Abstract
Objective
To compare the long-term outcomes of patients who had been randomly allocated to receive primary titanium cranioplasty or autologous bone graft following decompressive craniectomy.
Methods
Sixty-four patients had been previously enrolled and randomised to receive either their own bone graft or a primary titanium cranioplasty. Functional and cosmetic outcomes had previously been assessed at 1-year following the cranioplasty procedure. Hospital records and the Picture Archiving communication system were reviewed to determine how many patients had cranioplasty failure or associated complications such as seizures beyond 1 year—with a minimum of 24-month follow-up.
Results
Amongst the 31 patients in the titanium group (one patient had died), no patients had a partial or complete cranioplasty failure at 12 months follow-up and there had been no failures beyond 12 months. Amongst the 31 patients who had an autologous cranioplasty (one patient had died), 7 patients had complete resorption of the autologous bone such that it was adjudged a complete failure at 12-month follow-up. Five of these patients had had titanium augmentation and two patients declined further surgery. Both of these patients requested cranial augmentation for functional and cosmetic reasons subsequent to the 12-month follow-up. Another patient who had previously been noted to have moderate resorption at 12 months presented 1 year later with progressive bone flap resorption and also required subsequent augmentation for functional and cosmetic reasons. When follow-up was extended to a minimum of 24 months, use of titanium instead of autologous bone for primary cranioplasty resulted in a significant reduction in the number of patients who required rescue cranioplasty (0 vs 25%, 95% confidence interval [CI] 9.1–42.1%; p = 0.001). In addition, there were significantly less total hospital healthcare costs in those patients randomised to the titanium arm of the trial (difference = A$9999, 95%CI 2231–17,768; p = 0.015).
Conclusions
Bone resorption continued to occur beyond 12 months after autologous cranioplasty; use of primary titanium cranioplasty after decompressive craniectomy reduced the number of reoperations needed and the associated long-term total hospital costs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There continues to be considerable interest in the use of decompressive craniectomy in the management of neurological emergencies [6]. The results of recent randomised controlled trials have confirmed the significant survival advantage that can be achieved; however, evidence that outcome is improved when compared with those patients who survive following medical management has been less forthcoming [10, 15]. This may be for a number of reasons, not least of which is the morbidity associated with both the initial decompressive craniectomy and the subsequent cranioplasty [3, 7, 17].
One issue that remains a source of debate is the choice of the the most appropriate material with which to reconstruct the skull defect. Traditionally, the material most commonly used has been the patient’s own bone that has been stored in a refridgerated sterile container. This is because autologous bone is cheap, biocompatable, strong, radiolucent and has an ideal contour. However, it has been demonstrated that use of autologous bone is associated with a high failure rate due to either infection or bony resorption [2, 4, 7, 11, 13, 14]. When this occurs, the original bone flap has to be discarded and an alloplastic material has to be used. This subjects the patient to another surgical procedure with the attendent morbidity.
In order to address this issue, a number of alternative materials have been used, one of which is titanium [1, 16]. This has the the advantage of being strong and biocompatable, and advances in computer-assisted design and manufacturing has enabled technicians to produce large custom-made prefabricated plates [1]. Favourable long-term functional and aesthetic outcomes have been reported; however, one of the disadvantages of these plates has been cost.
This prompted the current investigators to conduct a prospective randomised controlled trial comparing autologous cranioplasty with primary titanium cranioplasty [9]. Patients were randomised to receive either autologous bone that had been stored in a refridgerator or primary titanium. At 1 year follow up, although five patients who had received an autologous cranioplasty required a secondary titanium cranioplasty due to severe bone flap resorption, overall primary titanium cranioplasty was cost-neutral, and no more cost effective, compared to autologous cranioplasty. This may have been because the study sample size was too small to detect a potential difference in cost-effectiveness. Alternatively, the follow-up period may have been inadequate such that significant differences that occurred beyond 12 months were not detected.
It is in that later regard that recent clinical findings may be significant because since the trial finished, a number of patients in the autologous arm of the trial have presented beyond 12 months follow up with severe autologous bone resorption such that they required a secondary titanium cranioplasty.
