Abstract
Background
As diffusion tensor imaging (DTI) is able to assess tissue integrity, authors used diffusion to detect abnormalities in trigeminal nerves (TGN) in patients with trigeminal neuralgia (TN) caused by neurovascular compression (NVC) who had undergone microvascular decompression (MVD). The authors also studied anatomical TGN parameters (cross-sectional area [CSA] and volume [V]). The study compared pre- and postoperative findings.
Methods
Using DTI sequencing on a 3-T MRI scanner, we measured the fraction of anisotropy (FA) and apparent diffusion coefficient (ADC) of the TGN in 10 patients who had undergone MVD for TN and in 6 normal subjects. We compared data between affected and unaffected nerves in patients and both nerves in normal subjects (controls). We then correlated these data with CSA and V. Data from the affected side and the unaffected side before and 4 years after MVD were compared.
Results
Before MVD, the FA of the affected side (0.37 ± 0.03) was significantly lower (p < 0.05) compared to the unaffected side in patients (0.48 ± 0.03) and controls (0.52 ± 0.02), and the ADC in the affected side (5.6 ± 0.34 mm2/s) was significantly higher (p < 0.05) compared to the unaffected side in patients (4.26 ± 0.25 mm2/s) and controls (3.84 ± 0.18 mm2/s). Affected nerves had smaller V and CSA compared to unaffected nerves and controls (p < 0.05). After MVD, the FA in the affected side (0.41 ± 0.02) remained significantly lower (p < 0.05) compared to the unaffected side (0.51 ± 0.02), but the ADC in the affected side (4.24 ± 0.34 mm2/s) had become similar (p > 0.05) to the unaffected side (4.01 ± 0.33 mm2/s).
Conclusions
DTI revealed a loss of anisotropy and an increase in diffusivity in affected nerves before surgery. Diffusion alterations correlated with atrophic changes in patients with TN caused by NVC. After removal of the compression, the loss of FA remained, but ADC normalized in the affected nerves, suggesting improvement in the diffusion of the trigeminal root.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trigeminal neuralgia (TN) is an example of a neurogenic facial pain condition. Classical TN requires the use of imaging to depict a potential neurovascular compression (NVC) of the trigeminal nerve (TGN) root [7]. Strong evidence suggests that the neuralgia is due to morphological and structural changes that are likely the result of chronic vascular compression [11, 38, 39].
High-resolution magnetic resonance imaging (MRI) sequences—three-dimensional (3D) T2-weighted, 3D time-of-flight (TOF), magnetic resonance angiography (MRA) along with 3D T1-weighted gadolinium (3D T1-Gad)—have been shown to be effective for depicting the fine trigeminal neurovascular anatomy, of paramount importance for planning microvascular decompression (MVD) [26, 28]. Additionally, structural abnormalities such as nerve deviation, groove formation, or atrophy can be seen in a large number of cases of TN [11]. Volume (V) and cross-sectional area (CSA) measurements obtained by MRI were significantly smaller in affected nerves than in unaffected nerves [29]. However, these morphological changes can be difficult to interpret.
Diffusion tensor imaging (DTI) enables the analysis of white matter integrity by means of in vivo measurements of molecular diffusivity [25]. There are numeric indexes to describe anisotropic diffusion and underlying tissue integrity [36]. Measuring the fraction of anisotropy (FA) presents a robust method of assessing the degree of directionality of diffusion (i.e., anisotropic diffusion) that occurs in a particular region [37]. Diffusivity, expressed as the apparent diffusion coefficient (ADC), is a quantitative measure of water motility (independent of orientation) in an individual voxel [37] and indicates the overall presence of obstacles to diffusion, correlating, not only with demyelination, but also with neuroinflammatory processes and/or neuroedema [2]. This method has been applied to the study of abnormalities in white matter tracts, independent of their cause.
This body of evidence has motivated several authors to examine structural abnormalities in the TGN of patients with TN using DTI parameters [3,4,5, 8, 9, 13, 18, 20, 31, 33,34,35, 41]. In 2011, our group reported in the literature that the affected nerve in patients had a decreased FA and an increased ADC compared to their unaffected nerve and compared to a control group [27]. These patients were subsequently submitted to MVD and harbored a potential NVC at imaging [27]. These alterations correlated with atrophic changes in patients with TN caused by NVC [27]. However, our work did not investigate diffusion parameters after nerve decompression. In the present study, we measured FA and ADC in both sides in the same patient group 4 years after MVD, verifying the long-term microstructural state of the TGN after removal of compression. The DTI parameters were then correlated with anatomical parameters (V and CSA) measured by 3-Tesla (3 T) MRI.
