Abstract
Most perennial herbaceous plants are able to reproduce vegetatively as well as sexually. Sometimes, such plants may lose the capacity for sexual reproduction. We have studied the case of sterility in triploid populations (2n = 3x = 45) of Gladiolus tenuis M.Bieb. in a considerable part of its area of distribution. Initially, we recorded the presence of a large clone of G. tenuis to the east of the Volga River, as a result of isozyme analysis. We also used AFLP fingerprinting to genotype 55 samples from 10 populations of G. tenuis and one population of the closely related G. imbricutus L. This analysis revealed an extremely low genetic diversity in sterile triploid populations of G. tenuis and a rather high genetic diversity in fertile tetraploid populations (2n = 4x = 60) over most of the area of this species. Genetic distances between fertile and sterile populations of G. tenuis were similar to those between different species of gladioli. It appears that a single sterile genotype has spread vegetatively over 800 km, propagating by daughter corms. The study of the reproductive features of G. tenuis suggests that the cause of sterility may be self-incompatibility between individuals of the clone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most perennial herbs and some woody plants can reproduce vegetatively. Vegetative reproduction allows the plants to quickly occupy new areas and does not depend on the availability of pollinators or conditions for the maturation and germination of seeds and spores. The plant structures responsible for vegetative propagation often contain a greater supply of nutrients than that of the seeds and spores. This gives a significant competitive advantage to young plants of vegetative origin in plant communities. In arctic-alpine conditions or on the edge of areas where seed propagation is hindered, the benefits of vegetative propagation are most evident in species naturally growing in this habitat and in plants growing at the limits of their distribution (Dorken and Eckert 2001; Eckert 2002).
Most clonal plants can maintain sufficiently high levels of genetic variability because they reproduce sexually from time to time (Ellstrand and Roose 1987; Hamrick and Godt 1989). However, there are some cases of complete loss of sexual reproduction, leading to formation of clones of various sizes (Dorken and Eckert 2001; Hollingsworth and Bailey 2000; Lynch et al. 1998; Peterson et al. 2010). Odd polyploidy, aneuploidy, hybridization, and other causes leading to lack of seed reproduction may contribute to the formation of sterile clones. A remarkable example is the king’s lomatia (Lomatia tasmanica V.M.Curtis)—locally endemic to Tasmania and represented by a single clone (genetically identical specimens). This species is triploid and characterized by a complete lack of seed reproduction and genetic diversity (Lynch et al. 1998). A sterile “megaclone” was detected in Belgian Gagea (Gagea spathacea Salisb.) in Central Europe (Pfeiffer et al. 2012). The authors consider the high ploidy and the putative hybrid origin as the reasons for the sterility of G. spathacea. The sterility of the flowering rush (Butomus umbellatus L.), introduced to America (Eckert 2002; Eckert et al. 2003), and Sedum bulbiferum Makino in Japan (Tsujimura and Ishida 2008) are associated with anorthoploidy. There are also many examples of asexual reproduction and the formation of clones in populations from peripheral parts of a species distribution area. Clones were detected in the northern populations of Decodon verticillatus (L.) Elliott (Dorken and Eckert 2001), in regional populations of Gagea bohemica (Zauschn.) Schult. & Schult.f. (Peterson et al. 2010) and in populations of Saxifraga cernua L. in different parts of its range (Gabrielsen and Brochmann 1998; Kapralov et al. 2006).
The genus Gladiolus includes species of different ploidal levels, all of them able to reproduce vegetatively. Two closely related species of this genus, G. tenuis M.Bieb. and G. imbricatus L., growing in the European part of Russia, are sometimes combined into a single species (Mayewsky 1964, 2006). The main part of the distribution area of G. tenuis covers the Crimea, the Caucasus, and some other southern regions of the European part of Russia. The Southern Urals and surrounding areas lay at its eastern limit. Because of the evidence for vegetative reproduction by small corm lets produced as offsets by the parent corms in the Southern Urals populations, we assumed that seed reproduction on the edge of the area may have been disturbed, resulting in the formation of clones. Using isozyme analysis, we studied several populations in the Orenburg Region and Southern Bashkortostan and found that they all belong to a single clone. Chromosome counts revealed that the clonal plants are all triploids (2n = 3x = 45). Fertile plants from the central part of the area of distribution of the species are, however, tetraploids (2n = 4x = 60).
