Abstract
Structural aspects of the seeds of Serjania communis Camb. and Urvillea ulmacea Kunth were described aiming to increase the knowledge about these organs in Sapindaceae. Ovules and seeds were collected at different stages of development and morphological and anatomical studies were performed according to standard techniques. Ovules are anatropous, tending or bowing to campilotropous, bitegmic, and pachychalazal; they also develop a pachychalazal, bitegmic, exotestal, and exalbuminous seed. Seeds are small, lenticular in S. communis and ellipsoid in U. ulmacea, and become campilotropous by means of the development of an invagination originating from the meristematic activity on the rapheal region. The exotesta consists of macrosclereids arranged in palisades. The other layers of the testa and the tegmen collapse up. In U. ulmacea, a cordiform aril is observed in the funicle and, as registered in the literature, it is similar to that found in Serjania inflata Poepp. & Endl. In other Serjania species there is no aril, but these two genera are considered phylogenetically close by many authors. Seed development in the studied species is similar and resembles other species of Paullinieae. Our results associated with those found in the literature seem to reinforce the transitional character of seed structures associated with fruit type in the tribe, as the reduction or absence of aril in indehiscent fruit of Serjania. However, seed ontogenetic studies must be extended to more species so as to allow reliable mapping of a larger number of characteristics of seeds on tribe phylogenies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Serjania Miller and Urvillea Kunth include around 230 and 16 species, respectively, of woody lianas; both are native to tropical and subtropical regions of the New World (Acevedo-Rodriguez 1993; Ferrucci 2006). The climbing habit, the presence of stipules, modified peduncles like tendrils, and nectariferous disc modified into four protrusive glands place these genera inside Paullinieae (Acevedo-Rodriguez 1993; Ferrucci 2006). This tribe belongs to Clade X of Sapindoideae, according to the phylogeny proposed by Buerki et al. (2009), together with Thouinieae and Sapindus oligophyllus Merr. & Chun. This clade is characterized by zygomorphic flowers, petals with a prominent scale, a unilateral disc, imparipinnate leaves, and the liana habit; the development of tendrils and stipules constituting synapomorphies for the group (Buerki et al. 2009).
Harrington et al. (2005) and Buerki et al. (2009) pointed the tribe Paullinieae as a monophyletic group that includes, besides Serjania and Urvillea, also Balsas Jime´nez Ram. & Vega, Cardiospermum L., Houssayanthus Hunz., Lophostigma Radlk., and Paullinia L. Paullinieae is the largest tribe of Sapindaceae (Sapindoideae) and comprises almost a quarter of all species from that family (Coulleri et al. 2012). According to Harrington et al. (2005), there is strong evidence for the monophyly of Cardiospermum, Paullinia, and Serjania¸ which is consistent with the recognition of Paullinieae.
Phylogenetically, Urvillea is closely related to Cardiospermum and its members are characterized by papery, inflated capsules, and seeds that usually have a dry aril. These two genera are distinguished from each other by the presence of pericarpic wings in Urvillea, and their complete absence in Cardiospermum (Urdampilleta et al. 2006; Buerki et al. 2009). In the study of Buerki et al. (2009), Serjania is related to Paullinia (BS 55) and the relationship between these two genera with the clade that includes Cardisopermum and Urvillea is strongly supported (BS 100).
Considering the circumscription of this family (Buerki et al. 2009, 2010), ontogenic studies of seeds are scarce and began to be important due to the taxonomic value of such structures since the first delimitations, as highlighted in Weckerle and Rutishauser (2005). Weckerle and Rutishauser (2005) pointed that in these studies the materials used were from herbarium and for that reason they did not analyze seminal characteristics in detail—including the aril structure. Thus, it becomes difficult to use the states of this character for phylogenetic discussions.