Methods
Participants
In a publication by our group [27], we conducted a prospective controlled study including 10 patients with a diagnosis of TN who underwent a surgical treatment by MVD and 6 normal control subjects. In the present work, we performed, in the same group of patients, a follow-up study 4 years after surgery to assess the effects of MVD on pain and the postoperative trigeminal root changes revealed by DTI. Ten patients (7 females and 3 males), with ages ranging from 26 to 68 years, were included in the study. Two patients had pain on the right side and eight on the left. The V1 division was affected in 2 patients, V2 in 8 and V3 in 6. The duration of the neuralgia before surgery averaged 5.7 years, ranging from 3.5 to 13 years. Six normal control subjects (3 females and 3 males), with ages ranging from 22 to 58 years, were also included in the study.
The study was approved by our institutional review board and was therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients and controls gave informed consent prior to inclusion in this study.
Imaging acquisition
Patients underwent an MRI examination using a 3-T MR scanner (Philips Achieva). Images were obtained using an eight-channel head coil with sensitivity encoding parallel processing capability, with the application of 32 non-collinear directions of diffusion gradients. Four years after MVD, all patients underwent the same imaging protocol of MRI that had been performed before surgery [27] to verify the trigeminal neurovascular anatomy and to obtain postoperative measurements of FA, ADC, V, and CSA. Two T2-weighted sequences were acquired in coronal and sagittal planes for accurate visualization of the TGN. These images were used for localization and exact graphical alignment of the slice position of the DTI sequence, parallel to and in the same plane as the TGN. For the acquisition of measurements of FA and ADC, we performed a DTI sequence (2D echoplanar imaging). In addition, we obtained a three-step protocol to assess the nerve decompression and to measure morphometric data [26, 28]: 3D driven equilibrium (DRIVE), 3D TOF-MRA, and 3D T1-Gad (T1-Gad) (Table 1).
Trigeminal root diffusion metric analysis
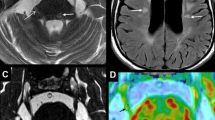
FA and ADC values were calculated for each voxel using the imaging software MedINRIA, available as a free download on the website: http://www-sop.inria.fr/asclepios/software/MedINRIA/[27]. Data were analyzed independently by 2 observers (J.A.R., P.R.L.L.) who were blinded to the surgical and clinical data. For statistical analysis, we used the mean values of the two observers. The inter-observer coefficients of variation for the average FA and ADC were less than 5%. To limit potential partial volume effects, the region of interest chosen for quantitative assessment of FA and ADC was the trigeminal root entry zone (TREZ) (Fig. 1). These parameters were compared with those obtained before surgery [27].
a Operative view of neurovascular compression (NVC) during microvascular decompression (MVD). The superior cerebellar artery (SCA) exerts pressure, leading to deformation at the superolateral aspect of the left trigeminal nerve (TGN). b Diffusion tensor imaging (DTI) showing examples of green box-shaped regions of interest used for quantitative analysis of DTI parameters in the trigeminal root entry zone (TREZ) before MVD [27]. There is an obvious difference between the 2 nerves, with a visibly reduced signal in the left TGN. c DTI showing that the reduction in the caliber of the TGN at the TREZ remains almost as important as in b 4 years after the surgical procedure
Trigeminal root morphometric analysis
Three-dimensional DRIVE images were transferred to a workstation for post-processing and analysis. Measurements of V and CSA for each TGN were performed using the imaging software MIPAV, with a free download available on the website http://mipav.cit.nih.gov/[29]. These morphometric parameters were calculated independently by the same observers (J.A.R., P.R.L.L.) who were blinded to the surgical and clinical data. For the statistical analysis, we used the mean values of the two observers. The inter-observer coefficients of variation for the average V and CSA were less than 5%. These parameters were compared with those obtained before surgery [27] and correlated to trigeminal root diffusion metric data.
Assessment of the effect of MVD on pain
The senior surgeon (M.S.) assessed all 10 patients in person at discharge (generally postoperative day 10), at the first outpatient visit (usually around postoperative day 70), and at 1, 2, 3, and 4 years after surgery. Four years after surgery, neuralgia was considered cured, and thus, the MVD a success, when relief was complete and all medication could be withdrawn. Neuralgia was estimated as partially relieved when some pain remained, but was well controlled by complementary medical therapy. The treatment was qualified as a failure when the pain persisted despite medical therapy.
Statistical analysis
The diffusion metric data (FA and ADC) and morphometric data (V and CSA) were compared in the affected and unaffected sides of patients 4 years after surgery. These results were also compared with those of the affected and unaffected sides of patients before surgery and compared with normal control subjects (controls) [27] using a paired-sample two-tailed t test. Results were expressed as mean ± SD.