Until recently isozyme analysis was useful in the study of clonal plants (Lynch et al. 1998; Tsujimura and Ishida 2008). In recent years, AFLP analysis has been applied to such studies. This technique generates highly polymorphic “fingerprints,” which permits identification of genets (Mueller and Wolfenbarger 1999). Also, unlike isozyme analysis, AFLP allows genetic analysis of polyploids. In this paper, we have used both types of analysis in the study of clonal Gladiolus. The aim of our study was to identify the genetic structure of populations and assess the reproductive system of G. tenuis in different parts of its distribution, to determine the boundaries of the putative vegetative clone and to compare its level of genetic variation with that of the fertile plants.
Materials and methods
Studied species
Gladiolus tenuis M.Bieb. (≡Gladiolus communis var.tenuis (M.Bieb.) Wahlb.) is a herbaceous perennial plant with an underground corm. It is propagated by seeds as well as vegetatively by daughter corms, which develop at the base of the parent plant. The corm is replaced annually. The main distribution area of G. tenuis covers Central and Eastern Europe, the southern part of the East European Plain, the Crimea, the Caucasus, and northwest Kazakhstan (Mugojar Mountains). In most of its range, G. tenuis is tetraploid (2n = 4x = 60), but on the eastern boundary of the area, in the Southern Urals, triploids (2n = 3x = 45) were found.
In carrying out the AFLP fingerprinting, the closely related species Gladiolus imbricatus L. was used as an out-group. Synonyms are Gladiolus apterus Klokov., Gladiolus rossicus Pers., Gladiolus galiciensis Schultes ex Besser., Gladiolus neglectus Schultes (Govaerts and Barker 2014). It differs from the G. tenuis in the large number of flowers in the inflorescence, winged seeds, and an absence of daughter corms. It is a European species, native to Central and Eastern Europe, including the European part of Russia, the Baltic States, and the Carpathians. The chromosome number of G. imbricatus is 2n = 4x = 60 (Fedorov 1969).
Study sites and sample collection
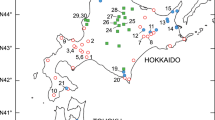
Plant material was collected from ten populations of G. tenuis from Orenburg, Volgograd, and Samara Regions, the Republic of Tatarstan, and the Republic of Bashkortostan. One population of G. imbricatus from the Republic of Belarus (Table 1; Fig. 1) was sampled for an out-group comparison in AFLP analysis. In all populations of G. tenuis, except population 3, there were more then 15–20 individuals. Only two individuals were in population 3, so we could not include this population in the isozyme analysis and the evaluation of reproductive system. In all populations, plants were sampled over a distance of at least 4 m. For isozyme and DNA analyses, we collected corms and leaves of gladioli. Extracts for isozyme analysis were prepared from fresh bulbs. The bulbs and leaves for AFLP fingerprinting were stored at −80 °C until DNA extraction.
