Abstract
This work aimed to understand ecological and evolutionary aspects of Juncus tenuis (Juncaceae) by analyzing its seed coat and seedling development. Mature seeds were germinated under controlled conditions and analyzed every 24 h for 60 days. Seeds and seedlings at different developmental stages were analyzed using a stereomicroscope, light microscopy, and scanning electron microscopy. The seed coat consists of the endotegmen, endotesta, and exotesta. The endotegmen is tanniferous, and the exotesta is mucilaginous. The germination percentage was low (28%), a result that may be related to specific light, humidity, and temperature requirements to overcome dormancy. Germination begins with the disruption of the seed coat and the emergence of a phaneromer, followed by the development of the collar rhizoids and the primary root. The anatomical analyses show that the formation of shoot and root apical meristems occurs only after the elongation of the phaneromer. The anatomy of the seedlings at early development is described for the first time for the family. The phaneromeric cotyledon is shared by Juncaceae and Thurniaceae and is most likely the ancestral condition in the cyperid clade (Poales). The presence of a mucilaginous exotesta is probably related to seed protection, dispersal, and germination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Juncaceae is one of the families of the order Poales, integrating the cyperid clade with Thurniaceae and Cyperaceae (Linder and Rudall 2005; Givnish et al. 2010, 2018; Bouchenak-Khelladi et al. 2014). Juncaceae is relatively small, including about 440 species widely distributed throughout the temperate and subtropical regions, but with few representatives occurring in the tropics (Kirschner et al. 2002a, b, c). The family is divided into eight genera, as follows: Juncus L. and Luzula DC., distributed worldwide; Distichia Nees & Meyen, Oxychloë Phil. and Patosia Buchenau, restricted to South America; Marsippospermum Desv. and Rostkovia Desv., which occur in South America and New Zealand (Kirschner et al. 2002a, b, c), and Oreojuncus Záv.Drábk. & Kirschner, occurring from Europe to Siberia and from Greenland to the United States (Drábková and Kirschner 2013).
Juncus is the largest genus in the family, with approximately 300 species (Kirschner et al. 2002b, c), which are usually perennial, rhizomatous herbs. Their fruits are loculicidal capsules, with numerous small, often reticulated seeds (Balslev 1996, 1998). The gynoecium consists of three united carpels, and placentation is parietal, on intruding septa; the ovules are anatropous and bitegmic, with each integument consisting of two cell layers (Balslev 1996, 1998; Oriani et al. 2012).
Juncus tenuis Willd. is native in North America but introduced to many areas and established in numerous regions over extratropical regions of both hemispheres (Kirschner et al. 2002c). The individuals grow in different environments, in exposed or shaded sites in soils ranging from sand to clay, under moist or drier conditions, on natural or disturbed areas (Kirschner et al. 2002c). Therefore, it is a key-species for studies on seed germination and seedling development.
The reproduction of Juncus tenuis and its dispersal were studied by Salisbury (1974), who reported the presence of a mucilaginous layer on wet seeds. According to Salisbury (1974), the dispersal of J. tenuis is facilitated by the ability of the seed coat to swell and become sticky, easily adhering to the dispersers. However, other dispersal strategies are also present in Juncus. Balslev (1998) proposed that the species of Juncus may have different dispersal strategies, including anemochory and/or hydrochory. The swelling of the testa upon moistening in some species appears to cause capsule dehiscence (Balslev 1998).
According to Zaman (1950), who studied the embryology of Juncus prismatocarpus R.Br. and J. effusus L., the seed coat is composed of endotegmen and endotesta; the outer layer of both outer and inner integuments is absorbed during the seed development. The exotesta becomes glandular at the micropylar end, and the endotegmen cells exhibit thickened inner walls and a red color content (Zaman 1950).
Although several studies have addressed the seedling development of monocots, few have focused on species of Juncaceae and its seedling anatomy remained unknown. Of the few papers found in the literature, Tillich (1995) describes the cotyledon of some species of Juncus and Luzula, which consists of an elongated, assimilating, and unifacial hyperphyll with a small distal haustorium, and a bifacial hypophyll forming a ventrally closed sheath. Tillich (2007) describes the morphology of the seedlings of J. maritimus Lam., Luzula nivea (L.) DC., Marsippospermum reichii Buch., and Rostkovia magellanica Hook, reporting the formation of a phaneromeric cotyledon and a short hypocotyl in Juncus and Luzula, whereas the hypocotyl is long in species of Marsippospermum and Rostkovia.
Tillich (2007) emphasizes that studies on seedling development in monocots are important to identify characters with taxonomic and phylogenetic value. These studies are of particular interest for Poales since species of this order have different cotyledon types. Tillich (2007) proposed a hypothesis for the cotyledon evolution in Poales, stating that the compact type would be the ancestral cotyledon state, which shifted directly to three other states: blade-like hypophyll, phaneromer, and coleoptile. In the cyperid clade, which comprises Juncaceae, Thurniaceae, and Cyperaceae, the first two families have a phaneromer, whereas in Cyperaceae, the cotyledon develops into a coleoptile (Tillich 2007). The phaneromer corresponds to the proximal part of the cotyledonary hyperphyll, which develops into a tubular structure that raises the seed well above the soil surface. In contrast, the coleoptile, which is also a tubular structure, is produced by the meristematic activity of the marginal tissue of the cotyledonary sheath and is usually short (Tillich 2007).
In this context, the present study analyzes the development of the seed coat and seedlings of J. tenuis with a morphological, micromorphological, and anatomical approach, aiming to provide new information about these processes in Juncaceae. The following questions were addressed: What is the origin of the mucilaginous layer of the seed coat of J. tenuis? How is the anatomical structure of the seed coat and its relation to dispersal? Does J. tenuis share the same morphological pattern of seedling development as other previously studied species of Juncaceae? Finally, how can the early seedling development be characterized from an anatomical perspective?
