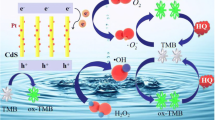
Abstract
The peroxidase-like activity of CdV2O6 nanorods has been considerably improved by modification with N, N-dicarboxymethyl perylene-diimide (PDI) as a photosensitizer. The peroxidase-like behaviors are evaluated by virtue of the colorless chromogenic substrate 3,3',5,5'-tetramethylbenzidine (TMB), which is fast changed into blue oxTMB in the presence of H2O2 in only 90 s. PDI-CdV2O6 exhibits high stability at elevated temperatures and PDI-CdV2O6 retains more than 70% of its catalytic activity over a wide range of 15 to 60 °C. The catalytic mechanism of PDI-CdV2O6 is ascribed to the synergistic interaction between PDI and CdV2O6 and the generation of •O2− radicals. Based on the enhanced peroxidase-like activity of PDI-CdV2O6, a selective colorimetric sensor has been constructed for H2O2 and pyrogallol (PG) with detection limits of 36.5 μM and 0.179 μM, respectively. The feasibility of the proposed sensing platform has been validated by detecting H2O2 in milk and pyrogallol in tap water.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural enzymes have been widely studied and applied owing to their high catalytic efficiency and substrate specificity [1]. However, they usually face with some disadvantages of high extraction and purification cost, low yield and strict storage conditions, restricting their practical application [2]. As we know, artificial nanozymes can overcome such shortcoming of natural enzymes. Thereof, nanozymes have aroused considerable interests [3]. Over the past decades, scientists have prepared numerous nanomaterials including metal oxides [4], metal sulfides [5], noble metals [6], carbon-based materials [7] and metal–organic frameworks [8]. However, how to improve the catalytic activity of nanozymes is an urgent task. Scientists have put forward different strategies for improving the catalytic activity of nanozymes. For example, various composites with the high activity have been prepared, such as Co-CeO2 [9], AgNPs@rGO [10], and Fe3O4@MoS2-Ag [11], etc. What’s more, to solve the agglomeration of inorganic nanoparticles, some supports were employed to disperse nanoparticles to expose more active sites and then improve the corresponding catalytic activity [12, 13]. Interestingly, some organic conjugate macromolecules as photosensitizers were used to modify the nanomaterials with semiconductivity [14, 15]. The synergistic effect between photosensitizers and nano-semiconductors would enhance the corresponding catalytic activity. Especially, Perylene diimide (PDI), as one of typical photosensitive molecules, was used to modify some inorganic nanomaterials to widen the absorption in visible region and widely used in organic photovoltaic [16] and electronic fields [17].
Pyrogallol (1,2,3-trihydroxybenzene, PG), a derivative of phenolic compounds, is widely used in pharmaceutical, cosmetic, plastic and other industries [18]. Also, PG is commonly used as an antioxidant and scavenger of reactive oxygen species, due to intrinsic reductive properties. Because of the wide application of PG in industry, it will inevitably cause pollution to the aqueous environment [19]. Therefore, it is necessary to develop a fast cheap convenient method for determination of PG. Until now, many methods including chromatographic [20], electrochemical [21], and chemiluminescence have been developed to detect PG [22]. Although the other methods (such as chromatography or electrochemistry) are simple and inexpensive and can be routinely performed by technicians, it is not met requirements for spot testing. Thus, the colorimetric method based on the artificial peroxidase mimics (one of nanozymes) is a good substitute. However, the nanozyme-based colorimetric method for determining PG requests for some highly active nanozymes. Based on the above strategies of the catalytic activity of artificial nanozymes, an organic molecule with the larger conjugate structure, N, N-dicarboxymethyl perylene-diimide (PDI), can be used as a functional molecule to modify inorganic nanomaterials to obtain a nanozyme with the enhanced catalytic activity. Considering the rich chemical valences of V, CdV2O6 nanorods are expected to possess the peroxidase-like activity. Thus, if PDI molecules are used to modify CdV2O6, the peroxidase-like activity of PDI-CdV2O6 composites will be greatly enhanced. To the best of our knowledge, there are few reports on the determination of PG by colorimetric method based on PDI-CdV2O6 nanozyme.