Locations of populations of Gladiolus tenuis and G. imbricatus as given in Table 1
Isozyme extraction and electrophoresis
To prevent degradation of isozymes, the extraction and subsequent operations were carried out at a temperature of 0–5° C. Plant material (about 100–150 mg) was homogenized with 0.8 ml of extraction buffer (Tris–HCl pH 7.5 with 1 % β-mercaptoethanol) at a temperature of 0–5 °C. Extracts were stored at −80 °C. Samples were electrophoretically separated in 6.4 % polyacrylamide gel in a vertical chamber with Tris–EDTA–borate buffer system (pH 8.0), as described previously (Semerikov et al. 2002). Populations 1 and 2 (Table 1) of G. tenuis were tested for 13 enzyme systems. Some of them appeared monomorphic or not clearly identifiable on gels under histochemical staining. Six polymorphic enzyme systems, alcohol dehydrogenase (ADH), 6-phosphogluconate dehydrogenase (6PGD), shikimate dehydrogenase (SKDH), phosphoglucoisomerase (PGI), glutamate oxaloacetate transaminase (GOT), and phosphoglucomutase (PGM) were used for the subsequent analysis. Due to polyploidy of G. tenuis, the bands could not be assigned to specific loci, being probably represented by product combinations from different loci. As a consequence, each isozyme showed a specific banding pattern and was recorded as a phenotype. The presence of identical band profiles in all isozyme systems, we interpreted as a single genotype (a clone). Genotypic diversity was evaluated using a modified Simpson index as suggested for clonal plants by Ellstrand and Roose (1987): D = D = 1 − {[Σn i (n i − 1)]/[N(N − 1)]}. Here n i means the number of plants identified in the sample from the i-th isozyme phenotype (and accordingly, the genotype); N stands for the total number of plants that were analyzed; the diversity index D varies from 0 in a population, consisting of a single genotype, to 1 in populations where each individual represents a unique genotype. Higher values of D correspond to greater clonal diversity within a population.
DNA isolation and AFLP fingerprinting
Genomic DNA was isolated using the CTAB method (Devey et al. 1996) from frozen (−80 °C) leaves and corms. AFLP analysis was carried out according to the standard type protocol (Vos et al. 1995) with modifications described in Samils et al. (2001) using a 3130 Genetic Analyzer (Applied Biosystems, USA) with fluorescently labeled EcoRI primers. In order to identify a combination of selective primers, 12 pairs of primers were tested. Five combinations with clear amplification profiles and an optimal number of fragments (EcoRIAGCNed + MseCTT, EcoRIACTFam + MseCAA, EcoRIACGJoi + MseCAA, EcoRIAGCNed + MseCAA, EcoRIACGJoi + MseCCTC.) were selected for further analyses. Fluorescently labeled products of each selective PCR were linked to the molecular weight standard GeneScan ROXTM 500 (Applied Biosystems, USA) prior to loading into the Analyzer. The chromatograms obtained were analyzed using the GeneMapper® version 4.0 program (Applied Biosystems, USA). The fragment lengths obtained were verified manually. Only loci that demonstrated unambiguous interpretation were accepted for the analysis, monomorphic loci were excluded. AFLP typing of the fragments was represented as a matrix of the band’s “presence” or “absence” with 1 or 0 coding, respectively.
Data analysis
We accounted for the following parameters of intrapopulation variability: the percentage of polymorphous loci (P), unbiased expected heterozygosity (Uhe), and Nei’s genetic distances (D) (Nei 1978) calculated from the allele frequencies of AFLP loci. All the parameters were analyzed using the GenALEx version 6 (Peakall and Smouse 2006), taking into account the Hardy–Weinberg equilibrium. The gene flow was calculated as follows: Nm = 0.25 × (1 − Fst)/Fst based on Wright’s statistics (Slatkin and Barton 1989). Analysis of molecular variance (AMOVA) (Weir and Cockerham 1984) was carried out according to the following hierarchy levels: between species, between groups of populations, between populations and within populations.
The ordination of the studied samples with the method of principal coordinates based on genetic distances was estimated using GenALEx. An alternative analysis of the population structure and assessment of the probability of hybrid nature of species was carried out using the Bayes’ algorithm based on the Hardy–Weinberg equilibrium model in STRUCTURE 2.2 (Pritchard et al. 2000; Falush et al. 2007). This approach allows estimation of the probability of dividing specimens into an optimal number of groups, K value, at which the logarithm of the posterior probability function (lnP(D)) reaches a plateau. The analyses were repeated five times for each of the five (2–6) К values tested. The admixture model was used, which takes into account the possible mixed origin of populations based on the independent frequencies of alleles between the clusters. The analysis was carried out with one million iterations. The burn-in point of the Markov chain was preliminarily chosen after 100,000 iterations.