Materials and methods
Botanical material
Mature seeds of J. tenuis were collected from the mature fruits of plants growing in open areas, roadsides, and human-impacted areas in the Lagoa Pequena, Florianópolis, Santa Catarina, Brazil (SisGen register number ADE51D9). The seeds were stored in closed paper bags and kept at room temperature. Flowers and fruits at different developmental stages were also collected, fixed in FAA 50 (37% formaldehyde, glacial acetic acid, 50% ethanol, 1:1:18 v/v) (Johansen 1940) for 24 h, and stored in 70% ethanol for the study of seed coat development. Vouchers were identified and deposited in the Herbarium Rioclarense (HRCB 77282) of the Instituto de Biociências at Universidade Estadual Paulista, Rio Claro, São Paulo, Brazil.
Germination and seedling development
For the analysis of seedling development, 100 mature seeds were placed to germinate in four Petri plates (25 seeds per plate). The Petri plates were lined with filter paper moistened with distilled water and stored in an air-conditioned room at a constant temperature (25 ± 2ºC) and fluorescent light (≈ 40 µmol.m-2.s-1 PAR).
The seeds were observed once every 24 h for 60 days, with germination identified as the rupture of the seed coat followed by the emergence of the phaneromer. The results were recorded by images obtained with a Canon Power Shot S80 digital camera coupled to a Leica EZ4 stereomicroscope. Seedlings at different developmental stages were fixed in FAA 50 for 24 h and then transferred to 70% ethanol for the anatomical study. The terminology adopted follows Tillich (2007), where definitions and examples for the use of the terms are given.
Light microscopy (LM)
Flowers, fruits, and seedlings at different developmental stages and fixed in FAA 50 were dehydrated in an n-butyl alcohol series. These materials were then embedded in 2-hydroxyethyl methacrylate (Leica Historesin Embedding Kit) (Gerrits and Smid 1983) and sectioned (5–7-µm thick) on a rotary microtome (Leica, RM 2245). Anatomical sections were stained with periodic acid-Schiff (PAS) reagent and 0.05% toluidine blue in sodium borate (O'Brien et al. 1964; Feder and O'Brien 1968), and then mounted on permanent slides with Entellan (Merck). Selected sections were registered under an image-capturing device (Leica, DFC 450) attached to a microscope (Leica, DM 4000B). Image processing was performed with the LAS digital imaging system (Leica Application Suite V4.0.0).
Scanning electron microscopy (SEM)
Seeds and seedlings at different developmental stages previously fixed in FAA 50 were dehydrated in an ethanol series (30%, 50%, 70%, 90%, and 100%) for 15 min in each solution, and then critical point dried (Balzers CPD 050). The dried samples were fixed in stubs and coated with gold by a Bal-Tec SCD 050 sputtering system. Observations were carried out under a Jeol JSM IT 300 microscope, and the images were obtained using the microscope image-capturing system.
Results
Seed coat development
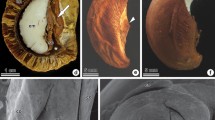
Juncus tenuis has a tricarpellary gynoecium with anatropous, bitegmic ovules, and parietal placentation. The outer and inner integuments have two layers of rectangular cells with a conspicuous nucleus and dense cytoplasm (Fig. 1a-c). Both integuments delimit the micropyle (Fig. 1b). In addition, a cuticle is observed between the outer and inner integuments and between the inner integument and the nucellus (Fig. 1a-c).
Seed coat development in Juncus tenuis. a–c Longitudinal sections of ovules in successive developmental stages, before a and after fertilization b, c. d, e Details of the ovule integuments after fertilization, in longitudinal and cross sections, respectively. f Cross section of the seed coat of the immature seed. g Longitudinal section of the seed coat of the mature seed. ct cuticle, en endosperm, ii inner integument, nu nucellus, ot outer integument. Symbols: square endotegmen, circle exotegmen, triangle endotesta, asterisk exotesta. Scale bars 50 µm a–c, 25 µm d–g
The integuments undergo modifications during endosperm and embryo development (Fig. 1d-g). After fertilization, the cells of the inner layer of the inner integument increase in size and accumulate phenolic compounds (Fig. 1c-e), giving rise to the tanniferous endotegmen (Fig. 1d-g). The cells of the outer layer of the inner integument do not undergo evident changes until the stage when the nucellus is absorbed by the developing endosperm (Fig. 1b-e). From that stage onward, these cells begin to disintegrate (Fig. 1f) and are completely absent in the mature seed (Fig. 1g). The cuticle between the inner and outer integuments becomes thicker and more sinuous, with changes in its chemical composition evidenced by changes in its color (Fig. 1f, g). The cells of the inner layer of the outer integument elongate, accommodating to the sinuosity of the cuticle (Fig. 1f, g). The cells of the outer layer of the outer integument, which form the exotesta, elongate tangentially (Fig. 1d-f). In the mature seed, they become mucilaginous by accumulating polysaccharides as indicated by the PAS-positive reaction (Fig. 1g).
In the mature seeds the seed coat comprises a tanniferous endotegmen, endotesta, and a mucilaginous exotesta (Fig. 1g). Numerous ellipsoid to ovoid seeds are formed per capsule, all yellow-orange and with a reticulated surface when dry (Fig. 2a, b). The mucilaginous exotesta can be observed under SEM (Fig. 3b); since the walls of its cells are thin, they usually break, leaving only parts of the anticlinal walls (Fig. 3a).