Among the classic studies describing the seed structure of some genera of Sapindaceae, the works of Corner (1976) and van der Pijl (1957, 1982) stand out, which did not include Serjania and Urvillea, which is one of the justifications for doing this work. Albiero et al. (2001) described the fruit and seed ontogenesis of Sapindus saponaria L. With regard to the tribe, it is very important to point out the works carried out by Milanez (1959), Mendonça et al. (1992), Weckerle and Rutishauser (2005), and Polo (2006) on morphology and ontogeny of Paullinia fruit and seeds. Weckerle and Rutishauser (2005) described six Paullinia species and three species of other genera—Serjania altissima Radlk., Cardiospermum halicacabum L., and Urvillea ulmacea Kunth. However, these descriptions did not emphasize the development of the seed coat. It is important to highlight here that there are few studies of detailed seminal ontogeny in Sapindaceae, especially in Paullinieae.
The presence of fleshy structures in the seeds has long been related with fruit dehiscence. In Sapindaceae, Corner (1976) states that the aril appears as a primary characteristic of capsular fruits (Alectryon Gaertn., Cupania L., Guioa Cav., Harpullia Roxb., and Paullinia). The aril is absent in the genera related to these cited above or even in species within those genera that have simplified and papyraceous capsules, or has been lost in tribes with indehiscent fruit such as Aphanieae, Lepisanthes, Melicocceae, Sapindeae, Schleichera, Thinouieae, and Thouinieae. According to Corner, in Paullinieae, the three situations seem to occur: Paullinia and Cardiospermum with capsular dehiscent fruit have arillate seeds; Urvillea with papyraceous consistency fruit has reduced aril; and in Serjania which presents a schizocarpic fruit with three samara-like mericarps, the aril is absent; whereas according to Weckerle and Rutishauser (2005), in Serjania inflata Poepp. & Endl. there is a reniform structure similar to the aril of U. ulmacea. This aspect can be associated with that discussed by Tanaka et al. (2014), who stated that the presence of dorsal wings, lighter spongy tissue, inflated loci, and the opening facilitated by the spongy tissue in septa in U. ulmacea may represent a transitional state between the samaroide mericarps to the schizocarpic fruit of Serjania and the septifragal capsule of Cardiospermum.
According to Weckerle and Rutishauser (2005), all studied Paullinieae species (except Serjania spp.) have seeds with arils, like a necklace or white-lobed structure, which is conspicuous and fleshy (Paullinia) or inconspicuous and dry (Cardiospermum and Urvillea). Polo (2006) found that in Paullinia trigonia Vell. the outer integument participates in the formation of fleshy structure in two regions of the seed, at the basal portion of the raphe and in the area near the micropyle, not evidencing any contribution of the funicle on the formation of the fleshy structure. This fleshy structure was designated by the author as sarcotesta, and the term aril as it has been described for the genus was not used.
Considering the importance of studies on the ontogeny of reproductive organs in Sapindaceae, the divergence in the structure present in seeds, such as the type of aril or sarcotesta, the objective of this paper is to describe the morphology and anatomy of seed development in Serjania communis and Urvillea ulmacea and answer the question: Is the seed structure associated with the transitional nature of the fruit type (indehiscent schizocarpic fruit to dehiscent capsule fruit) in the tribe?
Materials and methods
Sites for sampling S. communis and U. ulmacea (Sapindaceae) were located in urban forest remnants: Forest Garden “Dr. Luiz Teixeira Mendes” (23°26′01, 47″S and 51°57′56, 21″W) and “Bosque dos Pioneiros” (23°26′04, 36″S and 51°56′33, 04″W), both in the Maringá City, Paraná State, Brazil. Vouchers of the species were deposited as a taxonomic document at the Maringá State University Herbarium (HUEM), registered by the numbers 11.741 and 11.743, respectively.