Evaluation of the reproductive system in Gladiolus tenuis
Inflorescences of G. tenuis were fixed in alcohol–acetic acid fixative (3:1) and stored in 70 % alcohol. Male fertility was estimated by pollen stainability in acetocarmine (Jensen 1962), determined in a sample of 200 grains from each plant. The presence of pollination was evaluated by germination of pollen grains on the stigma, the pollen tube growth in the pistil, and pollen tube penetration into the ovule. These cytological features were evaluated under a fluorescence microscope (Leica DM 2500) after maceration of the pistils in 10 % KOH and staining of them with fluorochrome aniline blue. In each of nine populations (1, 2, 4–10), we have studied 10 plants.
An assessment of reproductive features was made for all populations of G. tenuis except population No. 3 (Table 1; Fig. 1), due to the small number of flowering individuals in it.
Results
Isozyme analysis
Isozyme analysis of tetraploid (2n = 4x = 60) populations (No. 1 and No. 2) revealed a high genotypic variability. Simpson’s index of genotypic diversity (D) is 0.91 and 0.98, respectively (Table 2). To the east of the Volga River, all the populations are triploid (2n = 3x = 45), monoclonal (D = 0), and represented by the same multilocus genotype.
AFLP fingerprinting
The use of five combinations of primers recovered 401 loci, according to which the studied samples were genotyped. The number of polymorphic loci by a primer pair varied from 37 to 146 (Table 3).
On average, for the populations of G. tenuis, the percentage of polymorphic loci in the investigated samples (Table 4) varied from 5.24 % (No. 1 and No. 7) to 18.45 % (No. 2). The only studied population of G. imbricatus was characterized by the highest level of polymorphism (38.4 % of polymorphic loci). The average polymorphism for groups of tetraploid populations of G. tenuis was 13.88 %, while for seven triploid populations it was 8.59 %.
Unbiased expected heterozygosity (Uhe) in tetraploid populations of G. tenuis ranged from 0.029 (No. 3) to 0.085 (No. 2). In triploid populations, it was lower, from 0.028 (No. 7) to 0.047 (No. 5). According to the results of AMOVA, the proportion of variability attributable to species differences (Storz 2005) between the two studied species of gladioli is 68.29 % and between two groups of G.tenuis, tetraploid fertile and triploid sterile populations, it is 66.36 % (Table 5). The share of the differences among populations dispersion of G. tenuis is only 4.85 %. The results of the principal coordinates analysis of the sample are shown in Fig. 2. The first three principal coordinates describe 46.60, 14.24, and 3.57 % of the total variability. The analysis shows clear separation of three groups of specimens: the population of G. imbricatus, the three tetraploid (No. 1, No. 2, and No. 3), and the seven triploid populations of G. tenuis. The greatest values of Nei’s distance (D) were shown between the populations of G. tenuis and G. imbricatus (0.271–0.352) (Table 6). The distance between the tetraploid populations of G. tenuis ranged from 0.045 to 0.130. The triploid populations of G. tenuis appear to be closely genetically related to one another (D ranges from 0.002 to 0.010), except for population No. 7, the distance to which is somewhat greater and varies from 0.029 to 0.036. Mean Nei’s genetic distances (D) between the different ploidy groups of populations of G. tenuis and G. imbricatus are shown in Table 7. As it is clear from the table, the distance between triploid and tetraploid groups of populations of G. tenuis is rather large and amounts to half the distance to the out-group species.
Bayesian analysis with STRUCTURE 2.2 under the admixture model revealed the maximum value of the logarithm of posterior probability function for K = 3 (three groups of genotypes) in all the program runs. This means that the sample may be divided with maximum posterior probability into three genetic clusters (Fig. 3).
The first cluster corresponds to the tetraploid populations of G. tenuis: No. 1, No. 2, and No. 3. The second cluster is represented by triploid populations of G. tenuis: No. 4–10; and the third one is composed by the samples from the population of G. imbricatus.
Reproductive features of Gladiolus tenuis
Some reproductive features of G. tenuis are presented in Table 8.