Morphological features of seedling development in Juncus tenuis. a, b Rupture of the seed coat and phaneromer emergence. c Phaneromer development. d Differentiation of the rhizoids. e Phaneromer elongation and beginning of primary root development. f Primary root elongation and shoot-born root in early development. g Seedling with the first developing eophyll and several shoot-born roots. eo eophyll, ph phaneromer, cr collar rhizoids, pr primary root, sr shoot-born root. Scale bars 1.0 mm a–c, 1.2 mm d, 1.5 mm e–g
Micromorphological features of seedling development in Juncus tenuis. a Beginning of seed germination. b Development of the phaneromer and beginning of the rhizoids differentiation; note the seed’s mucilaginous testa. c Seedling showing an elongated phaneromer and long, dense collar rhizoids. d Detail of the collar rhizoids and beginning of primary root development between the rhizoids. e Primary root development. f Developed primary root and beginning of shoot-born root development. g Seedling with the first developing eophyll. eo eophyll, ph phaneromer, cr collar rhizoids, pr primary root, sr shoot-born root. Scale bars 100 µm a, b, 200 µm c, f, 50 µm d, 20 µm e, 500 µm g
Germination and morphological aspects of seedling development
The germination percentage of J. tenuis was 28%. Table 1 shows the time for the appearance of the seedling structures.
Germination begins between 16–20 days after imbibition with the rupture of the seed coat tissues at the micropylar end, followed by the emergence of the phaneromer (Figs. 2a, b, 3a). The phaneromer curves toward the substrate and develops into a long, cylindrical structure that raises the seed (Figs. 2c, 3b). At one end of the phaneromer, where the extremely reduced hypocotyl-radicular axis is found, dense and long rhizoids are distinguishable two to four days after the phaneromer appears (Figs. 2d, e, 3c, d). Subsequently, the primary root begins to develop between the rhizoids (Figs. 2e, 3d, e). The primary root grows fast and becomes well-developed with many root hairs after few days (Figs. 2f, 3f). The formation of shoot-born roots starts two to six days after the emergence of the primary root (Figs. 2f, 3f). Six to ten days after the appearance of the first shoot-born root (35–38 days after germination), the first plumular leaf appears as a lanceolate eophyll (Figs. 2g, 3g). The first eophyll is fully expanded approximately 40 days after germination, by which time more shoot-born roots are formed (Fig. 2g).
Anatomical aspects of seedling development
After seed imbibition and before germination, mucilage secretion is observed at the micropylar end of the seed (Fig. 4a). In this region, the seed coat disrupts with the protrusion of the phaneromer (Fig. 4b, c). The cells of the phaneromer divide rapidly, forming a solid and cylindrical structure, which then elongates (Fig. 4d, e). The cells of the outermost layer at the end of the axis differentiate into the rhizoids (Fig. 4e). Concomitantly, the root apical meristem is formed, and both the root and stem poles become evident, positioned on the same orientation of the phaneromer (Fig. 4e, f). After the development of the primary root, the shoot apical meristem becomes organized (Fig. 4g). The rhizoids remain in the collar region, which corresponds to the transition between the hypocotyl and the primary root. The shoot-born roots, which have an endogenous origin, are formed from the division of hypocotyl cells (Fig. 4h, i). The cells of the shoot apical meristem divide, elongating the epicotyl and giving rise to the first plumular leaf primordium, both of which protected by the cotyledonary sheath (Fig. 4j).
Anatomical features of seedling development in Juncus tenuis. a Imbibed seed showing the mucilaginous testa and mucilage secretion in the micropylar region. b, c Phaneromer emergence. d Phaneromer development. e Collar rhizoids formation, and shoot and root poles differentiation. f Detail of the hypocotyl-radicle axis, with an organized root apical meristem. g Organization of the shoot apical meristem. h, i Primary root development and initiation of shoot-born root formation from hypocotyl cells. j Detail of the shoot apical meristem, with leaf primordia protected by the cotyledonary sheath. cs cotyledonary sheath, em embryo, m mucilage, ph phaneromer, pr primary root, cr collar rhizoids, rp root pole, sam shoot apical meristem, sp shoot pole, sr shoot-born root. Scale bars 100 µm a, b, 50 µm c, d, 60 µm e, 25 µm f–j
Discussion
Seed coat development and ecological considerations
The seed coat in J. tenuis is formed by three layers: the endotegmen, endotesta, and exotesta. The endotegmen is tanniferous, similarly to the seeds of representatives of other Poales families like the Xyridaceae (Oriani and Scatena 2014, 2017; Nardi et al. 2015); Eriocaulaceae (Scatena and Bouman 2001; Coan et al. 2010); Mayacaceae (Venturelli and Bouman 1986); Restionaceae (Rudall and Linder 1988); Centrolepidaceae (Hamann 1975); Rapateaceae (Venturelli and Bouman 1988); Poaceae (Nakamura et al. 2009); Bromeliaceae (Magalhães and Mariath 2012; Prado et al. 2014); as well as Thurniaceae, including Thurnia Hook.f. species (Thimm 1985) and Prionium serratum Baill. (formerly included in Juncaceae) (Munro and Linder 1997). In Cyperaceae, which forms the cyperid clade along with Juncaceae and Thurniaceae, the phenolic compounds are accumulated in the exotesta cells, as described for Rhynchospora Wild.species (Coan et al. 2008). Therefore, the tanniferous endotegmen, although common in Poales, is not a feature shared by all cyperids.
It is believed that the presence of phenolic compounds can inhibit germination (Mayer and Poljakoff-Mayber 1989), thus playing a regulatory role in the germination process. In addition, the phenolic compounds may act as defense against herbivores and play antibacterial and antifungal activities (Levin 1976).