The aim of this study was to review the clinical data in order to determine how many cranioplasties have failed beyond the initial 12-month follow up, incorporate this new data with the primary study analysis and recalculate the cost-effectiveness of performing a primary titanium cranioplasty.
Methods
The methodology and results of the primary analysis of this prospective randomised controlled trial have previously been published [9]. The trial had been approved by the Human Research Ethics committees from the two hospitals that provide neurosurgical services in Western Australia (EC 2012/126 and EC 2012-052). It was registered with Australian and New Zealand Clinical Trials Registry (ACTRN12612000353897).
The trial commenced recruitment in early 2012 and ended in late 2015. Sixty-four patients were enrolled and randomised to receive either their own bone that had been stored in a refrigerator at minus 80 °C or a primary titanium cranioplasty.
The inclusion and exclusion criteria, recruitment process and consent, randomisation process and manufacturing process for the titanium cranioplasties and the surgical technique have previously been reported.
Manufacture of custom-made titanium cranioplasties
A three-dimensional (3D) model of the skull was generated from a high-resolution helical multi-slice CT scan of the patient’s head. A model of the cranial defect was then created and a rapid-prototype mould was used to press the titanium plate. These were manufactured from medical-grade titanium sheet (ASTM-F67-98), ranging in thickness from 0.6 to 1.0 mm depending on the area to be covered and the depth of the pressing required. Each plate was try-fitted to the rapid-prototype model to ensure that the surface contours were smooth and the orientation was unambiguous (Fig. 1a, b).
Hospital record review
The hospital records of all patients involved in the trial were reviewed in order to determine how many patients had presented beyond the predetermined 12-month follow up. Note was made of the reasons for representation and all long-term complications. In addition, the Western Australian state-wide picture archiving communication system (PACS) was used to determine whether any patients had had further imaging performed at any other hospitals within Western Australia and the reason for requiring the imaging (e.g. seizures, possible reconstructive failure).
The original primary and secondary outcome measures were retained and the minimum follow-up period for all patients was 24 months after cranioplasty.
Outcome measures
Primary outcome measure—implant failure requiring reoperation
This may be due to either
-
Infection
This is defined as an infected cranioplasty that required removal of the implant and systemic antibiotic therapy.
-
Autologous bone flap resorption
This was assessed using CT scans following the immediate autologous cranioplasty, at the initial predetermined 12-month follow-up and at any time point thereafter when clinically indicated. The assessment was performed by the senior neurosurgeon (SH) using the original criteria.
Secondary outcome measures were as follows:
-
Adverse events (any time point beyond 12 months)
-
Long-term seizure activity
-
-
Long-term total hospital costs incurred by the two groups of patients
-
This included manufacture of the custom-made titanium cranioplasty plate (A$3500) (both as a primary and for those that failed as a secondary procedure) [1], total number of neurosurgical operations (A$25,000 per 2-h operation) and length of additional hospital days (A$1000 per ward day) including ICU stay (A$3500 per day) due to complications (excluding ongoing hospital inpatient requirements), total antibiotic-days and total theatre time [5].
-
The number of significant cost items were measured (e.g. costs of titanium plates), but the actual total costs were estimated using average costs (e.g. days in hospital) associated with each item as costed in Australia in 2015.
-
No cost analysis was incorporated for the bone flap storage. At the two neurotrauma institutions in Western Australia, the bone flaps are stored in-house within a designated bone flap refrigerator and as such, the financial implications are negligible.
-
Statistical analysis
Assuming p < 0.05 as statistically significant and a power of 80% for the study, we had previously calculated that 32 subjects in each group will be needed to demonstrate a 25% difference in rates of satisfactory cosmetic and functional outcomes due to bone resorption after primary cranioplasty. This was based on our previous experience that had demonstrated that over 32% of patients requiring decompressive craniectomy had complications related to either infections of the autologous cranioplasty or significant bone resorption compared to < 5% complication rate of using primary titanium cranioplasty [7, 8]. Overall, it had been calculated that a total of 64 patients were therefore required. All analyses had been calculated on an intention-to-treat basis. Categorical and continuous outcomes with skewed distributions were analysed by chi-square and Mann-Whitney tests, respectively. All analyses were conducted by SPSS for Windows (version 24.0, IBM, USA) and a p value < 0.05 was taken as significant.