Ovules and seeds at different developmental stages were fixed in FAA 50 (Johansen 1940) and stored in 70 % ethanol (Jensen 1962). The anatomical study was carried out in sections of 8 µm thick at different planes using a rotary microtome. Permanent slides were made with the botanic pieces embedded in hydroxyethyl + methacrylate Leica™, according to the manufacturer's protocol. Sections were stained with 0.05 % Toluidine Blue in buffer acetate, pH 4.7 (O’Brien et al. 1964 modified). The botanic material was also embedded in paraffin, sectioned, and stained with Astra Blue and Safranin (Gerlach 1969). Both cases were mounted in synthetic resin.
The following histochemistry tests were performed: phloroglucinol plus hydrochloric acid for lignified walls (Sass 1951); Sudan IV for lipid substances; lugol for starch; ferric chloride plus sodium carbonate for phenolic compounds (Johansen 1940), and Ruthenium Red for mucilage (Strasburger 1924).
Morphological and anatomical documentation was done by capturing images using the software Image Pro-Plus 4.0 (Media Cybernetics®). The scales referring to the pictures were obtained in the same optical conditions used for each case.
The terminology used in seed description was the one defined by Corner (1976).
Results
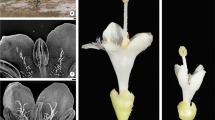
In both species, each carpel presents an erect ovule formed in axial placenta at the locule base (Fig. 1). The ovule is pachychalazal, bitegmic, anatropous tending or bowing to campilotropous. It has a short and thick funicle with obturator that surrounds it; the obturator has a papillose surface that secretes mucilage (positive reaction to ruthenium red and purple aspect with toluidine blue) and the major lobule at the side of the micropyle (Figs. 1–3). In U. ulmacea, above the obturator, the aril starts growing, evident like a small protrusion (Fig. 3). Ovule vascularization has a funical bundle (Fig. 4) which ramified into two bundles in the rapheal region. As the vascular tissue reaches the chalaza, the rapheal bundles are further ramified, showing circular arrangement in the periphery of all pachychalaza (Figs. 4–7).
Longitudinal (1–3) and cross (4–8) sections of the ovule of flowers at anthesis (1–3, 5, 8) and post-anthesis (4, 6, 7) of Serjania communis (2, 4, 7) and Urvillea ulmacea (1, 3, 5, 6, 8). 1 General view of the ovary showing the axial placentation and obturator in the funicle region. 2, 3 General view of the ovule evidencing rapheal and anti-rapheal limits on the pachychalaza (asterisk), meristematic region of the raphe, which originates the rapheal invagination (arrow), obturator and aril in the funicle region, and endostome formed by the inner integument. 4–6 General view showing the inner and outer integument, funicle, rapheal vascular bundles, and the reabsorption of the nucellus (arrow unfilled). 7, 8 General view showing the three regions of the pachychalaza (r1, r2, r3) and the pachychalazal bundles (ar aril, en endostome, fu funicle, ii inner integument, ob obturator, pt pachychalazal procambial bundles, rb rapheal bundle, r1 outer region, r2-middle region, r3 inner region, oi outer integument, vb vascular bundle)
The outer integument has about seven layers, and the inner approximately four layers of cubic cells, with thin walls, dense cytoplasm, and conspicuous nuclei (Figs. 2, 3). The three regions in the pachychalaza in U. ulmacea, may possibly differ: the outer region with five or six layers of isodiametric cells, periclinally flattened, in which the procambial strands are immersed; the middle region formed by isodiametric and vacuolated cells; and the inner region that presents smaller cells than the first two regions, of varied shapes (Fig. 8). In S. communis, the three regions of the pachychalaza are different, but the middle region has large cells with phenolic contents (Fig. 7). The nucellus has one or two layers of cubical parenchymatic cells with thin walls, dense content, conspicuous nuclei, and it shows signals of reabsorption highlighted by the degeneration of the cells between the inner tegument and the embryo sac (Figs. 5, 6). In both species, the micropyle consists of the endostome (Figs. 2, 3).