Discussion
The genus Gladiolus L. comprises 260–270 species, most of which originated in South Africa, and only 10 in Eurasia. Most of the South African species are diploids; the European species are polyploids (Goldblatt et al. 1993). As has already been noted above, G. tenuis is tetraploid in most of its area of distribution, as well is G. imbricatus. The studied tetraploid populations of G. tenuis combine vegetative and seed modes of reproduction, as follows from the repetition of isozyme and AFLP profiles among population samples. At the same time, infrapopulation genotypic diversity is rather high in these populations. The plants of the tetraploid populations invariably demonstrate high fertility of pollen and the presence of pollen tube growth in pistil.
On the contrary, samples studied from the eight triploid (2n = 3x = 45) populations of G. tenuis to the east of the Volga River possess the same isozyme genotype and probably represent a single clone. In these populations, we showed abnormalities in both the female and male reproductive systems. Namely, the level of pollen fertility is significantly lower than in the tetraploid populations (Table 8) and ovules usually degenerate at different stages of development. All these irregularities lead to the complete absence of seed reproduction in triploid populations. Reproduction of these plants can presumably occur only vegetatively, by means of daughter corms.
It is known that triploidy may not necessarily lead to complete sterility. According to the literature, triploids may vary as to pollen fertility. In triploid Datura stramonium L. (Solanaceae), pollen fertility determined by acetocarmine staining was 50–60 %, although only 15 % of them were able to germinate (Satina and Blakeslee 1937). In triploid Eupatorium macrocephalum Less. (syn. Campuloclinium macrocephalum, Asteraceae), fertility of pollen ranging from 46.64 to 54.83 % (Farco and Dematteis 2014). At the same time, some triploids, e.g., accessions of the Musa acuminata Colla (Musaceae) and plants of Taraxacum section Ruderalia have completely sterile pollen (Adeleke et al. 2004; Meirmans et al. 2006). As to seed propagation, it rarely occurs in triploid flowering rush Butomus umbellatus L. (Butomaceae) (Eckert et al. 2000) and breadfruit Artocarpus altilis (Parkinson) Fosberg (Moraceae) (Ragone 2001). Genetic diversity in populations of triploid Tulipa riparia indicates the haphazard occurrence of seed reproduction, though crossbreeding may probably happen only between different clones. Low pollen germination on stigmas and arrest of pollen tube growth in pistil columns indicate self-incompatibility of the triploid clone of G. tenuis, which could be the cause of its sterility.
The clone size and possible mechanisms of dispersal
The sterile triploid clone of G. tenuis is found over a distance of about 800 km from population No. 4 in Spassky District of the Tatarstan Republic in the North to population No. 10 in the Southeast of the Orenburg Region. Such a wide distribution is not unusual in clonal plants. For example, the clone of Gagea spathacea (Liliaceae) has spread over great distances in Europe (Pfeiffer et al. 2012), propagating by means of small underground bulbils. Pfeiffer et al. (2012) found that all the samples collected from locations up to 1500 km apart were genetically identical as to their AFLP profiles and DNA sequences.
There are two possible complementary mechanisms for spatial expansion of the G. tenuis vegetative propagules. The hydrochory is one of them, enabling the transfer of daughter corms by streams of water during heavy rains or floods. Another way of distribution of vegetative propagules depends on the activity and migrations of animals. Small mammals like field voles and zokors collect bulbs and corms of Tulipa, Gagea, and Crocus (Sludskiy 1978) and enable their local dispersal. It seems probable that the daughter corms of the gladioli can also be dispersed by the local activity of small mammals. Propagules can also spread over greater distances on the fur of larger mammals, as was shown for subsidiary bulbs of Gagea spathacea dispersed by boars (Pfeiffer et al. 2011). Boars could play a similar role in the distribution of daughter corms of Gladiolus, which may adhere to their fur. In late Pleistocene to early Holocene periods, other large mammals such as mammoths, whose fossils are known from late Pleistocene sediments between the Volga River and the Ural River, as well as in the Ural River valley (Smirnova 2004), could have participated in spreading the clone of G. tenuis.