Herein, CdV2O6 nanorods were firstly found to possess the peroxidase-like activity. Furthermore, a conjugate organic molecule, PDI was used to modify CdV2O6 nanorods to improve the catalytic activity and robust stability greatly. As expected, the peroxidase-like activity and robust stability of PDI-CdV2O6 were indeed higher than that of pure CdV2O6. Notably, PDI-CdV2O6 as the artificial nanozyme can effectively catalyze the chromogenic substrate TMB to be oxidized by H2O2 only in 90 s, and remains more than 70% catalytic activity over a wide range of 15 to 60 °C. The catalytic mechanism was investigated by free radical trapping and fluorescence experiments, respectively. Thus, a fast cheap colorimetric platform based on PDI-CdV2O6 nanorods was established for H2O2 and PG determination.
Experimental section
Materials
NH4VO3, CdCl2•2.5H2O, Ethylenediaminetetraacetic acid disodium salt (EDTA), isopropanol (IPA), terephthalic acid (TA), p-benzoquinone (PBQ), H2O2 (30 wt%), NaCl, BaCl2, KCl and urea, were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Pyrogallol (PG) was acquired from Shanghai Ekear Biotechnology Co., Ltd. 3,3,5,5-Tetramethylbenzidine (TMB), D-Histidine (His), D-Serine (Ser), L-Arginine (Arg), lactose, leucine, fructose and sucrose were purchased from Sigma-Aldrich Co., LLC. N, N-dicarboxymethyl Perylene-diimide (Fig. S1) was synthesis according the previous report [23].
Instruments
Details about instruments are presented in supporting information.
Preparation
Preparation of CdV2O6
The CdV2O6 nanorods was synthesized according to previous reports [24]. The detailed procedure is presented in supporting information.
Preparation PDI-CdV2O6
2 mg PDI was dissolved in 10 mL deionized water, and 100 mg CdV2O6 was added into 80 mL deionized water. Take 2 mL 0.4 mM PDI sample add to CdV2O6 solution. After 30 min of ultrasound at room temperature, the reaction system was moved into a 100 mL of autoclave and maintained for 2 h at 110 °C. Then, the precipitate was collected by centrifugation (10,000 r/min, 11,180 g) and washed with water and ethanol for several times. Finally, the product was dried overnight at 60 °C. Thus, PDI-CdV2O6 were successfully prepared.
Assay of the catalytic mechanism
To study the generation of active free radicals, holes (h+), superoxide radicals (•O2−), and hydroxyl radicals (•OH) are scavenged by EDTA, PBQ and IPA, respectively. Specifically, adding 200 μL scavengers into TMB-H2O2 reaction systems reacted for 90 s under the optimal conditions, and then recording the absorbance at 652 nm.
Fluorescent experiments were implemented by using TA (hydroxyterephthalic acid) as a fluorescence probe to further explore whether hydroxyl radicals (•OH) was produced. TA could easily react with •OH to form a high fluorescence product, dihydroxyterephthalic acid (HOTA). Specifically, phosphate buffer (pH = 4.0), the aqueous solution of PDI-CdV2O6 with different concentration (0.1–0.8 mg/mL), 25 mM H2O2 and fresh TA (5 mM) were added into quartz cuvette. After reacted for 30 min, the fluorescence spectra were reported. The fixed excitation wavelength is 315 nm, and the strongest signal appears around 440 nm.
The detailed experiments
Assays of the peroxidase-like activity, steady-state kinetics of PDI-CdV2O6 and colorimetric determination of H2O2 and PG, are provided in the supporting information.
Determination of H2O2 and PG in real samples
Determination of H2O2 in milk samples
Milk was purchased from our local supermarket (Qingdao, China). First of all, 5 mL milk was diluted to 50 mL with deionized water and centrifuged at 10,000 rpm to eliminate the organic components. Next, the supernatant was filtrated through a 0.45 µm filtered membrane and then different concentrations of H2O2 (200 μL) were added to the supernatant. Subsequently, 200 μL of 1 mM TMB was added to the reaction system containing buffer, PDI-CdV2O6 and the milk sample. After incubated for 90 s at the optimal temperature (45 oC), the absorbance (652 nm) of solutions was reported on a UV–vis spectrophotometer.