Differences in diffusion metric data (FA and ADC) in affected vs unaffected sides, calculated for each patient and expressed as a percentage, were compared before [27] and 4 years after MVD using an independent t test. Correlations between differences in affected vs unaffected sides, calculated for each patient and expressed as a percentage, were compared between FA and ADC values before [27] and 4 years after MVD. The Spearman correlation coefficient was calculated, and a linear regression curve was plotted using GraphPad Prism® statistical analysis software.
Finally, correlations between differences in affected vs unaffected sides, calculated for each patient and expressed as a percentage, were compared between loss of FA and loss of V, loss of FA and loss of CSA, increase in ADC and loss of V, and increase in ADC and loss of CSA before [27] and 4 years after MVD. The Spearman correlation coefficient was calculated, and a linear regression curve was plotted using GraphPad Prism® statistical analysis software.
Values of p ≤ 0.05 were considered as statistically significant differences.
Results
Surgical findings
NVC was found in all patients after complete exploration of the trigeminal root from the porus of the trigeminal Meckel’s cave to the TREZ at the pons. The superior cerebellar artery (SCA) was responsible for compression in 5 cases, the anterior inferior cerebellar artery (AICA) in 2 cases, and the basilar artery (BA) in 1 case. A vein was additionally compressing the nerve in 2 cases: the superior petrosal vein (SPV) in 1 case and the transverse pontine vein (TPV) in 1 case. NVC was located in the TREZ in 9 cases and the juxtapetrous (JP) segment in 1 case. The site of compression was superomedial (SM) to the root in 5 cases, inferior (INF) to the root in 4 cases, and superolateral (SL) to the root in 1 case. The severity of a neurovascular contact was graded using the system, introduced by Sindou et al. [38, 39]. The severity of NVC was grade I (mere contact) in 4 cases, grade II (displacement of the root) in 3 cases, and grade III (engrooving into the root) in 3 cases.
Trigeminal root diffusion metric measurements
Table 2 summarizes diffusion metric data (FA and ADC) extracted before MVD, published previously [27], and 4 years after surgery.
Before MVD, the mean FA in the affected side was significantly lower (p < 0.05) than the mean FA in the unaffected side of patients and the mean FA in both sides of the controls [27]. The mean ADC in the affected side was significantly higher (p < 0.05) than the mean ADC in the unaffected side of patients and the mean ADC in both sides of controls [27].
Four years after MVD, the mean FA in the affected side remained (0.41 ± 0.08, confidence interval [CI] 0.36–0.46) significantly lower (p < 0.05) than the mean FA in the unaffected side of patients (0.51 ± 0.07, CI 0.46–0.56) and the mean FA in both sides of the controls (0.52 ± 0.04, CI 0.48–0.56) (Fig. 2a). Four years after MVD, the mean FA in the affected side was similar (p > 0.05) to the mean FA in the affected side of patients before surgery [27] (Fig. 2b). Differences between FA in the affected side and the unaffected side, calculated for each patient and expressed as a percentage after MVD (18.98 ± 0.07%, CI 6.98–30.98) was similar to data before the procedure [27] (21.3 ± 18.96%, CI 7.73–34.87) (p = 0.6758) (Fig. 3).
a Graph showing a fraction of anisotropy (FA) data before [27] and after microvascular decompression (MVD). Before MVD, the mean FA in trigeminal nerves (TGN) in the affected side of patients (n = 10) was significantly lower (p < 0.05) than the mean FA in the TGN in the unaffected side of patients (n = 10) and the mean FA of the TGN in both sides of control subjects (n = 12). Four years after MVD, the mean FA in the affected side of patients (n = 10) was also significantly lower (p < 0.05) than the mean FA in the unaffected side (n = 10) and the mean FA in both sides of control subjects (n = 12). b Graph showing FA data connected by lines for each respective affected nerve of patients. Four years after MVD, the mean FA in the affected side was similar (p > 0.05) to the mean FA in the affected side of patients before surgery [27]. * = statistical significance
Graph showing that differences between the fraction of anisotropy (FA) in the affected side (n = 10) and the unaffected side (n = 10), calculated for each patient and expressed as a percentage, before surgery (21.3 ± 18.96%, CI 7.73–34.87) [27] were similar to those found 4 years after the procedure (18.98 ± 0.07%, CI 6.98–30.98)
Four years after MVD, the mean ADC in the affected side (4.24 ± 1.06 mm2/s, CI 3.48–4.99) had become similar (p > 0.05) to the mean ADC in the unaffected side of patients (4.01 ± 1.03 mm2/s, CI 3.27–4.75) and similar to the mean ADC in both sides of the controls (3.84 ± 0.43 mm2/s, CI 3.43–4.2) (Fig. 4a). Four years after MVD, the mean ADC in the affected side was lower (p < 0.05) than the mean ADC in the affected side of patients before surgery [27] (Fig. 4b). Differences in ADC between the affected side and the unaffected side of patients, calculated for each patient and expressed as a percentage, after MVD (7.4 ± 19.67%, CI − 6.66–21.47) was lower compared to data before the procedure [27] (28.17 ± 18.45%, CI 14.97–41.37) (p = 0.04) (Fig. 5).