Results
The initial study was from early 2012 to mid-2015. During that time period, 64 patients were randomised from a possible 105 patients who required a cranioplasty procedure. The patients randomised to either group had been similar in their baseline characteristics.
Primary titanium cranioplasty
Cranioplasty failure
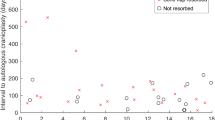
Amongst the 32 patients originally randomised to receive a primary titanium cranioplasty, there had been one death within the 12-month follow up. Since then, there have been no further deaths. No patients had presented with cranioplasty failure either within the predetermined 12-month follow up or at any time thereafter (Fig. 2).
Presentation beyond the predetermined 12-month follow up
Four patients presented to hospital with cranioplasty-related issues and had had a CT scan performed. Three patients had complained of headache. No cranial abnormality was found. One 40-year-old male who initially had a decompressive craniectomy following an assault presented on three further occasions following separate instances of assault. On each occasion, he had sustained significant head trauma but the titanium plate remained undamaged.
Primary autologous cranioplasty
Cranioplasty failure (Table 1(a and b))
Amongst the 32 patients originally randomised to receive a primary autologous cranioplasty, there had been one death within the 12-month follow-up. Since then, there have been no further deaths. In the predetermined 12-month follow-up period, five patients presented with complete cranioplasty failure due to bone flap resorption, and they each required further surgery to replace the resorbed bone with a titanium cranioplasty. Two other patients were adjudged to have complete cranioplasty failure due to bone flap resorption, but each declined further surgery. A 63-year-old lady felt that the cosmetic defect could be hidden by her hair and she was unconcerned regarding the lack of cranial protection. A 22-year-old indigenous female expressed cultural reasons for avoiding implantation of a metal object. She also had good hair coverage such that the cosmetic defect was minimally apparent. Beyond the predetermined 12-month follow-up, both patients changed their minds, predominantly because of increasingly severe postural headaches. A 40-year-old male was noted to have moderately severe resorption at 1 year and this subsequently progressed. He had augmentation with a titanium cranioplasty approximately 2 years following the initial autologous cranioplasty.
Presentation beyond the predetermined 12-month follow-up
Eight patients presented to hospital with cranioplasty-related issues and had a CT scan performed. Four patients had complained of headache. No cranial abnormality was found. Two patients presented following a seizure; a 22-year-old male presented with a first seizure and a 64-year-old male had a history of post-traumatic seizures. No cranial abnormality was found in either patient. A 41-year-old female on warfarin for a prosthetic heart valve presented 26 months following an autologous cranioplasty with an acute subdural haematoma having had a fall. This was contralateral to the previous cranioplasty and required evacuation. A 24-year-old male presented 3 years following autologous cranioplasty with a progressively worsening defect. He has made a poor neurological recovery and his parents are still considering their options. There were no infections in either the autologous or titanium cranioplasty arm of the trial patients beyond 12-month follow-up.
Overall differences in primary outcomes between the two groups
When follow-up was extended to a minimum of 24 months, use of titanium instead of autologous bone for primary cranioplasty resulted in significant reduction in the number of patients requiring rescue cranioplasty (0 vs 25%, 95% confidence interval [CI] 9.1–42.1%; p = 0.001) and total hospital healthcare costs (mean cost per patient A$33,344 vs A$43,343, respectively; and the difference = A$9999, 95%CI 2231–17,768, p = 0.015).
Discussion
This study represents the first prospective randomised controlled trial comparing autologous cranioplasty with an industrial patient-specific implant. The hypothesis upon which the trial was based was that primary titanium cranioplasty would improve cerebral protection by avoiding the frequent problem of bone resorption requiring reoperation, and this would offset the higher initial outlay cost of titanium plates.
The results of the trial at the initial predetermined 12-month follow up showed only a tendency to support this hypothesis which was not statistically significant. It was not intended to follow patients beyond 12 months because it had been anticipated that and all complications would be captured within that timeframe.