During seed development, the testa, tegmen, and pachychalaza do not increase the number of layers, but there is an increase in cell volume and the layers on the pachychalazal region become more evident (Figs. 9–16). At the anti-rapheal region, the tegmen has cuboidal cells with phenolic content and the first two layers of the mesotesta also have phenolic content (Figs. 10–12). An invagination appears in the ovule (Figs. 2, 3) due to high mitotic activity occurring in rapheal region and the seed becomes curvy. In this phase, the young seed is still anatropous (Fig. 9). The exotesta, in both species, is uniseriate throughout the seed development, and the cells of phenolic content start to show an anticlinal stretching (Figs. 10–18).
Longitudinal (9, 11, 12, 15, 16) and cross (10, 13, 14, 17, 18) sections of developing (9–16), developed (17), and mature (18) seeds of Serjania communis (9, 12–14, 17) and Urvillea ulmacea (10, 11, 15, 16, 18). 9 General view of the seed evidencing the rapheal and anti-rapheal limits of the pachychalaza (asterisk), meristematic rapheal region, which originates the rapheal invagination (double asterisk) and the cordiform embryo. 10–12 Details of the seed coat evidencing testa and tegmen. 13–16 Details of the seed coat in the pachychalaza showing the three distinct regions, the middle region being phenolic (r1, r2, r3). 17, 18 Details of the seed coat of the developed and mature seed, respectively, showing periclinal division in the region r1 of the pachychalaza, and exotesta with macrosclereids (eb embryo, en endostome, ex exotesta, pb pachychalazal bundles, rb rapheal bundle, r1 outer region, r2 medium region, r3 inner region, ts testa, tg tegmen)
The enlargement of mesophyll cells of the pachychalaza turns more distinct the three evident regions in the ovule: the outer region has six layers of isodiametric cells, slightly and periclinally flattened; the middle region has from four to five layers of isodiametric cells, larger than those in the outer region, slightly and anticlinally elongated and with phenolic content; and the inner region is formed by a variable number of layers of small cells in initiation of collapse (Figs. 14–16).
The non-specialized endotesta becomes indistinct from mesotesta along the development. The mesotesta and the tegmen, in both species, exhibit no specialization and become collapsed in mature seed (Fig. 18). The nucellus, little conspicuous, is slowly consumed. The endosperm is nuclear, presenting numerous free nuclei near the cordiform embryo that develops slowly (Figs. 19, 20).
Detail of longitudinal sections of developing (19, 20), developed (21), and mature (22, 23) seeds of Serjania communis (19) and Urvillea ulmacea (20–23). 19, 20 Micropyle region evidencing the rapheal invagination, endostome, and cordiform embryo. 21 Micropyle region evidencing the pachychalaza limit in the raphe (asterisk), cellularized endosperm being consumed, embryo, rapheal invagination, and radicle pocket. 22 Micropyle region evidencing the rapheal invagination and root cap. 23 Detail of the cotyledons highlighting the procambial strands (arrow) (ar aril, ct cotyledon, cr cap root, eb embryo, ed endosperm, eh hypocotyl-radicle axis, en endostome, fa funicle abscission region, ob obturator, ra raphe, ri rapheal invagination, rp radicle pocket, ts testa, tg tegmen)
The mature seed becomes a little campilotropous by the development of an invagination that causes a slight curving of the seed (Figs. 19–22). It is pachychalazal, bitegmic, and exotestal. The exotesta, including the pachychalazal region, is formed by macrosclereids, arranged in palisade covered with thick cuticle. The remaining testa and tegmen collapse up. In the immature seed, the pachychalazal integument is still quite distinct, but there is evidence of collapse, mainly in the inner region (Figs. 17, 18). At the end of seed development, the nucellus was completely used and the endosperm, after the cellularization, is reabsorbed (Figs. 21, 22).
Seeds of S. communis are lenticular (Fig. 24) and the seeds of U. ulmacea are ellipsoid (Fig. 25), with smooth surface, showing red to brown color. In U. ulmacea, near the hilum, in the pre-rapheal region, the reduced aril is cordiform in shape (Fig. 26). Next the maturation, an abscission line, which will originate the hilum, is observed between the obturator and the aril (Fig. 21).