a Graph showing the apparent diffusion coefficient (ADC) data before [27] and after MVD. Before MVD, the mean ADC in the affected side (n = 10) was significantly higher (p < 0.05) than the mean ADC in the unaffected side (n = 10) and the mean ADC in both sides of control subjects (n = 12). Four years after MVD, the mean ADC in the affected side (n = 10) was similar (p > 0.05) to the mean ADC in the unaffected side (n = 10) and the mean ADC in both sides of control subjects (n = 12). b Graph showing ADC data connected by lines for each respective affected nerve of patients. Four years after MVD, the mean ADC in the affected side was lower (p < 0.05) than the mean ADC in the affected side of patients before surgery [27]. * = statistical significance
Graph showing that differences between the apparent diffusion coefficient (ADC) in the affected side (n = 10) and the unaffected side (n = 10), calculated for each patient and expressed as a percentage, before surgery (28.17 ± 18.45%, CI 14.97–41.37) [27] were higher than those found 4 years after the procedure (7.4 ± 19.67%, CI − 6.66–21.47). * = statistical significance
Before MVD [27], the Spearman correlation coefficient showed a strong negative correlation (r = − 0.7295, p = 0.0202) between loss of anisotropy and the corresponding increase in ADC in the affected side compared to the unaffected side of patients (Fig. 6a). Four years after MVD, the Spearman correlation coefficient did not show correlation (r = − 0.0303, p = 0.9460) between loss of anisotropy and the corresponding increase in ADC in the affected side compared to the unaffected side of patients (Fig. 6b).
a Linear regression plot derived from the relationship between the differences in the fraction of anisotropy (FA) and the apparent diffusion coefficient (ADC), in affected nerves vs unaffected nerves in patients with trigeminal neuralgia (TN) before microvascular decompression (MVD) [27]. The Spearman correlation coefficient showed a strong negative correlation (r = − 0.7295, p = 0.0202) between loss of anisotropy and the corresponding increase in diffusivity. b Linear regression plot derived from the relationship between the differences in FA and ADC, in affected nerves vs unaffected nerves in patients with TN 4 years after MVD. The Spearman correlation coefficient did not show correlation (r = − 0.0303, p = 0.9460) between loss of anisotropy and the corresponding increase in diffusivity
Trigeminal root morphometric measurements
Table 3 summarizes morphometric data (V and CSA) extracted before MVD, published previously [27] and 4 years after surgery. Before and after the surgical procedure, the mean V of the affected side was significantly smaller (p < 0.05) than the mean V of the unaffected side of patients and the mean V of both sides in controls (Table 3). Before and 4 years after the surgical procedure, the mean CSA of the affected side was significantly smaller (p < 0.05) than the mean CSA of the unaffected side of patients and the mean CSA of both sides in controls (Table 3).
Correlation of diffusion metric and morphometric data on the trigeminal root
As shown in our first publication [27], the Spearman correlation coefficient showed a strong positive correlation between loss of anisotropy and the corresponding loss of V in the affected side compared to the unaffected side in patients (Fig. 7a) and loss of anisotropy and the corresponding loss of CSA in the affected side compared to the unaffected side in patients (Fig. 7b) before MVD. The Spearman correlation coefficient showed a strong negative correlation between increased ADC and loss of V in the affected side compared to the unaffected side in patients (Fig. 7c) before MVD [27]. A strong negative correlation was also shown between increased ADC and loss of CSA in the affected side compared to the unaffected side in patients (Fig. 7d) [27].
Correlations of differences between affected and unaffected sides, calculated for each patient and expressed as a percentage, were compared between diffusion tensor imaging (DTI) and morphological data on the trigeminal nerve (TGN). Before MVD [27], linear regression plots revealed that the Spearman correlation coefficient showed: a a strong positive correlation (r = 0.8303, p = 0.0047) between the loss of FA and the corresponding loss of V, b a strong positive correlation (r = 0.9273, p = 0.0003) between the loss of FA and the corresponding loss of CSA, c a strong negative correlation (r = − 0.8085, p = 0.0072) between the increase in ADC and the corresponding loss of V, and d a strong negative correlation (r = − 0.7416, p = 0.0174) between the increase in ADC and the corresponding loss of CSA. Four years after MVD, linear regression plots revealed that the Spearman correlation coefficient showed: e no correlation (r = 0.6364, p = 0.0544) between the loss of FA and the corresponding loss of V, but the p value was slightly higher than 0.05, f a strong positive correlation (r = 0.8545, p = 0.0029) between the loss of FA and the corresponding loss of CSA, g no correlation (r = − 0.2242, p = 0.5367) between the increase in ADC and corresponding loss of V, and h no correlation (r = − 0.0181, p = 0.9730) between the increase in ADC and the corresponding loss of CSA
Four years after MVD, the Spearman correlation coefficient did not show correlation, but the p value was slightly higher at 0.05, between loss of anisotropy and the corresponding loss of V in the affected side compared to the unaffected side in patients (Fig. 7e). The Spearman correlation coefficient showed a strong positive correlation between loss of anisotropy and the corresponding loss of CSA in the affected side compared to the unaffected side in patients (Fig. 7f). In contrast, the Spearman correlation coefficient did not show correlation between differences in ADC and the corresponding loss of V in the affected side compared to the unaffected side in patients (Fig. 7g), nor differences in ADC and the corresponding loss of CSA in the affected side compared to the unaffected side in patients (Fig. 7h).