The results of the current study demonstrate that a longer period of formal clinical and radiological assessment should have been considered in order to fully appreciate the success or otherwise of cranial reconstruction with either titanium or autologous bone. Overall, the results of the current study would seem to favour the use of primary titanium over autologous bone when considering cranial reconstruction following decompressive craniectomy; however, before this position can be adopted, there are a number of issues that require consideration.
First, notwithstanding the relatively high rate of failure due to bone resorption, it should be noted that this was by no means always the case. In many cases, the autologous cranioplasty was seen to provide an excellent restorative contour with radiological evidence of bone fusion, at least at the anterior aspect of the reconstruction. What remains to be established is the reason why some bone flaps are successful and others fail so dramatically. Age would appear to be an important factor, and the results of this study would certainly support the use of primary titanium in younger patients. What remains to be established is the ideal material in the paediatric population and whilst this question is outside the remit of the current study, it remains an important research topic [4].
Second, notwithstanding the cost benefit demonstrated in this study, it should be noted that the costing of the custom-made titanium plates is probably less than the market prices, not only for titanium but also for other materials that are currently available. The plates have been manufactured in the Department of Medical Engineering and Physics at Royal Perth Hospital for more than 20 years and are produced at cost price with little need to take into account factors such as overhead expenses and profit margins that would need to be considered by a private company.
It must be acknowledged that recent advances in the direct 3D printing of metal implants has the potential to make patient-specific implants more widely available and at a lesser cost and although the initial costs of these plates may be high, the wider adoption of this technology is expected to eventually reduce the cost [12]. As such, the cost benefit demonstrated in this study may need to be recalculated depending on local circumstances, choice of reconstructive material and manufacturing costs thereafter.
Finally, there are the limitations of this study. Whilst the predetermined follow up of 12 months may have been too short, we did not allow for follow up beyond this period. As such, the long-term outcome in many of the patients has not been formally assessed, and as such, we are unable to provide certain important clinical data such as the mean length of follow up. We have assumed that there have been no failures in the titanium arm of the study beyond 12 months; however, it must be accepted that this has not been definitively assessed. Likewise, there may have been ongoing resorption beyond 12 months in patients randomised to the autologous arm of the trial. As has been previously noted [14], many patients with significant resorption do not spontaneously report this occurrence and were unaware that this could be a potential problem. Over recent years, the practice at the two neurotrauma centres in Perth Western Australia has been modified such that all patients who have an autologous cranioplasty have a fine-cut CT scan at 1 year specifically to assess the integrity of the skull. Whether this surveillance period needs to be extended to 2 years and beyond has yet to be established but will need to be considered in the light of the current study findings. A final issue that needs to be considered is the ideal material with which to reconstruct skull defects. The results of this study suggests that titanium has significant advantages over autologous bone; however, there are many other commercially available materials such as methylmethacrylate, ceramics, carbon fibre, and PEEK. Further studies will be required in order to evaluate the efficacy of each material in terms of functional outcome and cost-effectiveness, and this must be the focus of ongoing research in his area.
Conclusion
Use of primary autologous bone for a reconstructive cranioplasty remains a viable option but bone resorption is a significant problem especially in young patients. This resorption continued to occur beyond 12 months in a small number of patients. The use of primary titanium cranioplasty after decompressive craniectomy reduced the number of reoperations needed and the associated long-term total hospital costs.