The number of layers that constitute the seed coat is a taxonomic trait that differentiates Poales families and even genera, as reported for Xyridaceae (Oriani and Scatena 2014, 2017; Nardi et al. 2015). As shown in the present study for J. tenuis, the seed coat is composed of endotegmen, endotesta, and exotesta, with the exotegmen being absorbed during seed development. In the seeds of J. prismatocarpus and J. effusus, Zaman (1950) observed the absorption of the outer layer of both outer and inner integuments, thus the seeds do not have the exotegmen and exotesta. In species of Cyperaceae, the exotesta is present and forms the seed coat along with the endotegmen, but neither of these layers is mucilaginous (Makde and Bhuskute 1987; Coan et al. 2008).
The number of layers in the outer integument is variable for Cyperaceae, as shown in species of HypolytrumPers.–with three cell layers–and Rhynchospora–with two cell layers (Coan et al. 2008); the last character state is the most common for this family (Makde and Bhuskute 1987; Zhang 1999). However, this variation in the number of layers of the outer integument is not significant; during seed development, some layers disintegrate, and in the mature seed, the testa is composed only of the exotesta, with one layer of thin-walled cells (Coan et al. 2008). In Thurnia (Thurniaceae), the testa is formed by multiple layers, with lignified cells, whereas the endotegmen and exotegmen cells are thin-walled and filled with tannins, making the limits of the cells almost imperceptible (Thimm 1985). The seed coat composed of exotesta, exotegmen, and endotegmen is therefore shared by Juncaceae and Thurniaceae, whereas in Cyperaceae, the seed coat comprises only the exotesta and endotegmen. The reduction in the number of cell layers of the seed coat in Cyperaceae is probably related to the presence of indehiscent fruits (nutlets).
The anatomical characteristics of the seed coat in cyperids may also be related to environmental factors, since these species occur in different environments. The species of Thurnia, for instance, are usually found in wet areas and creek banks, suggesting that the presence of the testa with multiple layers of lignified cells is an adaptation to prevent the seed from rotting in the water, allowing it to remain viable for a longer period. On the other hand, most Cyperaceae species are found in open and dry areas, and their seeds, having few layers in the seed coat, are protected by the pericarp since their fruits are indehiscent. Studies of the anatomy of the Cyperaceae fruit wall show that it is generally composed of lignified cells in the mesocarp (Shah 1968; Ragonese et al. 1984; Browning and Gordon-Gray 1993, 1996; Coan et al. 2008). Thus, the mechanical protection of the seed is transferred to the pericarp, while the testa, whose cells contain phenolic compounds (Coan et al. 2008), provides chemical protection against predators and pathogens. For J. tenuis and other Juncaceae species occurring in dry and open areas with high light exposure, a mucilaginous testa may help protect the seed against desiccation, since their fruits are dehiscent (loculicidal capsules), and there is no layer with lignified cells composing the seed coat. We believe that the cuticle layer between the tegmen and the testa, which thickens during seed maturation, provides mechanical protection.
Because the mucilaginous layer of the seeds of Juncus tenuis corresponds to the exotesta, it may be termed sarcotesta (Corner 1976). Among the Poales, seeds with a mucilaginous testa have also been reported for Bromelioideae species (Bromeliaceae) (Silva et al. 2020). In these species, as is in J. tenuis, the number of layers in the outer integument does not increase during seed development, but its cells do increase in size by accumulating hydrophilic substances, resulting in a mucilaginous testa. The mucilaginous nature of the seed testa in J. tenuis was observed soon after imbibition, when the mucilage increased the seed’s adherence to the substrate. The occurrence of a sticky substance adhering the seeds to the substrate was also observed by Kraus et al. (1996) in germination experiments of Paepalanthus Mart. species (Eriocaulaceae) and, according to the authors, this feature facilitates the imbibition.
Studies on the functions of the seed coat mucilage of a number of angiosperms suggest that it has multiple ecological roles. These include facilitation of seed hydration, mediation of germination under waterlogged conditions, regulation of seed dormancy by reduction of oxygen diffusion, and promotion of seed dispersal by attachment to animals (Western 2012; Yang et al. 2012). The precise role of mucilages appears to be dependent on species and their environmental context (Western 2012). Since representatives of J. tenuis generally occur in open environments under high light exposure and also in disturbed areas, it is believed that the mucilaginous exotesta regulates germination by keeping a sufficient concentration of water around the seed, as well as protecting against desiccation. Furthermore, as already demonstrated for other species of angiosperms, the presence of a mucilaginous seed coat facilitates germination in soils with low water potential (Young et al. 1970; Sun et al. 2012), such as the sandy soils of grassland vegetation, where many species of Juncaceae grow.
In addition to regulating germination and protecting the seed against desiccation, we believe that the mucilaginous seed coat may also affect seed dispersal, since the fruiting and seed dispersal of J. tenuis occur in the rainy season (Salisbury 1974; Balslev and Stefano 2015). Thus, when the seeds come into contact with water, they swell up and form large viscous masses, causing the capsules to open, a mechanism previously described by Balslev (1996, 1998) for other species of Juncus. Furthermore, depending on the hydration level, the mucilage can become extremely sticky, working as an adhesive and increasing seed adherence (Kreitschitz et al. 2015). This mechanism also facilitates dispersal by fixing the seeds to dispersal agents and then to the substrate, increasing the probability of germination.
We believe that there is also a relationship between seed size and the need for light as an ecological strategy for germination since small seeds tend to sink less, remaining in the surface layers of the soil, which may facilitate the emergence of the small seedlings. The mucilaginous seed coat increases seed adherence to surface soil particles and is also important for early seedling development.