Pain relief over time
At discharge, on the 10th postoperative day, all patients reported neuralgia relief. At the outpatient visit on postoperative day 70, 9 patients were pain-free and 1 still had some background pain which was well controlled with medical treatment. At the 1-, 2-, 3-, and 4-year postoperative visits, the success rate was 90%, corresponding to 9 patients who were totally pain-free and 1 patient (10%) who was partially relieved.
Discussion
This study is the first long-term evaluation of trigeminal root abnormalities by DTI conducted in a homogenous series 4 years after MVD in patients with classical TN. The study shows the persistence of loss of FA and recovery of ADC in the affected nerve after surgical decompression.
DTI abnormalities in the trigeminal nerve root due to neurovascular compression
Several studies examining DTI metrics comparing the affected and unaffected sides in patients with classical TN and controls have revealed a significant decrease in FA values [3,4,5, 8, 9, 18, 20, 31, 33,34,35] and a significant increase in ADC values [5, 33]. Three other studies did not find differences in ADC values between affected and unaffected sides in patients and controls [13, 34, 35]. Five articles on specific DTI studies on TN caused by NVC found a significant decrease in FA values [18, 31, 33,34,35] and one found a significant increase in ADC values [33] in the affected nerves. Two studies compared diffusion abnormalities in patients with TN before and after surgery [9, 20], but with a different population of patients and a different protocol and data acquisition method.
Liu et al. studied 16 consecutive patients with classical TN in a controlled protocol, with 3 T DTI sequences with the application of 12 non-collinear directions of diffusion gradients. Their study demonstrated that FA was significantly lower in the affected side, while radial diffusivity (RD) and mean diffusivity (MD) were significantly higher in the affected side compared to the unaffected side [31]. Lummel et al., using a 3-T DTI sequence with the application of 15 non-collinear directions, compared diffusion alterations in 12 patients with TN caused by multiple sclerosis (MS), 12 patients with idiopathic TN caused by NVC, and 12 normal control subjects [33]. The authors found that FA was significantly lower in the affected side compared to the unaffected side in patients with idiopathic TN or normal control subjects [33]. Recently, Lutz et al. analyzed diffusion abnormalities in 81 patients with TN who underwent MVD in a blinded fashion. DTI analyses revealed significantly lower FA values within the vulnerable zone of the affected TGN compared with the contralateral side [35].
Fujiwara et al. did not find significant differences between the absolute FA and ADC values for the affected and unaffected sides or between the absolute FA and ADC values in patients with classical TN and control subjects. In addition, the mean variance of FA values between both sides in the patient group was not significantly different from that in the control group [13].
With the exception of the last authors [13], these abovementioned results reinforced evidence of pathological changes such as axonopathy, axonal loss, demyelination, and residual myelin debris in histological studies of trigeminal fascicles collected from patients with NVC near the TREZ [10, 19, 23]. The elevation in diffusivity in the TREZ of affected nerves indicates the presence of obstacles to diffusion, probably because of axonal loss and focal demyelination. Reduced anisotropy could result from damage and removal of highly aligned cellular structures such as axons or from focal endoneurial injury resulting from NVC. These phenomena may explain the theory of focal demyelination of the sensory axons at the site of the NVC [32] and that ephaptic “short circuits” are responsible for neuralgia, as hypothesized by Gardner [14]. Demyelinated nerve fibers are also known to be sensitive to tiny deformations [40], so that pulsatile compression by a vessel could, at least in theory, also initiate axonal impulses that spread ephaptically within the TREZ [32]. Demyelination has also been found in well-documented experimental models of acute [15] or chronic [12] compression of central white matter.
Postoperative DTI changes after microvascular decompression
Little information has been published on long-term DTI abnormalities after surgical treatment. In our study, all patients underwent MVD with a complete exploration of the TGN and detection of direct neurovascular contact in all cases [27]. Nine patients were pain-free and only 1 was partially relieved. Four years after MVD, DTI was applied in a 3-T MRI machine with the application of 32 non-collinear directions of diffusion gradients (Table 1). With DTI, multiple images are collected so that the signal can be sensitized to diffusion in different directions, building up multiple measurements for each voxel in the brain [22].