Comparison of the results obtained by isozyme and AFLP analyses
The samples from the seven populations of G. tenuis collected to the east of the Volga River appeared to be completely identical in nine isozyme polymorphic loci. However, as was previously mentioned, isozyme analysis is not very sensitive. Besides, this method does not allow analysis of the allelic composition in polyploids and, consequently, the genetic structure of a population. AFLP method allows analysis of polyploids, as ploidy does not affect the interpretation of the results. AFLP data often show greater polymorphism in clonal plant populations compared to isozyme markers. AFLP analysis can identify a large number of highly reproducible markers, overcoming the problems inherent in both isozyme and RAPD methods, and is, therefore, particularly suitable for obtaining individual “fingerprints.” However, AFLP fingerprints may not be absolutely identical even between two replicates of the same plant or a genet.
Therefore, a certain threshold of similarity between clones needs to be established for a particular study. To calculate this, various factors such as the biological features of a species and the sensitivity of the method may be taken into account. In various studies, thresholds of similarity for clonal populations are in the range of 0.95–1.00, most often being set to 0.98 (Arens et al. 1998; Douhovnikoff and Dodd 2003). Perhaps, this is the reason for differences identified in our samples from the group of triploid populations, where genetic distances range from 0.002 to 0.010. Population No. 7 from Southwest Bashkortostan seems to be an exception, since its distance from other populations of the clone varies from 0.029 to 0.036. This may be due to a somatic mutation that occurred in this population. Pfeiffer et al. (2012) explain small differences in the AFLP profile and sequences of several samples from the giant clone of Gagea spathacea by somatic mutations.
Genetic differentiation between populations and species in Gladiolus
Bayesian analyses in STRUCTURE and principal component analysis all suggest considerable differentiation of the sterile triploid population from the fertile tetraploid. The distances between the three sexual and the seven clonal populations of G. tenuis are similar to those between them and G. imbricatus (i.e., out-group). The gene flow between populations of G. tenuis is estimated as very low, about 0.116 or one-tenth individual per generation, and probably only reflects the existing gene exchange between sexual populations. Bayesian analyses in STRUCTURE 2.2 in all the runs under the admixture model revealed three genetic groups (K = 3) with maximum posterior probability. These three identified clusters clearly correspond to the two groups of G. tenuis (sexual and clonal) and to G. imbricatus.G. tenuis and G. imbricatus have a number of morphological differences and are regarded as two separate species in the World Checklist (Govaerts and Barker 2014). However, some authors do not recognize G. tenuis as an independent species lumping it with G. imbricatus (Mayewsky 1964, 2003; Komarov 1935). According to our results, the recognition of these species is correct, as there are significant genetic differences between them.
Conclusions
It appears that Gladiolus tenuis represents quite an unusual phenomenon, the transition of some populations of the species to a triploid level accompanied by a complete loss of sexual propagation and the formation of an extensively distributed (about 800 km) vegetative clone. High genetic similarity of the clonal populations leads us to propose that the transition from tetraploid to triploid level occurred only once. Since then, the triploid sterile genotype seems to have spread widely via vegetative propagation. Genetic differences between the sexual tetraploid and the clonal triploid populations of G. tenuis are quite significant and comparable to those between the two closely related species G. tenuis and G. imbricatus. The genome reorganization that occurred in the triploid may be the reason for such a difference. More research is needed to understand the origin of the triploid clone of G. tenuis and the mechanisms of its spread and survival.