The embryo of both species is curved and the embryonic axis is short, showing in the radicle a root cap at the beginning of the differentiation process (Fig. 22). Cotyledons are fleshy, constituted by isodiametric meristematic cells and procambial strand immersed in fundamental meristem (Fig. 23). In S. communis, both cotyledons are flat and parallel to the seed axis (Figs. 27, 29). In U. ulmacea, the outer cotyledon is curved and the inner one biplicated (Figs. 28–30). The embryonic reserve in both species basically consists of lipids, showing small starch grains dispersed in the seed coat.
Discussion
In S. communis and U. ulmacea, the ovules are anatropous tending or bowing to campylotropous as in most Sapindaceae described by Corner (1976) in which the latter state occurs at least after fertilization. In Paullinieae, Weckerle and Rutishauser (2005) described campylotropous ovules for the tribe. These contradictions can be due to differences in developmental stages at which the ovules had been described.
In both species, there is a funicular obturator with secretory surface, a structure that, according to Corner (1976), among Sapindales, has been reported in Rutaceae and Sapindaceae. In Sapindaceae the obturator, it may have funicular or placental origin and cover or not the micropyle (Corner 1976). A papillose obturator is also found in S. saponaria (Albiero et al. 2001). Bouman (1984) stated that the papillose obturator has the function of guiding the pollen tube to the micropyle, and it is connected with the transmitting tissue, which was also emphasized by Weckerle and Rutishauser (2005). For the species described in this work, the obturator maybe have this function, but floral biology studies are necessary to be sure. The obturator surface is smooth in S. communis and papillose in U. ulmacea. The occurrence of papillose obturator in P. alata G. Don, P. pachycarpa Benth., P. dasystachya Radlk., Cardiospermum halicacabum L., U. ulmacea (Weckerle and Rutishauser 2005), and S. saponaria (Albiero et al. 2001) can be a characteristic that reinforces the proximity of the species from tribe Paullinieae with the other groups in the Clade X (Paullinieae) from Buerki et al. (2009) proposal, besides the species sampled being S. oligophyllus. A detail investigation from the seed ontogeny from more species in the Clade X and species from other clades in Sapindaceae can reinforce or refute this point of view.
In mature seeds of S. communis and U. ulmacea, the mesotesta, endotesta, and tegmen become quite crushed, and only the exotesta consisting of macrosclereids in palisade is visible. This exotestal seed is described by Corner (1976) in Sapindaceae, by Albiero et al. (2001) in S. saponaria, by Weckerle and Rutishauser (2005) in all Paullinieae species studied, and in P. trigonia by Polo (2006). According to Corner (1976), exotestal palisade of imperfect Malpighian cells, some with linea lucida (Sapindus L.), suggests a connection to a leguminous ancestry. This affirmation makes sense because seed with Malpighian cells is a character state shared by Fabales (eurosids I) and Sapindales (eurosids II) (APG III 2009); however there is variation according to the position of this layer (testa or tegmen). The layer of Malpighian cells in the testa or tegmen is constant in the descriptions presented in Corner (1976), as well in the studies of seed ontogeny in Chorisia speciosa A. St.Hil. (Malvales) (Marzinek and Mourão 2003), and in other species from another family in Sapindales (Pinto et al. 2003 in Guarea macrophylla Vahl). It is important to highlight that studies like the one realized in this work, with a bigger amount of species in eurosids I and II, allow us to explain better the affirmation of Corner (1976).
The seed is pachychalazal in S. communis and U. ulmacea. It is interesting to note that both Weckerle and Rutishauser (2005) and Polo (2006) did not refer to the occurrence of pachychalaza in the species they described, despite the record in the tribe by Corner (1976) for Cardiospermum and Paullinia. In C. halicacabum, Corner (1976) stated that pachychalaza has the same structure of the testa, except for the presence of vascular bundles. In the species studied in this work, pachychalaza is much broader than the testa, and we could distinguish three regions. This fact reinforces the importance of ontogenetic studies in the definition of seminal structures, so they can be correctly used in the phylogenetic discussions.