References
Day RE, Guy DT, Kop AM, Morrison DA (2012) The Royal Perth Hospital method for the design and manufacture of titanium cranioplasty plates. Br J Oral Maxillofac Surg 50:376–387
Dünisch P, Walter J, Sakr Y, Kalff R, Waschke A, Ewald C (2013) Risk factors of aseptic bone resorption: a study after autologous bone flap reinsertion due to decompressive craniotomy. J Neurosurg 118:1141–1147
Gooch MR, Gin GE, Kenning TJ, German JW (2009) Complications of cranioplasty following decompressive craniectomy: analysis of 62 cases. Neurosurg Focus 26:E9
Grant GA, Jolley M, Ellenbogen RG, Roberts TS, Gruss JR, Loeser JD (2004) Failure of autologous bone-assisted cranioplasty following decompressive craniectomy in children and adolescents. J Neurosurg 100(2 Suppl Pediatrics):163–168
Ho KM, Honeybul S, Lind CR, Gillett GR, Litton E (2011) Cost-effectiveness of decompressive craniectomy as a lifesaving rescue procedure for patients with severe traumatic brain injury. J Trauma 71:1637–1644
Honeybul S, Ho KM (2013) The current role of decompressive craniectomy in the management of neurological emergencies. Brain Inj 27:979–991
Honeybul S, Ho KM (2011) Long-term complications of decompressive craniectomy for head injury. J Neurotrauma 28:929–935
Honeybul S, Ho KM (2012) How “successful” is calvarial reconstruction using frozen autologous bone? Plast Reconstr Surg 130:1110–1117
Honeybul S, Morrison DA, Ho KM, Lind CR, Geelhoed E (2017) A randomized controlled trial comparing autologous cranioplasty with custom-made titanium cranioplasty. J Neurosurg 126:81–90
Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, Anderson I, Bulters DO, Belli A, Eynon CA, Wadley J, Mendelow AD, Mitchell PM, Wilson MH, Critchley G, Sahuquillo J, Unterberg A, Servadei F, Teasdale GM, Pickard JD, Menon DK, Murray GD, Kirkpatrick PJ, RESCUEicp Trial Collaborators (2016) Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med 375:1119–1130
Matsuno A, Tanaka H, Iwamuro H, Takanashi S, Miyawaki S, Nakashima M, Nakaguchi H, Nagashima T (2006) Analyses of the factors influencing bone graft infection after delayed cranioplasty. Acta Neurochir 148:535–540
Park EK, Lim JY, Yun IS, Kim JS, Woo SH, Kim DS, Shim KW (2016) Cranioplasty enhanced by three-dimensional printing: custom-made three-dimensional-printed titanium implants for skull defects. J Craniofac Surg 27:943–949
Schuss P, Vatter H, Oszvald A, Marquardt G, Imöhl L, Seifert V, Güresir E (2013) Bone flap resorption: risk factors for the development of a long-term complication following cranioplasty after decompressive craniectomy. J Neurotrauma 30:91–95
Stieglitz LH, Fung C, Murek M, Fichtner J, Raabe A, Beck J (2015) What happens to the bone flap? Long-term outcome after reimplantation of cryoconserved bone flaps in a consecutive series of 92 patients. Acta Neurochir 157:275–280
Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, Amelink GJ, Schmiedeck P, Schwab S, Rothwell PM, Bousser MG, van der Worp HB, Hacke W, DECIMAL, DESTINY, and HAMLET investigators (2007) Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol 6:215–222
Wiggins A, Austerberry R, Morrison D, Ho KM, Honeybul S (2013) Cranioplasty with custom-made titanium plates-14 years experience. Neurosurgery 72:248–256
Yang XF, Wen L, Shen F, Li G, Lou R, Liu WG, Zhan RY (2008) Surgical complications secondary to decompressive craniectomy in patients with a head injury: a series of 108 consecutive cases. Acta Neurochir 150:1241–1247
Funding
Partially funded by Western Australian State Health Research Advisory Council ($129,500). This funding was used to cover the costs of the titanium plates manufactured at Royal Perth Hospital. The sponsor had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
Stephen Honeybul—literature search, study design, data collection, manuscript preparation.
David Anthony Morrison—titanium plate manufacture, study design, manuscript preparation.
Kwok M. Ho—study design, data analysis, statistical analysis, manuscript preparation.
Christopher RP Lind—study design, data analysis, manuscript preparation.
Elizabeth Geelhoed—study design, data analysis, cost analysis, manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed were in accordance with the ethical standards of the two hospitals that provide neurosurgical services in Western Australia (Royal Perth Hospital; EC 2012/126 and Sir Charles Gairdner Hospital; EC 2012-052) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Australian and New Zealand Clinical Trials Registry No. 12612000353897
Rights and permissions
About this article
Cite this article
Honeybul, S., Morrison, D.A., Ho, K.M. et al. A randomised controlled trial comparing autologous cranioplasty with custom-made titanium cranioplasty: long-term follow-up. Acta Neurochir 160, 885–891 (2018). https://doi.org/10.1007/s00701-018-3514-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-018-3514-z