integration. This makes HA a biological material differently from all the other materials used for cranioplasty (PEEK, PMMA, titanium...) which are materials not integrated into the skull and acting as a foreign body.
There are very few published papers with a good CT follow-up study to demonstrate osteointegration. Furthermore, to my knowledge, this is the first study to demonstrate a relationship between CT data and gadolinium MRI data. MRI can show the presence of small new vessels within the cranioplasty than confirming bone colonisation.
The results of the study with a long follow-up in an adult population show that in a majority of cases, osteointegration did occur even if to a different degree.
With the limit of the small number of cases and of the retrospective data collection, these patients show that it is possible and feasible to use a biological material for cranial reconstruction.
The authors have described one case of HA cranioplasty fracture 2 years after implantation. There is an important message never presented before: we can probably measure with gadolinium MRI (this is the only message never presented before the presence of osteointegration was already reported in a few cases in live patients).
Franco Servadei
Milan, Italy
This article is part of the Topical Collection on Neurosurgical technique evaluation
Rights and permissions
About this article
Cite this article
Maenhoudt, W., Hallaert, G., Kalala, JP. et al. Hydroxyapatite cranioplasty: a retrospective evaluation of osteointegration in 17 cases. Acta Neurochir 160, 2117–2124 (2018). https://doi.org/10.1007/s00701-018-3694-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-018-3694-6