In S. communis and U. ulmacea, vascularization is typical of pachychalazal seeds (Corner 1976), in which the vascular supply of the raphe is divided a little before entering the seed into several branches which are further ramified in the pachychalaza, closing over the expanded hypostasis, being absent in the anti-raphe and in the free portion of the testa.
The nucellus is not a conspicuous layer in the ovules of S. communis and U. ulmacea and rapidly disorganizes in the developing seed, unlike the description of Corner (1976), in which the nucellus extends up long before the absorption by the endosperm in species of this family. Initially, the nuclear endosperm of S. communis and U. ulmacea becomes cellularized, and it is subsequently reabsorbed by the developing embryo that fills the seed, as described by Corner (1976) for Sapindaceae in general and Polo (2006) for P. trigonia.
In S. communis, the aril is absent. This structure also does not occur in S. altissima, but in S. inflata, a reniform structure is found at the seed base, similar to U. ulmacea (Weckerle and Rutishauser 2005). These two genera are considered phylogenetically close by several authors (Acevedo-Rodriguez 1993; Weckerle and Rutishauser 2005). In Sapindaceae, there are arillate, exarillate, and sarcotestal seeds; structures which absent in Serjania and Urvillea (Corner 1976), an aspect not confirmed by Weckerle and Rutishauser (2005) in the two genera (they described in S. inflata and in U. ulmacea as confirmed in the present study).
The aril of U. ulmacea is funicular, but Weckerle and Rutishauser (2005) described, for this species and C. halicacabum, that the aril is built in the basal part of the chalaza. However, Corner (1976) described the aril as funicular in C. halicacabum. Probably, the descriptions made by Weckerle and Rutishauser (2005) have not taken in account the aril ontogeny, like the one realized in U. ulmacea. An extensive discussion on the use of the term aril and sarcotesta in Sapindaceae is presented by Polo (2006). This author named partial sarcotesta the fleshy structure that differentiates at the basal portion of the raphe and in the area near the micropyle, in Paullinia, contrary to the term aril described for the genus by Weckerle and Rutishauser (2005). All these contradictions are due to the lack of detailed analysis on seed ontogenesis making the seed appendages not reliable in phylogenetic discussions.
The transitional character of aril loss associated to the loss of the fruit dehiscence in Sapindaceae and in Paullinieae, as described by Corner (1976), is reinforced in the present study. The same way, this transition is present in the work of Weckerle and Rutishauser (2005), when the authors cite the presence of a reniform structure in S. inflata similar to the aril in U. ulmacea. As highlighted in the introduction, the anatomical transitional character of the loss of dehiscence in the fruit of Paullinieae was also described and discussed by Tanaka et al. (2014), who stated that the presence of dorsal wings, lighter spongy tissue, inflated loci, and the opening facilitated by the spongy tissue in septa in U. ulmacea may represent a transitional state between the schizocarpic fruit with three samara-like indehiscents mericarps of Serjania and the septifrage dehiscent capsule of Cardiospermum. Tanaka et al. (2014) affirmed that ontogenic studies of more species belonging to these three genera can sustain these affirmations. This consideration is also valid to seminal ontogeny, since studies of this aspect in other species of Serjania can reveal different degrees in aril size or even its absence. The biggest difficulty is to collect a representative number of species with all the developmental phases that are necessary to realize an ontogenetic study like the one done in this work.