Determination of PG in tap water
The tap water, from our laboratory from Shandong University of Science and Technology, was treated by being centrifuged (10,000 r/min) and filtered through a 0.2 μm membrane. Subsequently, different concentrations of PG solutions were added into the treated tap water. The standard addition method was used to evaluate the practicability of the method.
Results and discussion
PDI-CdV2O6 characterization
Figure 1a shows the X-ray diffraction (XRD) spectra of CdV2O6-PDI and CdV2O6 nanorods, respectively. The characteristic planes (-201), (110), (-202), (201), (111), (-311), (-310), (400), (003), (311), (-403), (020), (-204), (203) are attributable to CdV2O6 (JCPDS card no. 22–0134). Notably, the diffraction peaks of PDI-CdV2O6 are similar to those of CdV2O6, suggesting that the introduction of PDI hardly affect the crystalline structure of CdV2O6.
The surface compositions of PDI-CdV2O6 were explored by the X-ray photoelectron spectroscopy (XPS), displayed in Fig. 1b. The total XPS spectrum reveals the presence of elements Cd, V, O and N. In the Cd 3d spectra (Fig. 1c), the peaks located at 411.3 and 404.6 eV are well corresponding to Cd 3d3/2 and Cd 3d5/2. In Fig. 1d, there are two peaks at 524.30 and 516.9 eV corresponding to the V 2p1/2 and V 2p2/3, respectively. In addition, the O 1 s spectrum (Fig. 1e) exhibits three peaks at 529.67, 530.8 and 532.28 eV, which can be assigned to the lattice oxygen, oxygen vacancy and absorbed water [25], respectively. Specially, the existence of oxygen vacancy is very important for improving the peroxidase-like activity of PDI-CdV2O6. Notably, in the N 1 s spectrum (Fig. 1f), the peaks at 399.8 eV can be assigned to − N − C bonds of PDI, verifying PDI molecules are successfully modified on CdV2O6 nanorods.
FT-IR spectra (Fig. S2) was further confirm that the introduction of PDI molecule on CdV2O6 and the coordination interaction between them. Figure 2 shows SEM and TEM images of CdV2O6 and PDI-CdV2O6, respectively. From Fig. 2a and 2c, the prepared CdV2O6 exhibits the rod-like structure with the diameter range of 50–200 nm and an average length of 1 μm (± 0.245). Also, compared with that of CdV2O6, the morphology of PDI-CdV2O6 nanorods has rarely significantly changed (Fig. 2b and 2d), because of a small amount of PDI molecules introduced.
Peroxidase-like behaviors of PDI-CdV2O6
The potential peroxidase-like activity of the PDI-CdV2O6 nanorods were investigated by using TMB-H2O2 reaction systems. The visible absorption spectra were recorded in the wavelength range of 500–800 nm. As presented in Fig. 3, no obvious color change of four systems (system a, b, c and d) is found, while the experimental system e (CdV2O6 + H2O2 + TMB) appears a light blue color, indicating that CdV2O6 possesses a weak peroxidase-like activity. Interestingly, an obvious blue change can be observed in system f, suggesting that PDI-CdV2O6 possesses a stronger peroxidase-like activity than that of CdV2O6. That is to say, the peroxidase-like activity of CdV2O6 is indeed greatly enhanced by the introduction of PDI molecules, ascribed to the synergistic interaction between PDI and CdV2O6.
Optimal conditions
The catalytic activity of natural peroxidase (HRP) and reported artificial peroxidases were easily affected by pH and temperature. Thus, we studied comparatively the influences of pH and temperature on the catalytic activity of CdV2O6 before and after introduction of PDI (Fig. S3 and S4, number of experiment n = 3). The optimal conditions are pH = 4 and 45 °C. Relevant experimental results are discussed in the supporting information.