DeSouza et al. studied patients who underwent gamma knife radiosurgery (GKRS, 15 patients) or MVD (10 patients) before and 2 to 6 months after treatment and 14 controls [9]. The authors established a cutoff of at least a 75% reduction in preoperative pain to distinguish effective from ineffective treatment [9]. After effective treatment (a group of 6 patients who had GKRS and 9 patients who had MVD), the FA abnormality in the affected side resolved such that FA increased and was no longer significantly different from the unaffected side or controls [9]. However, FA remained significantly lower in the affected side compared to the unaffected side and controls in the ineffective treatment group (a group of 9 patients who had GKRS and 1 patient who had MVD) [9]. The authors suggested that surgical treatment can effectively resolve pain by normalizing trigeminal root abnormalities [9]. The main criticism of the DeSouza et al. study was the link between patients who underwent different surgical treatments and early DTI metrics extraction after surgical treatment (2 to 6 months), in which tissue changes were not yet fully established. MVD would influence TREZ abnormalities by removing the source of the compression and the subsequent pathophysiology at this location [9]. Radiosurgery would produce analgesia by injuring trigeminal fibers just enough to impede the aberrant signaling of these neurons [9]. Studies examining the effects of radiation on peripheral nerves have found that radiation doses in the range used for TN radiosurgery can decrease the excitability of cells by partially blocking sodium and nerve conduction [16].
In the Hodaie et al. study on 5 TN patients who benefited from radiosurgery, treatment resulted in a 47% drop in FA values at the target with no significant change in FA outside the target, demonstrating highly focal changes after treatment. This suggests that radiosurgery primarily affects myelin [20]. In their study, postoperative imaging was performed at 6–7 months (3 patients) and at 12–14 months (2 patients) after surgery [20]. DTI abnormalities could be related to radiation in the trigeminal root and might interfere with DTI data. In the literature, it has been hypothesized that, over time, the axonal injury induced by radiosurgery would resolve and be accompanied by remyelination and normalization of membrane channels, including those at the TREZ [16].
In our study, FA values remained lower in the affected side of patients despite the root decompression. The way FA reveals the level of myelin in tissue structure and packing density of axons within a voxel is that FA values drop when demyelination and axonal loss is are present [1, 30]. We hypothesize that nerve damage caused by chronic pulsatile vascular compression can be definitive because of irreversible lesions in TGN myelin. Little information is available about the long-term completeness of remyelination in the central nervous system (CNS). Harrison and McDonald reported, in an experimental cat model, that the thickness of myelin around remyelinated fibers after spinal cord compression appears to increase over a period of months, but even after 18 months, remyelinated fibers with inappropriately thin sheaths may remain numerous [17]. Remyelination in the CNS is apparently a progressive but incomplete process [17]. The possibility that some of the thin myelin sheaths may indicate partial demyelination rather than remyelination cannot be excluded [19]. Aberrant remyelination seems to be the most likely explanation for this phenomenon, given that the process involves CNS rather than peripheral nervous system (PNS) axons, and that the nerve roots appear entirely normal, both proximal and distal to the region of compression. This is was found in histological trigeminal fascicles collected from patients with NVC near the TREZ [19]. The chronic compression might also create focal endoneurial vascular injury, with resulting ischemia.
In our study, ADC increased after nerve decompression. This recovery of ADC values suggests an improved conduction and reduced edema of the trigeminal root after surgery.
Trigeminal root atrophy persists after microvascular decompression
Morphometric abnormalities persisted after surgery, with measurements of V and CSA of the affected nerve being 25.3% and 23.5% smaller, respectively, than those of unaffected nerves in patients. These results, obtained non-invasively and confirmed by two blinded observers, are concordant with observations made during surgery [38], and with the studies of authors who measured morphometric parameters using different noninvasive imaging methods [11, 24, 29].
Technical limitations
In regard to limitations of the study, firstly, the partial volume effect specifically caused by the small size of the root that is bathed in cerebrospinal fluid may confound DTI measurements. Secondly, severe image degradation by involuntary patient movement (causing ghosting or artifactual image) and the magnetic susceptibility effects with large discontinuities in bulk magnetic susceptibility (such as those occurring at tissue-air interfaces, producing local magnetic field gradients) could degrade and distort DTI [6, 21]. Thirdly, due to the proximity of the Teflon plate to the TGN at the pontocerebellar angle, consequently introducing the possibility of imaging artifacts, it was difficult to perform the measurements of the morphometric parameters after surgery. Finally, the small number of cases is also one of the limitations in this study.