The monophyly of Cardiospermum, Paullinia, and Serjania was supported in the study of Harrington et al. (2005) and the close relationship of Cardiospermum and Urvillea by Urdampilleta et al. (2006). In a more recent study, working with more molecular markers, Buerki et al. (2009) confirmed the monophyly of the tribe Paullinieae. In this phylogenetic proposal, the authors had a 55 % degree of reliability between the relationship of Serjania (with indehiscent schizocarpic fruit and vestigial aril in S. inflata) and Paullinia (with dehiscent capsule fruit and seeds with fleshy appendices), while the degree of reliability between these two genera and the branch that includes Cardiospermum and Urvillea is 100 %.
In this sense, studies with fruit and seed ontogeny including more species of the tribe Paullinieae can demonstrate in a more consistent way the transitions between dehiscence to indehiscence, with the subsequent loss of the fleshy appendice in the group or even reversals or parallelisms that may have occured in the tribe.
In S. communis and U. ulmacea, the seeds become slightly campylotropous through the development of a partial septum, arising from meristematic activity in the rapheal region adnate to the outer integument from the ovule, which causes a slight curving of the seed. This development is described in Sapindaceae by Corner (1976) and also by Weckerle and Rutishauser (2005) and Polo (2006), resulting in a radicle chamber (radicle pocket) that holds the short embryonic radicle.
According to Acevedo-Rodriguez (1993), the size and shape of seeds in Paullinieae are important characteristics at the species level, with the embryo being straight or curved in various degrees and the cotyledons being generally thick, straight, or curved. In S. communis, embryo is type 1, which is in accordance with one of the types described by the above-mentioned author in Serjania section Platycoccus and characterized by both cotyledons which are flat and parallel to the axis of the fruit.
Weckerle and Rutishauser (2005) described straight (Serjania) or curved (Cardiospermum) cotyledons and embryo with the outer cotyledon curved and the inner biplicate in U. ulmacea, which was confirmed in the present work for this species and that classifies the species into type 2 cotyledon proposed for Serjania (Acevedo-Rodriguez 1993).
Although recent researches on the reproductive organs of Sapindaceae define differential morphological and anatomical seed characters, contradictory descriptions are found in many structures, for example the type of fleshy appendix, which could be elucidated only by ontogenetic analysis as performed herein. Studies on seed ontogeny in the tribe Paullinieae can ensure the reliable use of its structural characters and mapping on current molecular phylogenetic purpose revealing transitional states among genera of Paullinieae.
The transitional character of the presence of aril associated with the fruit type (dehiscent or indehiscent) in the tribe Paullinieae is reinforced. Species with capsule fruit type present a well-developed aril. In U. ulmacea, the aril is poorly developed and is associated with indehiscent fruit, but have tissues that allow an easy disintegration of the pericarp. In Serjania, with indehiscent schizocarp fruit with free samara-like mericarps, the aril is absent or very small, as reported in the literature, which can strengthen the gradual transition of these characteristics between these two genera, whose position has varied in the phylogenies of Paullinieae.