In a manner similar to Alyssum minus (L.) Rothm. (Brassicaceae) (Sun et al. 2012), we believe that the mucilage of the seeds of J. tenuis plays an important role in seed dispersal, adhesion of seeds to the substrate, and hydration, as well as functioning as a water reservoir for germination, especially in conditions of water stress. This feature probably explains the wide distribution of J. tenuis through different habitats, including disturbed environments such as roadsides, trails, and even sidewalks in urban areas in the Americas, Japan, New Zealand, and Europe (Balslev and Stefano 2015). According to Salisbury (1974), humans can also affect the dispersion of J. tenuis by taking the seeds adhered to the footwear or tires, which is facilitated by the presence of the mucilaginous seed coat, meaning that human activity and urban mobility can help expand the species’ distribution. Also according to Salisbury (1974), the minute size of the seeds, combined with their shape prevent them from injury by the moving footwear or rotating tires to which they adhere, while such movements may disrupt the seed-aggregates resulting from the mucilaginous seed coat. The relationship between the presence of seeds with mucilage and the ability to colonize disturbed areas has already been demonstrated in other herbaceous species of Brassicaceae, Euphorbiaceae, Lamiaceae, Onagraceae, and Plantaginaceae, in which mucilage has been identified as an ecological advantage since such seeds do not require soil to germinate (Young and Evans 1973), including increasing their invasive potential (Bangle et al. 2008).
Germination and seedling development in an ecological and evolutionary context
Juncus tenuis seeds are small, with many of their features visible only under a stereomicroscope. Seed mass affects several aspects of plant ecology (Moles et al. 2005). Small-seeded species can produce more seeds for a given amount of energy than those with large seeds (Leishman et al. 2000; Moles et al. 2005, 2007).
Species with small seeds are often associated with open (Leishman and Westoby, 1994; Seiwa and Kikuzawa, 1996) or disturbed environments (Fenner 1995), as observed for J. tenuis. Although this species has a widespread distribution and grow in different conditions, its germination percentage is low. We believe that the seeds require special conditions to germinate, such as specific light, humidity, and temperature, as reported for other monocot species including members of Xyridaceae and Eriocaulaceae (Garcia and Oliveira 2007) as well as Velloziaceae (Mota and Garcia 2012), which also grow in open environments with high light exposure. The lack of specific conditions may thus explain the low germination percentage observed for J. tenuis in our experiment and in the experiments by Salisbury (1974) with seeds of J. tenuis from Hampshire (in which 39% of 6800 seeds germinated) and from Sussex (in which 32% of 700 seeds germinated). Since this species is perennial and have rhizomes densely branched (Kirschner et al. 2002c), the vegetative propagation may be one of its strategies to maintain the natural populations.
As described for J. tenuis, seedling development in Juncaceae is marked by the rupture of the seed coat at the micropylar region followed by the development of the phaneromer. The cells of the proximal end of the cotyledonary hyperphyll divide, forming an elongated tubular structure (phaneromer), which pushes the embryonic axis out of the seed. The shoot and root apical meristems are only evident after the development of the phaneromer. This late development of both meristems is not a common feature in angiosperms and is described here for Juncaceae for the first time.
After the development of the phaneromer and before the emergence of the primary root, dense and long rhizoids appear at the collar region for fixation of the seedling to the substrate and absorption of water and nutrients for seedling development, while the primary root is still undeveloped. These rhizoids differ from the root hairs, as the former differentiate from the epidermis of the collar region (Tillich 2007), whereas the latter differentiate from the protoderm, originating from the root apical meristem. The collar rhizoids, the first structure to come into contact with the substrate, is considered a derived character that increases the likelihood of seedling establishment (Tillich, 2000). Unlike some Poales, in which the primary root does not develop or aborts early in development (Kraus et al. 1996; Tillich 2007; Corredor et al. 2015; Mascarenhas and Scatena, 2021), the primary root and shoot-born roots of Juncaceae develop a few days after the formation of the collar rhizoids and replace them in the function of fixation and absorption.
With respect to the cotyledon, Tillich (2007) identifies two regions: the hypophyll, corresponding to its basal and bifacial portion, and the hyperphyll, which is the distal and unifacial portion. The hyperphyll can be entirely haustorial, retained in the seed, or elongated, forming an apocole or a phaneromer. The apocole is short and restricted to hypogeal palm seedlings of which the plumule is buried into the soil during germination (Tillich 2007). By comparison, the phaneromer is more elongated and raises the seed well above the soil surface, as occurs in Juncaceae and other monocot families (Tillich 2007).
In J. tenuis, only the most distal portion of the hyperphyll is retained inside the seed during seedling development, with a haustorial function, while the proximal portion of the hyperphyll elongates, forming the phaneromer. The phaneromer is a specialized structure, which, in addition to the considerable elongation between the seed and the cotyledon sheath, is the first assimilated organ in the seedling (Tillich 2007). The presence of the phaneromer can be considered an ecological adaptation favoring germination, since this structure raises the seed well above the ground surface, facilitating light exposure and, consequently, promoting seedling development.
In Poales, the phaneromer occurs in Juncaceae and Thurniaceae (cyperid clade), in Typhaceae (an early diverging family), and in the families of the restiid clade (Restionaceae, Centrolepidaceae, and Anarthriaceae) (Tillich 2007). According to Tillich (2007), the phaneromer has derived from the compact cotyledon, which is the ancestral condition for the order (Tillich 2007). Thus, considering the occurrence of the phaneromer in distinct clades of Poales, it probably had several independent origins during the evolution of the order. Because the phylogenetic studies based on morphological and molecular data show Thurniaceae as a sister group of the clade formed by Juncaceae and Cyperaceae (e.g., Bremer 2002; Linder and Rudall 2005; Chase et al. 2006; Givnish et al. 2010, 2018; Bouchenak-Khelladi et al. 2014), the phaneromeric cotyledon is most likely the ancestral character state in the cyperid clade.
