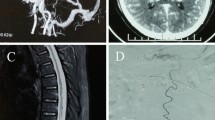
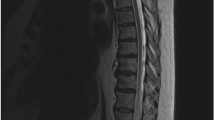
Abstract
Background
Spinal dural arteriovenous fistulas (SDAVFs) are abnormal arteriovenous shunts between a radicular artery and the radicular vein, located in the dorsal surface of the dura sleeve, which drains in a retrograde manner into the coronal venous plexus of the spinal cord without an interposed capillary network. This result is a venous hypertension that reduces spinal cord perfusion and leads to ischemia and edema. Spontaneous resolution is extremely rare and, once symptomatic, the typical course is further progression with increased neurological impairment. Therefore, once a fistula is diagnosed, treatment is recommended.
Method
The fistula is placed at the level of intervertebral foramen and surgical ligation is performed through a laminectomy. After dural opening, the area is inspected, and the arterialized vein is identified and ligated.
Conclusions
Laminectomy and arteriovenous fistula ligation is a safe and reliable approach for accessing and treating spinal dural arteriovenous fistulas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Relevant anatomy
Arterial vascularization of the spinal cord is provided by an anterior spinal artery and two posterior spinal arteries, with a variable supply from medullary branches of the metameric radiculo-meningeal arteries. Venous drainage is provided by the longitudinal spinal veins linked together and to the epidural network [1,2,3]. A spinal radicular branch supplying the dura and the nerve root as a radiculomeningeal artery is present at each segment and follows the course of the corresponding nerve root (Fig. 1). Spinal dural arteriovenous fistulas (SDAVFs) are predominantly located around the area of the intervertebral foramen within the dura, and arterial supply is provided via a posterior radiculomeningeal branch of the corresponding segmental radicular artery that supplies the dura at every level [3,4,5,6,7]. Venous drainage consists of a posterior radicular vein, a tributary of the radiculomedullary vein, which coalesces with normal spinal cord venous drainage in a retrograde fashion. The venous outflow through the medullary vein and venous plexus is located on the dorsal surface of the spinal cord in 80–90% of the cases [2].
Arterial vascularization of the spinal cord is provided by an anterior spinal artery and two posterior spinal arteries. Venous drainage is provided by the longitudinal spinal veins linked together and to the epidural network. A spinal radicular branch supplying the dura and the nerve root as a radiculomeningeal artery is present at each segment and follows the course of the corresponding nerve root
Description of the technique
Position
The patient is positioned prone on a Jackson table with care so as to minimize the degree of intraabdominal pressure. In patients with high- or mid-thoracic dural arteriovenous fistulas, radiopaque markers are useful for facilitating intraoperative identification of the correct level (Fig. 2). An arterial line is placed and we recommend a mean blood pressure of 90 mmHg to provide adequate spinal cord perfusion.
Incision
Skin is injected with local anesthetic and epinephrine to minimize bleeding. Although the procedure can be performed through an hemilaminectomy, we prefer a one-level laminectomy because it allows for easier watertight dura closure, especially in the case of elderly patients with friable dura and in overweight patients because of the depth of the surgical field.
Dural opening
A fluoroscopy confirmation of the level is mandatory before the opening of the dura. Under microscopic magnification, dura mater is opened along the midline, while leaving the arachnoid intact, and dura tack-up sutures are applied to retract the dural edges. This maneuver will minimize epidural bleeding and increase the field of view. To minimize the arachnoid disruption, under the microscope using high magnification, the area of the intravertebral foramen is explored in the subdural space between the dura and the intact arachnoid.
Intradural stage and microscope dissection
The proximal portion of the arterialized vein is identified as it emerges from the dura. It is dissected free from the adjacent nerve roots and arachnoid adhesions, and the short, proximal segment is isolated (Fig. 3). It is important to note that occasionally the radiculomedullary artery feeding the fistula provides a small arterial branch to the corresponding nerve root. At this point a temporary aneurism clip can be applied on the arterialized vein. An immediate change of both color and turgor of the vein, distal to the occlusion, can be appreciated. Indocyanine green (ICG) fluorescein angiography is helpful in confirming exposure of the arterialized vein by showing rapid and early filling. The arterialized draining vein has a typical appearance as it emerges from the dura (Fig. 3). The arterialized draining vein is then lifted away from the nerve root, coagulated with low power bipolar, and divided. We try to avoid closure of the draining vein with a surgical clip to minimize postoperative artifacts on magnetic resonance imaging (MRI).
Indications
Open microsurgery is an important therapeutic modality of treatment for most dural AV fistulas, despite advances in endovascular techniques. Surgery has the highest rate of complete occlusion, and, therefore we prefer it for patients with advanced myelopathy to provide rapid and reliable elimination of the fistula. Microsurgery is also the best option in situations where superselective catheterization of the feeding artery reveals contribution to the vascularization of the spinal cord as well.
How to avoid complications
Some patients with advanced myelopathy might have problems with mobilization, so careful periprocedural deep vein thrombosis (DVT) prophylaxis is imperative to prevent the risk of DVT formation and pulmonary emboli. After closure of the vein, there is theoretical risk of retrograde venous thrombosis which could involve veins that participate in drainage of the normal spinal cord. To minimize this risk, we insist that the patient is well hydrated and begins a low-dose aspirin regimen during the immediate postoperative period. Watertight dural closure is imperative to prevent postoperative cerebrospinal fluid (CSF) fistula.
