Abstract
A 13-year-old girl with a large left fronto-parietal hard-tissue replacement patient-matched implant (HTR®-PMI) cranioplasty—since she suffered from a traumatic brain injury (TBI) 6 years ago—had a new severe TBI that detached and fractured the implant as well as caused a left subdural hematoma and a large frontal contusion. The hematoma and contusion were removed and the implant was substituted by a provisional titanium mesh. To the best of our knowledge, this is the first case reported about an HTR®-PMI fracture. It is theorized that the bone ingrowth into the macroporous implants, like those of hydroxyapatite, gives strength and resistance to the implant. But in the case we describe, no macroscopic bone ingrowth was detected 6 years after implantation and the traumatic force that impacted over the cranioplasty exceeded its properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Case report
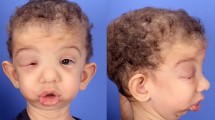
We describe the case of a 13-year-old girl with a large left fronto-parietal hard-tissue replacement patient-matched implant (HTR®-PMI) cranioplasty that was placed after having suffered a severe traumatic brain injury (TBI) at the age of 7 requiring left decompressive craniotomy followed by frozen autologous bone cranioplasty. But, almost 1 year later, a chronic wound infection made necessary the removal of the autologous plasty and 3 months of perioperative antibiotic treatment. Nine months after the removal, and without any evidence of wound infection, a definitive custom-made HTR®-PMI was implanted (Fig. 1). The sequels of her accident were facial palsy, right hemiparesis, impairment in psychomotor and language development, and left encephalomalacia. The wound did not develop any new complication.
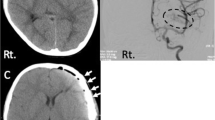
Six years later, she suffered a new TBI after falling down the stairs and hit her head violently against the edge of a step. At admission, she had a GCS score of 8/15 and presented a left III nerve palsy. Urgent CT scan showed left frontal subdural hematoma, uncal herniation, convexity subarachnoidal hemorrhage, left longitudinal petrous fracture, pneumocephalus, and frontal lobe contusion. The HTR®-PMI implant was detached and fractured (Figs. 2 and 3).
The patient underwent removal of the implant, evacuation of the intradural clot, and polar frontal lobectomy. No macroscopic bone ingrowth into the HTR®-PMI cranioplasty was observed. In the same procedure, a new cranioplasty was performed by using a titanium mesh (Fig. 4).
Three months after surgery, the patient gradually recovered to her previous neurological status and was fully conscious with her previous speech difficulty and right hemiparesis.
Discussion
The ideal material for cranioplasty must meet the requirements of biocompatibility to avoid rejection, of bioactivity to reduce the risk of infection, of biomechanical strength to ensure the protection of the brain, and finally, it must afford a cosmetically acceptable look [1].
Among the materials used for heterologous cranioplasties, polymethylmethacrylate (PMMA) seems to be the most commonly used (53 %), followed by the titanium mesh (26 %), macroporous dense hydroxyapatite (10 %), polyetheretherketone (PEEK) (2 %), titanium plate (2 %), and other materials (2 %) [1].
Hard-tissue replacement polymer patient-matched implant (HTR®-PMI; Walter Lorenz Surgical, Jacksonville, FL) is an alloplastic material first used in the 1970s in dentoalveolar reconstructions [8], and broadly well recognized as an appropriate implant for reconstruction of cranial defects offering excellent esthetic results when autologous bone cannot be used. It is made from non-resorbable biocompatible material composed of PMMA spherical macrobeads of 700 to 850 μm in diameter that are fussed together with polyhydroxyethylmethacrylate (PHEMA) and coated with calcium hydroxide. The addition of barium sulfate renders the implant radiopaque [8].
This alloplastic implant possesses several characteristic features such as the following: possibility of patient-specific anatomic fit; hydrophilic surface that enables pre-operative antibiotic impregnation; a negatively charged surface (−8 to −15 mV [2]) inhibiting bacterial adhesion; and 20 to 30 % material porosity (pore diameter of 250–500 μm) allowing vascular, soft-tissue, and bone ingrowth [2]. It possesses rigidity and considerable compressive strength (5,000 lb/in.2 in molded form) comparable with that of traditional acrylic [2–5, 8]. Cranioplasty is designed preoperatively by using a 3-dimensional CT scan of the patient’s cranial defect. At the time of surgery, the implant is secured by using metal or resorbable fixation.
There are few reports of HTR®-PMI outcomes in cranial reconstruction. Those authors reported minimal complications and excellent esthetic results [3, 4, 8–10]. Cranioplasty following decompressive craniectomy is associated with a complication rate not well determined in the literature. That ranges from 14–22 to 33.8 % depending on authors [6, 7]. Complications reported by Gooch et al. in their series [6] of 62 cranioplasties (92 % used autologous bone) were as follows: wound infection, 11.3 %; wound dehiscence, 3.2 %; epidural hematoma, 1.6 %; subdural hematoma, 1.6 %; bone reabsorption, 6.4 %; sunken bone plate, 1.6 %; status epilepticus, 1.6 %; intraop hemodynamic instability, 1.6 %; hydrocephalus, 1.6 %; and deep vein thrombosis, 3.2 % [6]. Of the cases with complications, 76 % required another operation to address their previous cranioplasty.
Complications reported for alloplastic cranioplasties are similar to those reported for autologous bone. Nassiri et al.’s series [8] of 21 HTR®-PMI cranioplasties (the largest retrospective study of HTR®-PMI outcomes to date) identified 5 complications: 1 implant exposure (4.8 %), 1 soft-tissue infection (4.8 %), and 3 implant infection (14.3 %). Eppley et al. [4] reported no postoperative complication or infection and good reconstructive results in 14 patients.