References
Acevedo-Rodriguez P (1993) Systematics of Serjania (Sapindaceae) part. I: a revision of Serjania sect. Platycoccus. Mem N Y Bot Gard 67:1–93
Albiero ALM, Bacchi EM, Mourão KSM (2001) Caracterização anatômica das folhas, frutos e sementes de Sapindus saponaria L. (Sapindaceae). Acta Sci 23:549–560. doi:10.4025/actascibiolsci.v23i0.2733
APG, III (The Angiosperm Phylogeny Group) (2009) An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc 161:105–121
Bouman F (1984) The ovule. In: Johri BM (ed) Embryology of angiosperms. Springer, Berlin
Buerki S, Forest F, Acevedo-Rodríguez P, Callmander MW, Nylander JAA, Harrington M, Sanmartín I, Küpfer P, Alvarez N (2009) Plastid and nuclear DNA markers reveal intricate relationships at subfamilial and tribal levels in the soapberry family (Sapindaceae). Mol Phylogenet Evol 51:238–258. doi:10.1016/j.ympev.2009.01.012
Buerki S, Lowry PP II, Alvarez N, Razafimandimbison SG, Küpfer P, Callmander MW (2010) Phylogeny and circumscription of Sapindaceae revisited: molecular sequence data, morphology and biogeography support recognition of a new family, Xanthoceraceae. Plant Ecol Evol 143:148–159. doi:10.5091/plecevo.2010.437
Corner EJH (1976) The seeds of dicotyledons. Cambridge University Press, Cambridge
Coulleri JP, Dematteis M, Ferrucci MS (2012) A new insight into Serjania Mill. Sapindaceae, Paullinieae) infrageneric classification: a cytogenetic approach. Plant Syst Evol. doi:10.1007/s00606-012-0675-8
Ferrucci MS (2006) A new species of Urvillea (Sapindaceae) from northwestern Venezuela. Brittonia 58:83–87
Gerlach G (1969) Botanische mikrotechnik. Georg Thieme Verlag, Stuttgard
Harrington MG, Edwards KJ, Johnson SA, Chase MW, Gadek PA (2005) Phylogenetic Inference in Sapindaceae sensu lato using plastid matK and rbcLDNA sequences. Syst Bot 30:366–382. doi:10.1600/0363644054223549
Jensen WA (1962) Botanical histochemistry: principles and practice. W. H. Freeman, San Francisco
Johansen DA (1940) Plant microtechnique. McGraw-Hill, New York
Marzinek J, Mourão KSM (2003) Morphology and anatomy of the fruit and seed in development of Chorisia speciosa A. St.-Hil.-Bombacaceae. Rev Bras Bot 26:23–34
Mendonça MS, Noda H, Corrêa MPF (1992) Aspectos morfológicos da semente e da germinação do guaraná (Paullinia cupana var. sorbillis (Mart.) Ducke). Rev U A Série Ciên Ag 1:71–82
Milanez FR (1959) Anatomia do fruto do guaraná. Arq Jard Bot Rio de Janeiro 16:57–100
O’Brien TP, Feder N, Mccully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373
Pinto DD, Mourão KSM, Souza LA, Moscheta IS (2003) Morfo-anatomia do fruto e da semente em desenvolvimento de Guarea macrophylla Vahl. (Meliaceae). Act Cient Venez 54:238–246
Polo SHO (2006) Estrutura e Desenvolvimento de Sementes de Paullinia L. (Sapindaceae). Thesis. Universidade Estadual de Campinas, Campinas
Sass JE (1951) Botanical microtechnique, 2nd edn. State College Press, Iowa
Strasburger E (1924) Handbook of practical botany. MacMillan, New York
Tanaka BM, Pinto DD, Mourão KSM (2014) Ontogeny of the pericarp of Serjania communis Camb. and Urvillea ulmacea Kunth (Sapindaceae) with emphasis on the dispersion apparatus. Act Sci 4:457–465. doi:10.4025/actascibiolsci.v36i4.20666
Urdampilleta JD, Ferrucci MS, Torezan JMD, Vanzela ALL (2006) Karyotype relationships among four South American species of Urvillea (Sapindaceae: Paullinieae). Pl Syst Evol 258:85–95. doi:10.1007/s00606-005-0393-6
van der Pijl L (1957) On the arilloids of Nephelium, Euphoria, Litchi and Aesculus, and the seeds of Sapindaceae in general. Act Bot Neerl 6:618–641
van der Pijl L (1982) Principles of dispersal in higher plants. Springer, New York
Weckerle CS, Rutishauser R (2005) Gynoecium, fruit and seed structure of Paullinieae (Sapindaceae). Bot J Linn Soc 147:159–189
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanaka, B.M.M., Pinto, D.D. & Mourão, K.S.M. Seed ontogeny of Serjania communis and Urvillea ulmacea and its relationship to transitional characters in Paullinieae (Sapindoideae, Sapindaceae). Braz. J. Bot 39, 885–894 (2016). https://doi.org/10.1007/s40415-016-0279-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-016-0279-6