References
Adeleke MTV, Pillay M, Bosa E (2004) Relationships between meiotic irregularities and fertility in diploid and triploid Musa L. Cytologia 69:387–393
Arens P, Coops H, Jansen J, Vosman B (1998) Molecular genetic analysis of black poplar (Populus nigra L.) along Dutch rivers. Molec Ecol 7:11–18. doi:10.1046/j.1365-294x.1998.00316
Devey ME, Bell JS, Smith DN, Neale DB, Moran GF (1996) A genetic linkage map for Pinus radiata based on RFLP, RAPD and microsatellite markers. Theor Appl Genet 92:673–679. doi:10.1007/BF00226088
Dorken ME, Eckert CG (2001) Severely reduced sexual reproduction in northern populations of a clonal plant, Decodon verticillatus (Lythraceae). J Ecol 89:339–350. doi:10.1046/j.1365-2745.2001.00558
Douhovnikoff V, Dodd RS (2003) Intra-clonal variation and a similarity threshold for identification of clones: application to Salix exigua using AFLP molecular markers. Theor Appl Genet 106:1307–1315. doi:10.1007/s00122003-1200-9
Eckert CG (2002) The loss of sex in clonal plants. Evol Ecol 15:501–520. doi:10.1023/A:1016005519651
Eckert CG, Massonnet B, Thomas JJ (2000) Variation in sexual and clonal reproduction among introduced populations of flowering rush, Butomus umbellatus (Butomaceae). Canad J Bot 78:437–446. doi:10.1139/b00-019
Eckert CG, Lui K, Bronson K, Corradini P, Bruneau A (2003) Population genetic consequences of extreme variation in sexual and clonal reproduction in an aquatic plant. Molec Ecol 12:331–344. doi:10.1046/j.1365-294X.2003.01737
Ellstrand NC, Roose ML (1987) Patterns of genotypic diversity in clonal plant species. Amer J Bot 74:123–131. doi:10.2307/2444338
Falush D, Stephens M, Pritchard JK (2007) Inference of population structure using multilocus genotype data: dominant markers and null alleles. Molec Ecol Notes 7:574–578. doi:10.1111/j.1471-8286.2007.01758
Farco GE, Dematteis M (2014) Meiotic behavior and pollen fertility in triploid and tetraploid natural populations of Campuloclinium macrocephalum (Eupatorieae, Asteraceae). Pl Syst Evol 300:1843–1852. doi:10.1007/s00606-014-1011-2
Fedorov AA (ed) (1969) Chromosome numbers of flowering plants [In Russian]. Nauka, Leningrad
Gabrielsen TM, Brochmann C (1998) Sex after all: high levels of diversity detected in the arctic clonal plant Saxifraga cernua using RAPD markers. Molec Ecol 7:1701–1708. doi:10.1046/j.1365-294x.1998.00503
Goldblatt P, Takei M, Razzaq ZA (1993) Chromosome cytology in tropical African Gladiolus (Iridaceae). Ann Missouri Bot Gard 80:461–470
Govaerts R, Barker C (2014) Gladiolus. World checklist of selected plant families. Royal Botanic Gardens, Kew: Retrieved 2014-06-13. http://apps.kew.org/wcsp/qsearch.do
Hamrick JL, Godt MJW (1989) Allozyme diversity in plant species. In: Brown AHD, Clegg MT, Kahler AL, Weir BS (eds) Plant population genetics, breeding and genetic resources. Sinauer Associates, Sunderland, Massachusetts
Hollingsworth ML, Bailey JP (2000) Evidence for massive clonal growth in the invasive weed Fallopia japonica (Japanese Knotweed). Bot J Linn Soc 133:463–472. doi:10.1111/j.1095-8339.2000.tb01589.x
Jensen WA (1962) Botanical Histochemistry: Principle and Practice. Freeman and Company, San Francisco
Kapralov MV, Gabrielsen TM, Sarapultsev IE, Brochmann C (2006) Genetic enrichment of the arctic clonal plant Saxifraga cernua at its southern periphery via the alpine sexual Saxifraga sibirica. Molec Ecol. 15:3401–3411. doi:10.1111/j.1365-294X.2006.03024.x
Komarov VL (ed) (1935) Flora of the USSR, vol 4 [In Russian]. Publishing House of Academy of Sciences of the USSR, Leningrad
Lynch AJ, Barnes RW, Cambecedes J, Vaillancourt RE (1998) Genetic evidence that Lomatia tasmanica (Proteaceae) is an ancient clone. Austral J Bot 46:25–33. doi:10.1071/BT96120
Mayewsky PF (1964) Flora of the middle belt of the European part of the USSR [In Russian]. Kolos, Leningrad