Conclusions
In J. tenuis, the seed coat is formed by a tanniferous endotegmen, endotesta, and a mucilaginous exotesta. We suggest that the mucilaginous exotesta plays multiple roles, facilitating seed hydration and promoting seed dispersal. The tanniferous endotegmen probably serves as chemical protection for the seed, while mechanical protection is provided by the thick cuticle between the tegmen and the testa.
Our results show that J. tenuis shares the same pattern of seedling development and the same type of cotyledon as the other species of the family. Germination is marked by the rupture of the seed coat at the micropylar region and the protrusion of the phaneromer. The shoot and root poles become evident later, only after the elongation of the phaneromer. This study provides new anatomical data on the seed coat and seedling development in Juncaceae, contributing to understand the ecology and evolution of the family.
References
Balslev H (1996) Juncaceae. Fl Neotrop Monogr 68:1–167
Balslev H (1998) Juncaceae. In: Kubitzki K (ed) Flowering Plants. Monocotyledons. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 252–260. https://doi.org/10.1007/978-3-662-03531-3_26
Balslev H, Stefano RD (2015) The family Juncaceae in Mexico. Acta Bot Mex 111:61–164
Bangle DN, Walker LR, Powell EA (2008) Seed germination of the invasive plant Brassica tournefortii (Sahara mustard) in the Mojave Desert. W N Amer Naturalist 68:334–342. https://doi.org/10.3398/1527-0904(2008)68[334:SGOTIP]2.0.CO;2
Bouchenak-Khelladi Y, Muasya AM, Linder HP (2014) A revised evolutionary history of Poales: origins and diversification. Bot J Linn Soc 175:4–16. https://doi.org/10.1111/boj.12160
Bremer K (2002) Gondwanan evolution of the grass alliance of families (Poales). Evolution 56:1374–1387. https://doi.org/10.1111/j.0014-3820.2002.tb01451.x
Browning J, Gordon-Gray KD (1993) Studies in Cyperaceae in Southern Africa. 21: The taxonomic significance of the achene and its embryo in Bolboschoenus. S African J Bot 59:311–318. https://doi.org/10.1016/S0254-6299(16)30734-7
Browning J, Gordon-Gray KD (1996) Studies in Cyperaceae in Southern Africa. 30: Aspects of the relationship between Cyathocoma and Costularia. S African J Bot 62:250–257. https://doi.org/10.1016/S0254-6299(15)30653-0
Chase MW, Fay MF, Devey DS, Maurin O, Ronsted N, Davies TJ, Pillon Y, Pertersen G, Seberg O, Tamura M, Asmussen CB, Hilu K, Borsch T, Davis JI, Stevenson DW, Pires JC, Givnish TJ, Sytsma KJ, McPherson MA, Graham SW, Rai HS (2006) Multigene analyses of monocot relationships: a summary. Aliso 22:63–75. https://doi.org/10.5642/aliso.20062201.06
Coan AI, Alves MV, Scatena VL (2008) Comparative study of ovule and fruit development in species of Hypolytrum and Rhynchospora (Cyperaceae, Poales). Pl Syst Evol 272:181–195. https://doi.org/10.1007/s00606-007-0636-9
Coan AI, Stützel T, Scatena VL (2010) Comparative embryology and taxonomic considerations in Eriocaulaceae (Poales). Feddes Repert 121:268–284. https://doi.org/10.1002/fedr.201000016
Corner EJH (1976) The seeds of dicotyledons. Cambridge University Press, Cambridge
Corredor BAD, Escobar DFE, Scatena VL (2015) Seed morphology and post-seminal development in species of Comanthera (Eriocaulaceae). Rev Biol Trop 63:1127. https://doi.org/10.15517/rbt.v63i4.16956
Drábková LZ, Kirschner J (2013) Oreojuncus, a new genus in the Juncaceae. Preslia 85:483–503
Feder N, O’ Brien TP, (1968) Plant microtechnique: some principles and new methods. Amer J Bot 55:123–142. https://doi.org/10.2307/2440500
Fenner M (1995) Ecology of seed banks. In: Kigel J, Galili G (eds) Seed development and germination. Academic Press, New York, pp 507–543
Garcia Q, Oliveira P (2007) Germination patterns and seed longevity of monocotyledons from the Brazilian campos rupestres. Seed Sci Biotech 1:35–41
Gerrits PO, Smid L (1983) A new, less toxic polymerization system for the embedding of soft tissues in glycol methacrylate and subsequent preparing of serial sections. J Microscopy 132:81–85. https://doi.org/10.1111/j.1365-2818.1983.tb04711.x
Givnish TJ, Ames M, McNeal JR, McKain MR, Steele PR, Pamphilis CW, Graham SW, Pires JC, Stevenson DW, Zomlefer WB, Briggs BG, Duvall MR, Moore MJ, Heaney JM, Soltis DE, Soltis PS, Thiele K, Leebens-Mack JH (2010) Assembling the tree of the monocotyledons: plastome sequence phylogeny and evolution of Poales. Ann Missouri Bot Gard 97:584–616. https://doi.org/10.3417/2010023