The key to complete obliteration of the dural fistula is permanent interruption of the proximal portion of the draining vein. To prevent recanalization, the coagulated vein must be divided. Leaving a subfacial drain should be avoided to prevent aspiration of CSF. For unknown reasons, patients with type I spinal dural AV fistula have increased outflow resistance of blood draining from the intradural compartment to the epidural plexus. It is debatable whether this increased venous outflow resistance is related to the pathophysiology of the fistula or is a consequence of it.
Perioperative considerations
Intraoperative monitoring can be utilized during the procedure, although we do not think it is necessary if the proper steps described above are closely followed. We usually obtain an immediate postoperative MRI with MR angiography and repeat the study after 3-6 months. Postoperative angiography is not necessary as postoperative MRI with dramatic improvement of the flow voids and decreased vascular enhancements are adequate markers of complete fistula exclusion. There is no correlation between the degree of radiological improvement on follow-up MRI and improvement/resolution of clinical symptoms. Clinical status prior to surgery or embolization has been shown to be the most important predictor for patient outcome [4].
Specific information for patient
Patients must be informed that both endovascular and surgical ligation of the fistula are valuable therapeutic options. However, surgical treatment is associated with higher incidence of complete obliteration and lower risk of recurrences. Patients must understand that the primary goal of treatment is to arrest the progression of symptoms and that improvement can occur up to 24 months following successful treatment of the fistula. Patients uniformly experience improvement or at least stabilization, although not normalization, of motor symptoms. Patients with advanced myelopathy might experience a subjective worsening of their motor symptoms months after closure of the fistula. This is usually related to the superimposed effects of progressively increasing muscle tone and spasticity and often improves with medical and pharmacological treatment. Sensory symptoms improve in about 50% of patients, while bladder dysfunction only improves in about one-third of patients.
Summary of key points
-
1.
Type I SDAVFs are mostly localized in the region of the intervertebral foramen.
-
2.
Effective and definitive exclusion of the fistula results only from interruption of the proximal portion of the draining vein as it emerges from the dura.
-
3.
In patients with high or mid-thoracic dural arteriovenous fistulas, radiopaque markers are useful for facilitating intraoperative identification of the correct level
-
4.
Surgical treatment consists of isolation, coagulation, and division of the proximal draining vein after intradural exposure.
-
5.
The dura should be open while leaving the arachnoid intact.
-
6.
Through the intact arachnoid, the area of the neuroforamen is explored and the arterialized draining vein is identified as it emerges from the dura.
-
7.
The arterialized draining vein is freed of any arachnoid connection and coagulated close to its point of emergence with low-power cautery and then divided.
-
8.
ICG fluorescein angiography is helpful in confirming exposure of the arterialized vein by showing rapid and early filling.
-
9.
After closure of the vein there is a theoretical risk of retrograde venous thrombosis, which could involve veins that participate in drainage of the normal spinal cord. To minimize this risk, we insist that the patient be well hydrated and will begin a low-dose aspirin regimen during the immediate postoperative period.
-
10.
After definitive treatment of the fistula, motor symptoms improve in most patients, although do not necessarily normalize. Sensory symptoms improve in approximately 50% of patients, while improvement in bladder dysfunction occurs only in about 33% of cases.
References
Amanieu C, Hermier M, Peyron N, Chabrol A, Deiana G, Manera L (2014) Spinal dural arteriovenous fistula. Diagn Interv Imaging 95:897–902
Bortolotti C, Morales-Valero SF, Lanzino G (2014) Spinal dural arteriovenous fistulae. Contemp Neurosurg 36:1–5
Krings T, Geibprasert S (2009) Spinal dural arteriovenous fistulas. AJNR Am J Neuroradiol 30:639–648
Marcus J, Schwarz J, Singh IP, Sigounas D, Knopman J, Gobin YP, Patsalides A (2013) Spinal dural arteriovenous fistulas: a review. Curr Atheroscler Rep 15:335
Saladino A, Atkinson JL, Rabinstein AA, Piepgras DG, Marsh WR, Krauss WE, Kaufmann TJ, Lanzino G (2010) Surgical treatment of spinal dural arteriovenous fistulae: a consecutive series of 154 patients. Neurosurgery 67:1350–1357 discussion 1357-1358
Steinmetz MP, Chow MM, Krishnaney AA, Andrews-Hinders D, Benzel EC, Masaryk TJ, Mayberg MR, Rasmussen PA (2004) Outcome after the treatment of spinal dural arteriovenous fistulae: a contemporary single-institution series and meta-analysis. Neurosurgery 55:77–87 discussion 87-78
Takai K, Komori T, Taniguchi M (2015) Microvascular anatomy of spinal dural arteriovenous fistulas: arteriovenous connections and their relationships with the dura mater. J Neurosurg Spine 23:526–533
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Informed consent was obtained for the procedure illustrated. However, no institutional review board approval, or patient consent is required per institutional policy for retrospective, single-cases in which no identifiable patient information is shared.
Electronic supplementary material
The authors demonstrate the steps for the successful access, cautery and division of a symptomatic spinal dural arteriovenous fistula in an 82-year-old man using a one-level laminectomy. (MP4 180,883 kb)
Rights and permissions
About this article
Cite this article
Sorenson, T., Giordan, E., Cannizzaro, D. et al. Surgical ligation of spinal dural arteriovenous fistula. Acta Neurochir 160, 191–194 (2018). https://doi.org/10.1007/s00701-017-3381-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-017-3381-z