All the biomaterials seem to maintain their volume over time. The porosity may be a significant factor in determining bone ingrowth into the implant. PMMA is nonporous, and no bone ingrowth is expected. Cement paste implants tend to contain micropores, and there is less long-term bone ingrowth. Biomaterials with macroporous architecture such as ceramic forms of hydroxiapatite, HTR®, porous polyethylene (Medpor®), bioactive glasses (NovaBone®), and demineralized bone paste have demonstrated bone ingrowth in clinical or experimental studies.
It is difficult to attribute many of the complications solely to the implant material itself, and there is much overlap between surgical technique, host response, and potential toxicity of the implant [1, 11].
To the best of our knowledge, there are only two cases of custom alloplastic cranioplasty fractures reported [1, 12]. In both of them, the implant was custom-made macroporous hydroxyapatite and the different severity of the fracture could be mediated by the different time from implantation and the colonization by osteoblasts. In the first case reported [1], a custom-made macroporous hydroxyapatite prosthesis was fractured during the immediate postoperative period after a generalized seizure leading to a traumatic head injury. An epidural hematoma under the fractured cranioplasty was also diagnosed. Removal of the implant and the epidural hematoma was followed 3 months later by a custom PEEK cranioplasty. Authors disclose that macroporous hydroxyapatite implants have weak mechanic resistance, especially before colonization of the implant by osteoblasts. In the other case reported [12], the patient suffered a cranial trauma 11 months after a custom macroporous hydroxyapatite prosthesis implantation. It caused a slight sinking and fracture of the prosthesis, with partial detachment at the edges. The patient was treated conservatively, with complete clinical recovery in just a few days. The bone growth throughout the pores of the plasty 11 months after implantation could explain the lesser severity of this case.
Current literature data do not allow to establish an average time at which the prosthesis is likely to be as strong as the surrounding bone in case of an impact. Micro-radiographic studies have detected in ceramic hydroxiapatite prosthesis extensive compact bone, cancellous bone, and bone marrow in appropriate geometry at 9 months after implantation [12].
To the best of our knowledge, no fractures of HTR®-PMI have been reported until now. In the case we describe, although the fracture occurred 6 years after an accurately fixed HTR®-PMI implantation and no infections, resorption, or movement was detected in this period, no evidence of macroscopic bone ingrowth was observed during surgical removal. Indeed, the cranioplasty got detached from the skull after the TBI and showed similar properties to nonporous materials. An explanation for this lack of bone ingrowth into the implant is that the residual post-infectious condition after the autologous cranioplasty removal might have influenced in the loss of vitality in the neighboring bone.
We also consider that the hemiparesis that the patient suffered when she fell downstairs made her not to be able to cushion the impact against the steps, so the traumatic force over the cranioplasty was high enough to exceed its properties as the cause of the fracture.
References
Adetchessi AT, Pech-Gourg G, Metellus P, Fuentes S (2012) Fracture of macroporous hydroxyapatite prosthesis. Neurochirurgie 58(6):382–385
Cho YR, Gosain AK (2004) Biomaterials in craniofacial reconstruction. Clin Plast Surg 31(3):377–385
Eppley BL (2002) Craniofacial reconstruction with computer-generated HTR patient-matched implants: use in primary bony tumor excision. J Craniofac Surg 13(5):650–657
Eppley BL, Kilgo M, Coleman JJ (2002) Cranial reconstruction with computer-generated hard-tissue replacement patient matched implants: Indications, surgical technique, and long-term followup. Plast Reconstr Surg 109:864–871
Eppley BL, Sadove AM, German RZ (1990) Evaluation of HTR polymer as a craniomaxillofacial graft material. Plast Reconstr Surg 86:1085–1092
Gooch MR, Gin GE, Kenning TJ, German JW (2009) Complications of cranioplasty following decompressive craniectomy: analysis of 62 cases. Neurosurg Focus 26(6):E9
Moreira-Gonzalez, A, Jackson, IT, Miyawaki, T, Barakat, K, DiNick V (2003) Clinical outcome in cranioplasty: critical review in long-term follow-up. J Craniofac Surg 14 (2):144–153 (Erratum in: J. Craniofa. Surg 14 (5):816).
Nassiri N, Cleary DR, Ueeck BA (2009) Is cranial reconstruction with a hard-tissue replacement patient-matched implant as safe as previously reported? A 3-year experience and review of the literature. Oral Maxillofac Surg 67(2):323–327
Petersen T, Hollins R (2002) Cranial reconstruction with computer-generated hard-tissue replacement patient-matched implants: indications, surgical technique, and long-term follow-up. Arch Facial Plast Surg 5(6):533–34, Comment on: Plast Reconstr Surg 2002;109:864–871
Roberson JB, Rosenberg WS (1997) Traumatic cranial defects reconstructed with the HTR-PMI cranioplastic implant. J Craniomaxillofac Trauma 3:8–13
Rubin JP, Yaremchuk MJ (1997) Complications and toxicities of implantable biomaterials used in facial reconstructive and aesthetic surgery: a comprehensive review of the literature. Plast Reconstr Surg 100:1336–1353
Staffa G, Nataloni A, Compagnone C, Servadei F (2007) Custom-made cranioplasty prostheses in porous hydroxyapatite using 3D design techniques: 7 years experience in 25 patients. Acta Neurochir 149(2):161–170
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
López González, A., Pérez Borredá, P. & Conde Sardón, R. Fracture of a HTR-PMI cranioplastic implant after severe TBI. Childs Nerv Syst 31, 333–336 (2015). https://doi.org/10.1007/s00381-014-2493-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-014-2493-5