Mayewsky PF (2006) Flora of the middle zone of the European part of the USSR [In Russian]. Association of Scientific Publications KMK, Moscow
Meirmans PG, Den Nijs JC, Van Tienderen PH (2006) Male sterility in triploid dandelions: asexual females vs. asexual hermaphrodites. Heredity 96:45–52. doi:10.1038/sj.hdy.6800750
Mueller UG, Wolfenbarger L (1999) AFLP genotyping and fingerprinting. Trends Ecol Evol 14:389–394
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Molec Ecol Notes 6:288–295. doi:10.1111/j.1471-8286.2005.01155
Peterson A, Harpke D, Peruzzi L, Tison JM, John H, Peterson J (2010) Gagea bohemica (Liliaceae), a highly variable monotypic species within Gagea sect. Didymobulbos. Plt Biosyst 144:308–322. doi:10.1080/11263500903374625
Pfeiffer T, Klahr A, Heinrich A, Schnittler M (2011) Does sex make a difference? Genetic diversity and spatial genetic structure in two co-occurring species of Gagea (Liliaceae) with contrasting reproductive strategies. Pl Syst Evol 292:189–201. doi:10.1007/s00606-010-0404-0
Pfeiffer T, Klahr A, Peterson A, Levichev IG, Schnittler M (2012) No sex at all? Extremely low genetic diversity in Gagea spathacea (Liliaceae). Flora 207:372–378. doi:10.1016/j.flora.2012.03.002
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Ragone D (2001) Chromosome number and pollen stainability of three species of Pacific Island breadfruit (Artocarpus, Moraceae). Amer J Bot 88:693–696
Samils B, Lagercrantz U, Lascoux M, Gullberg U (2001) Genetic structure of Melampsora epitea populations in Swedish Salix viminalis plantations. Eur J Pl Pathol 107:399–409
Satina S, Blakeslee AF (1937) Chromosome behavior in triploid Datura stramonium. I. The male gametophyte. Amer J Bot 24:518–527
Semerikov VL, Belyaev AY, Lascoux M (2002) The origin of Russian cultivars of red clover (Trifolium pratense L.) and their genetic relationships to wild populations in the Urals. Theor Appl Genet 106:127–132
Slatkin M, Barton NH (1989) A comparison of three indirect methods of estimating average levels of gene flow. Evolution 43:1349–1368
Sludskiy AA (ed) (1978) Mammals of Kazakhstan. Rodents (gerbils, voles, the Siberian zokor), vol 1, part 3 [In Russian]. Nauka, Alma-Ata
Smirnova OV (ed) (2004) East European forests: history in the Holocene and the present, vol 1 [In Russian]. Nauka. Moscow
Storz JF (2005) Using genome scans of DNA polymorphism to inter adaptive population divergence. Molec Ecol 14:671–688. doi:10.1111/j.1365-294X.2004.02437.x
Tsujimura N, Ishida K (2008) Isozyme variation under different modes of reproduction in two clonal winter annuals, Sedum rosulato-bulbosum and Sedum bulbiferum (Crassulaceae). Pl Spec Biol 23:71–80. doi:10.1111/j.14421984.2008.00215
Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucl Acids Res 23:4407–4414
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370. doi:10.2307/2408641
Acknowledgments
We would like to thank the staff, especially Svetlana Semerikova and Vladimir Semerikov, of the Molecular Ecology of Plants Lab of the Institute of Plant and Animal Ecology where we carried out the analyses. We also thank Galina Klinkova (Volgograd State Teaching University), Mikhail Knyasev and Evgeniy Philippov (Botanical Garden RAS, Yekaterinburg) for their help in collecting the samples and Ivan Schanzer (MHA, Moscow), Keith Chamberlain (Rothamsted Research, UK), and Irina Belyaeva (Kew Gardens, UK) for editing the English translation and helpful comments. The work was supported by Act 211 Government of the Russian Federation, Agreement No. 02.A03.21.0006.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Mark Mort.
Rights and permissions
About this article
Cite this article
Kutlunina, N., Permyakova, M. & Belyaev, A. Genetic diversity and reproductive traits in triploid and tetraploid populations of Gladiolus tenuis (Iridaceae). Plant Syst Evol 303, 1–10 (2017). https://doi.org/10.1007/s00606-016-1347-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-016-1347-x