Conclusions
The DTI technique was used to investigate TGN microstructure secondary to a NVC in patients with TN 4 years after MVD. Long term after the removal of compression, loss of FA persisted, but ADC normalized in the affected nerves, suggesting an improvement in conduction sensitivity and reduction of edema in the trigeminal root after surgery. The re-establishment of diffusion could well be the reason for pain relief after MVD. Furthermore, from a practical standpoint, DTI metrics could be an effective biomarker for confirmation of aggressiveness of a potential NVC found on imaging and could become an additional diagnostic tool for ascertaining its compressive behavior, thus helping in decision making.
References
Acosta-Cabronero J, Williams GB, Pengas G et al (2010) Absolute diffusivities define the landscape of white matter degeneration in Alzheimer’s disease. Brain 133(Pt 2):529–539
Basser PJ, Pierpaoli C (1996) Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reason 213:560–570
Chen DQ, DeSouza DD, Hayes DJ et al (2016) Diffusivity signatures characterize trigeminal neuralgia associated with multiple sclerosis. Mult Scler 22(1):51–63
Chen F, Chen L, Li W et al (2016) Pre-operative declining proportion of fractional anisotropy of trigeminal nerve is correlated with the outcome of micro-vascular decompression surgery. BMC Neurol 16:106
Chen ST, Yang JT, Yeh MY et al (2016) Using diffusion tensor imaging to evaluate microstructural changes and outcomes after radiofrequency rhizotomy of trigeminal nerves in patients with trigeminal neuralgia. PLoS One 11(12):e0167584
Cottier JP, Barantin L, Destrieux C et al (2005) Cerebral diffusion tensor imaging and brain fiber tracking: principles and current limitations. Feuill Radiol 45(3):191–199
Cruccu G, Finnerup NB, Jensen TS et al (2016) Trigeminal neuralgia: new classification and diagnostic grading for practice and research. Neurology 87(2):220–228
DeSouza DD, Hodaie M, Davis KD (2014) Abnormal trigeminal nerve microstructure and brain white matter in idiopathic trigeminal neuralgia. Pain 155:37–44
DeSouza DD, Davis KD, Hodaie M (2015) Reversal of insular and microstructural nerve abnormalities following effective surgical treatment for trigeminal neuralgia. Pain 156(6):1112–1123
Devor M, Govrin-Lippmann R, Rappaport H (2002) Mechanism of trigeminal neuralgia: an ultrastructural analysis of trigeminal root specimens obtained during microvascular decompression surgery. J Neurosurg 96:532–543
Erbay SH, Bhadelia RA, O’Callaghan M et al (2006) Nerve atrophy in severe trigeminal neuralgia: non-invasive confirmation at MR imaging – initial experience. Radiology 238:689–692
Fish CJ, Blakemore WF (1983) A model of chronic spinal cord compression in the cat. Neuropathol Appl Neurobiol 9:109–120
Fujiwara S, Sasaki M, Wada T et al (2011) High-resolution diffusion tensor imaging for the detection of diffusion abnormalities in the trigeminal nerves of patients with trigeminal neuralgia caused by neurovascular compression. J Neuroimaging 21:102–108
Gardner (1962) Concerning the mechanism of trigeminal neuralgia and hemifacial spasm. J Neurosurg 19:947–958
Gledhill RF, Harrison BM, McDonald WI (1973) Demyelination and remyelination after acute spinal cord compression. Exp Neurol 38:472–487
Gorgulho A (2012) Radiation mechanisms of pain control in classical trigeminal neuralgia. Surg Neurol Int 3:S17–S25
Harrison BM, McDonald WI (1977) Remyelination after transient experimental compression of the spinal cord. Ann Neurol 1:542–551
Herweh C, Kress B, Rasche D et al (2007) Loss of anisotropy in trigeminal neuralgia revealed by diffusion tensor imaging. Neurology 68:776–778
Hilton DA, Love S, Gradidge T et al (1994) Pathological findings associated with trigeminal neuralgia caused by vascular compression. Neurosurgery 35:299–303
Hodaie M, Chen DQ, Quan J et al (2012) Tractography delineates microstructural changes in the trigeminal nerve after focal radiosurgery for trigeminal neuralgia. PLoS One 7:e32745
Horsfield MA, Jones DK (2002) Applications of diffusion-weighted and diffusion tensor MRI to white matter diseases – a review. NMR Biomed 15:570–577
Johansen-Berg H, Rushworth MF (2009) Using diffusion imaging to study human connectional anatomy. Annu Rev Neurosci 32:75–94
Kopp N, Adabotti J, Sindou M (1994) Histological study (photon and electron microscopic) of trigeminal rootlet specimen in patients with idiopathic trigeminal neuralgia operated on for vascular decompression (13 cases). Acta Neurochir 109:129
Kress B, Schindler M, Rasche D et al (2005) MRI volumetry for the preoperative diagnosis of trigeminal neuralgia. Eur Radiol 15:1344–1348
Le Bihan D (1995) Molecular diffusion, tissue microdynamics and microstructure. NMR Biomed 8(7–8):375–386