Givnish TJ, Zuluaga A, Spalink D, Gomez MS, Lam VKY, Saarela JM, Sass C, Iles WJD, Sousa DJL, Leebens-Mack J, Pires JC, Zomlefer WB, Gandolfo MA, Davis JI, Stevenson DW, Pamphilis C, Specht CD, Graham SW, Barrett CF, Ané C (2018) Monocot plastid phylogenomics, timeline, net rates of species diversification, the power of multi-gene analyses, and a functional model for the origin of monocots. Amer J Bot 105:1888–1910. https://doi.org/10.1002/ajb2.1178
Hamann U (1975) Neue Untersuchungen zur Embryologie und Systematik der Centrolepidaceae. Bot Jahrb Syst Pflanzengesch Pflanzengeogr 96:154–191
Johansen DA (1940) Plant microtechnique. McGraw-Hill Book Company, New York
Kirschner J, Balslev H, Ceska A, Swab JC, Edgar E, Garcia-Herran K, Hämet-Ahti L, Kaplan Z, Novara LJ, Novikov VS, Wilton A (2002a) Juncaceae 1: Rostkovia to Luzula, Species Plantarum: Flora of the world Part 6. Australian Biological Resources Study, Canberra
Kirschner J, Balslev H, Clemants SE, Ertter B, Álvarez MCFC, Hämet-Ahti L, Miyamoto F, Noltie HJ, Novara LJ, Novikov VS, Simonov SS, Snogerup S, Wilson KL (2002b) Juncaceae 2: Juncus subg. Juncus, Species Plantarum: Flora of the world Part 7. Australian Biological Resources Study, Canberra
Kirschner J, Balslev H, Brooks RE, Clemants SE, Ertter B, Hämet-Ahti L, Álvarez MCFC, Novara LJ, Novikov VS, Simonov SS, Snogerup S, Wilson KL, Zika PF (2002c) Juncaceae 3: Juncus subg. Agathryon, Species Plantarum: Flora of the world Part 8. Australian Biological Resources Study, Canberra
Kraus JE, Scatena VL, Lewinge ME, Trench KUS (1996) Morfologia externa e interna de quatro espécies de Paepalanthus Kunth (Eriocaulaceae) em desenvolvimento pós-seminal. Bol Univ Sao Paulo, Bot 15:45–53
Kreitschitz A, Kovalev A, Gorb SN (2015) Slipping vs sticking: water-dependent adhesive and frictional properties of Linum usitatissimum L. seed mucilaginous envelope and its biological significance. Acta Biomat 17:152–159. https://doi.org/10.1016/j.actbio.2015.01.042
Leishman MR, Westoby M (1994) The role of large seed size in shaded conditions: experimental evidence. Funct Ecol 8:205–214. https://doi.org/10.2307/2389903
Leishman MR, Wright IJ, Moles AT, Westoby M (2000) The evolutionary ecology of seed size. In: Fenner M (ed) Seeds: the ecology of regeneration in plant communities. CAB International, Wallingford, pp 31–57
Levin DA (1976) The chemical defenses of plants to pathogens and herbivores. Annual Rev Ecol Syst 7:121–159. https://doi.org/10.1146/annurev.es.07.110176.001005
Linder HP, Rudall PJ (2005) Evolutionary history of Poales. Annual Rev Ecol Evol Syst 36:107–124. https://doi.org/10.1146/annurev.ecolsys.36.102403.135635
Magalhães RI, Mariath JEA (2012) Seed morphoanatomy and its systematic relevance to Tillandsioideae (Bromeliaceae). Pl Syst Evol 298:1881–1895. https://doi.org/10.1007/s00606-012-0688-3
Makde KH, Bhuskute SM (1987) Embryology of Kyllinga monocephala (Cyperaceae) and its systematic position. Pl Syst Evol 156:143–150. https://doi.org/10.1007/BF00936069
Mascarenhas AAS, Scatena VL (2021) Seed morphology and post-seminal development in Leiothrix Ruhland (Eriocaulaceae, Poales). Feddes Repert 132:9–19. https://doi.org/10.1002/fedr.202000022
Mayer AM, Poljakoff-Mayber A (1989) The germination of seeds. Pergamon Press, Oxford
Moles AT, Ackerly DD, Webb CO, Tweddle JC, Dickie JB, Westoby M (2005) A brief history of seed size. Science 307:576–580. https://doi.org/10.1126/science.1104863
Moles AT, Ackerly DD, Tweddle JC, Dickie JB, Smith R, Leishman MR, Mayfield MM, Pitman A, Wood JT, Westoby M (2007) Global patterns in seed size. Global Ecol Biogeogr 16:109–116. https://doi.org/10.1111/j.1466-822x.2006.00259.x
Mota LAS, Garcia QS (2012) Germination patterns and ecological characteristics of Vellozia seeds from high-altitude sites in south-eastern Brazil. Seed Sci Res 23:67–74. https://doi.org/10.1017/S0960258512000256
Munro SL, Linder HP (1997) The embryology and systematic relationships of Prionium serratum (Juncaceae : Juncales). Amer J Bot 84:850–860
Nakamura AT, Longhi-Wagner HM, Scatena VL (2009) Desenvolvimento de óvulo, fruto e semente de espécies de Poaceae (Poales). Revista Brasil Bot 32:165–175. https://doi.org/10.1590/s0100-84042009000100016
Nardi KO, Scatena VL, Oriani A (2015) Development of ovule, fruit and seed of Xyris (Xyridaceae, Poales) and taxonomic considerations. Bot J Linn Soc 177:619–628. https://doi.org/10.1111/boj.12265
O’Brien TP, Feder N, McCully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373
Oriani A, Scatena VL (2014) Ovule, fruit and seed development in Abolboda (Xyridaceae, Poales): implications for taxonomy and phylogeny. Bot J Linn Soc 175:144–154. https://doi.org/10.1111/boj.12152
Oriani A, Scatena VL (2017) Ovule, fruit, and seed development of Orectanthe sceptrum and its systematic relevance to Xyridaceae (Poales). Int J Pl Sci 178:104–116. https://doi.org/10.1086/689617