Leal PRL, Hermier M, Froment JC et al (2010) Preoperative demonstration of the neuro-vascular compression characteristics with special emphasis on the degree of compression, using high resolution magnetic resonance imaging. A prospective study, with comparison to surgical findings, in 100 consecutive patients who underwent micro-vascular decompression for trigeminal neuralgia. Acta Neurochir 152(5):817–825
Leal PR, Roch JA, Hermier M et al (2011) Structural abnormalities of the trigeminal root revealed by diffusion tensor imaging in patients with trigeminal neuralgia caused by neurovascular compression: a prospective, double-blind, controlled study. Pain 152:2357–2364
Leal PRL, Hermier M, Souza MA et al (2011) Visualization of vascular compression of the trigeminal nerve with high-resolution 3-T MRI: a prospective study comparing pre-operative imaging analysis to surgical findings in 40 consecutive patients who underwent micro-vascular decompression for trigeminal neuralgia. Neurosurgery 69(1):15–26
Leal PR, Barbier C, Hermier M et al (2014) Atrophic changes in the trigeminal nerves of patients with trigeminal neuralgia due to neurovascular compression and their association with the severity of compression and clinical outcomes. J Neurosurg 120:1484–1495
Liu Y, Duan Y, He Y et al (2012) A tract-based diffusion study of cerebral white matter in neuromyelitis optica reveals widespread pathological alterations. Mult Scler 18(7):1013–1021
Liu Y, Li J, Butzkueven H et al (2013) Microstructural abnormalities in the trigeminal nerves of patients with trigeminal neuralgia revealed by multiple diffusion metrics. Eur J Radiol 82:783–786
Love S, Hilton DA, Coakham HB (1998) Central demyelination of the Vth nerve root in trigeminal neuralgia associated with vascular compression. Brain Pathol 8:1–12
Lummel N, Mehrkens JH, Linn J et al (2015) Diffusion tensor imaging of the trigeminal nerve in patients with trigeminal neuralgia due to multiple sclerosis. Neuroradiology 57:259–267
Lutz J, Linn J, Mehrkens JH et al (2011) Trigeminal neuralgia due neurovascular compression: high-spatial-resolution diffusin-tensor imaging reveals microstructural neural changes. Radiology 258:524–530
Lutz J, Thon N, Stahl R et al (2016) Microstructural alterations in trigeminal neuralgia determined by diffusion tensor imaging are independent of symptom duration, severity, and type of neurovascular conflict. J Neurosurg 124(3):823–830
Pierpaoli C, Basser PJ (1996) Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med 36:893–906
Pierpaoli C, Jezzard P, Basser PJ et al (1996) Diffusion tensor MR imaging of the human brain. Radiology 201(3):637–648
Sindou M, Howeidy T, Acevedo G (2002) Anatomic observations during microvascular decompression for idiopathic trigeminal neuralgia with correlations between topography of pain and site of the neurovascular conflict: prospective study in a series of 579 patients. Acta Neurochir 144:1–12
Sindou M, Leston J, Decullier E et al (2007) Microvascular decompression for trigeminal neuralgia: long-term effectiveness and prognostic factors in a series of 362 consecutive patients with clear-cut neurovascular conflicts who underwent pure decompression. J Neurosurg 107:1144–1153
Smith KJ, McDonald WI (1980) Spontaneous and mechanically evoked activity due to central demyelinating lesion. Nature 286(5769):154–155
Wilcox SL, Gustin SM, Eykman EN et al (2013) Trigeminal nerve anatomy in neuropathic and non-neuropathic orofacial pain patients. J Pain 14(8):865–872
Acknowledgments
We are grateful to Ms. Valérie Constans for linguistic editing assistance.
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Contributions
PRLL participated in the acquisition of data and design of the clinical protocol, drafted the ethics applications and regulatory submissions, interpreted the data, and wrote the manuscript. JAR, MH, and YB participated in the acquisition of data. MS participated in the interpretation of data, coordinated the selection of patients, drafted the ethics applications and regulatory submissions, interpreted the data, and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial or non-financial interests in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Functional Neurosurgery - Pain
Rights and permissions
About this article
Cite this article
Leal, P.R.L., Roch, J., Hermier, M. et al. Diffusion tensor imaging abnormalities of the trigeminal nerve root in patients with classical trigeminal neuralgia: a pre- and postoperative comparative study 4 years after microvascular decompression. Acta Neurochir 161, 1415–1425 (2019). https://doi.org/10.1007/s00701-019-03913-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-019-03913-5