Oriani A, Stützel T, Scatena VL (2012) Contributions to the floral anatomy of Juncaceae (Poales - Monocotyledons). Flora 207:334–340. https://doi.org/10.1016/j.flora.2012.03.001
Prado JPC, Schimidt EC, Steinmacher DA, Guerra MP, Bouzon ZL, Vesco LLD, Pescador R (2014) Seed morphology of Vriesea friburgensis var. paludosa L.B. Sm. (Bromeliaceae). Hoehnea 41:553–562. https://doi.org/10.1590/2236-8906-08/2013
Ragonese AM, Guaglianone ER, Struttmatter CD (1984) Desarrolo del pericarpio com cuerpos de silice de dos especies de Rhynchospora Vahl (Cyperaceae). Darwiniana 25:27–41
Rudall PJ, Linder HP (1988) Megagametophyte and nucellus in Restionaceae and Flagellariaceae. Amer J Bot 75:1777–1786. https://doi.org/10.2307/2444732
Salisbury EJ (1974) The reproduction of Juncus tenuis (Juncus macer) and its dispersal. Trans Bot Soc Edinburgh 42:187–190. https://doi.org/10.1080/03746607408685277
Scatena VL, Bouman F (2001) Embryology and seed development of Paepalanthus sect. Actinocephalus (Koern.) Ruhland (Eriocaulaceae). Pl Biol 3:341–350. https://doi.org/10.1055/s-2001-16467
Seiwa K, Kikuzawa K (1996) Importance of seed size for the establishment of seedlings of five deciduous broad-leaved tree species. Vegetatio 123:51–64. https://doi.org/10.1007/BF00044887
Shah CK (1968) Development of pericarp and seed coat in the Cyperaceae. Naturaliste Canad 95:39–48
Silva KR, Stützel T, Oriani A (2020) Seed development and its relationship to fruit structure in species of Bromelioideae (Bromeliaceae) with fleshy fruits. Bot J Linn Soc 192:868–886. https://doi.org/10.1093/botlinnean/boz111
Sun Y, Tan DY, Baskin CC, Baskin JM (2012) Role of mucilage in seed dispersal and germination of the annual ephemeral Alyssum minus (Brassicaceae). Austral J Bot 60:439–449. https://doi.org/10.1071/BT11314
Thimm U (1985) Zur Embriologie, Blüten- und Fruchtanatomie der isolierten Juncales-Gattungen Prionium and Thurnia. MSc Thesis, Ruhr-Universität Bochum, Bochum
Tillich HJ (2007) Seedling diversity and the homologies of seedling organs in the order Poales (monocotyledons). Ann Bot (Oxford) 100:1413–1429. https://doi.org/10.1093/aob/mcm238
Tillich HJ (1995) Seedlings and systematic in monocotyledons. In: Rudall PJ, Cribb PJ, Cutler DF, Humphries CJ (eds). Monocotyledons: systematic and evolution. Royal Botanical Gardens, Kew, pp 303–352
Tillich HJ (2000) Ancestral and derived character states in seedlings of monocotyledons. In: Wilson KI, Morrison DA (eds). Monocots: systematics and evolution. Commonwealth scientific and industrial research organisation, Melbourne, pp 221–228
Venturelli M, Bouman F (1986) Embryology and seed development in Mayaca fluviatilis (Mayacaceae). Acta Bot Neerl 35:497–516. https://doi.org/10.1111/j.1438-8677.1986.tb00489.x
Venturelli M, Bouman F (1988) Development of ovule and seed in Rapateaceae. Bot J Linn Soc 97:267–294. https://doi.org/10.1111/j.1095-8339.1988.tb01584.x
Western TL (2012) The sticky tale of seed coat mucilages: production, genetics, and role in seed germination and dispersal. Seed Sci Res 22:1–25. https://doi.org/10.1017/S0960258511000249
Yang X, Baskin JM, Huang Z (2012) More than just a coating: ecological importance, taxonomic occurrence and phylogenetic relationships of seed coat mucilage. Perspect Pl Ecol Evol Syst 14:434–442. https://doi.org/10.1016/j.ppees.2012.09.002
Young JA, Evans RA (1973) Mucilaginous seed coats. Weed Sci 21:52–54
Young JA, Evans RA, Gifford RO, Eckert RE Jr (1970) Germination characteristics of three species of Cruciferae. Weed Sci 18:41–48
Zaman BYB (1950) The embryology of Juncus prismatocarpus Br. and J. effusus Linn. Proc Indian Acad Sci 31:223–234
Zhang SR (1999) The embryology of the genus Kobresia (Cyperaceae). Acta Bot Yunnan 21:466–470
Acknowledgements
The authors thank the Laboratório de Microscopia Eletrônica “Elliot Watanabe Kitajima” of Escola Superior de Agricultura “Luiz de Queiroz” (ESALQ/USP) for the use of the SEM facilities, and Renato Salaroli for his technical assistance.
Funding
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico–CNPq [process number 170850/2017–0 to L.R.O.S. and process number 157531/2018–0 to A.O.] and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–CAPES–Finance Code 001 [PNPD grant to A.O.].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Ferhat Celep.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silva, L., Oriani, A. Ecological and evolutionary aspects of seed coat and seedling development in Juncus tenuis (Juncaceae, Poales). Plant Syst Evol 308, 31 (2022). https://doi.org/10.1007/s00606-022-01825-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00606-022-01825-z