Steady-state kinetics
To further investigate the peroxidase-like behaviors of PDI-CdV2O6, steady-state kinetics experiments were carried out. Fig. S5a and S5b show the typical Michaelis–Menten plots towards substrates TMB and H2O2, respectively. The kinetic parameters (Km and Vmax) were calculated and listed in Table 1, according to the double-reciprocal linear Weaver Burk plots (Fig. S5c and S5d). As we know, Km is associated with the affinity between peroxidase mimics and substrates. As shown in Table 1, the Km values of PDI-CdV2O6 nanorods with TMB and H2O2 are 0.129 and 2.053 mM, respectively, much lower than that of HRP [26] and other artificial enzymes. Specifically, the Km value of PDI-CdV2O6 nanorods for TMB is 62 times lower than that of AgVO3 [27], 10 times lower than that of NiV2O6 [28] and 6.5 times lower than that of ZnFeO4 [29], suggesting a much stronger affinity of PDI-CdV2O6 towards TMB.
Catalytic mechanism
According to the previous studies, one of the catalytic mechanisms of artificial peroxidase mimics is ascribed to active species. Above all, to determine whether •OH occurred in the catalytic reaction, TA was selected as a fluorescence probe, which can easily react with •OH to produce a remarkable fluorescent hydroxy terephthalate acid. As seen from Fig. 4a, the fluorescence intensity decreases with increasing of the amount of PDI-CdV2O6, indicating no •OH production during the catalytic reaction.
Furthermore, active free radicals capture experiments were carried out by using PBQ, EDTA and IPA as scavengers to capture •O2−, h+ and •OH, respectively. As seen from Fig. 4b, the catalytic activity of PDI-CdV2O6 is decreased apparently after adding PBQ and decreased slightly after adding EDTA, while IPA have negligible effect during the catalytic reaction. Thereof, •O2− can be verified to be the primary active species during the catalytic reaction in the presence of PDI-CdV2O6.
To further explore the catalytic mechanism, the electron transport direction between CdV2O6 and PDI is obtained by measuring the forbidden bandwidth (Eg) and valence band (VB) of CdV2O6 with UV–vis diffuse reflection and XPS. Eg and VB value of CdV2O6 are determined to be 2.53 and 2.14 eV (Fig. 5c and 5d), as well as the conduction band (CB) is calculated to be -0.39 eV.
(a) A dose–response plot depending of the absorbance at 652 nm on the concentration of H2O2 from 0.05 mM to 10 mM; (b) The corresponding linear calibration plot of H2O2; (c) A dose–response plot depending on the absorbance of ox-TMB at 652 nm on the concentration of PG from 1 μM to 80 μM; (d) The corresponding linear calibration plot of PG
Based on the experimental data above, the catalytic mechanism of PDI-CdV2O6 was proposed (Scheme 1). As a typical photosensitizer, PDI molecules have relatively strong absorption in the visible region. Initially, the CB of CdV2O6 is higher than that of PDI, so electrons can be transferred from CdV2O6 to PDI under the irradiation of natural light, forming an electric field and facilitating the separation of electron and hole. The holes in VB of PDI with strong oxidation ability are transferred to CdV2O6 to further oxidize H2O2, generating H2O and O2. Thus, because a lot of oxygen vacancies existed in PDI-CdV2O6, the transferred electrons are captured by oxygen dissolved in the solution, and accelerates the generation of reactive oxygen (•O2−), which can easily catalyze the substrate TMB into blue oxTMB. The reducibility of pyrogallol with different concentration can fade the blue color of oxTMB to different extent. Thus, the concentration of pyrogallol can be determined, according to the intensity of absorption of oxTMB at 652 nm.
Determination of H2O2 and PG
Based on the excellent peroxidase-like activity of PDI-CdV2O6, a facile colorimetric sensing platform was established to determination H2O2 and PG, respectively. From Fig. 5a, it can be found that the absorbance increases gradually as increasing the concentration of H2O2. Figure 5b shows a good linear correlation between the absorbance and H2O2 concentration in the range of 50–100 μM (R2 = 0.9993) and the detection limit (LOD) is calculated to be 36.5 μM (LOD = 3 s/k). The comparison of LOD with other sensors based on different nanomaterials (PA/Cu3(PO4)2•3H2O [30], TPyP-CuS [31] NiO/a-Fe2O3 [32], CuS [33], PDDA-AgNPs [34] and Ir NPs [35]), shown in Table S1.
Pyrogallol, one of the phenol hydroxyl groups, as a reductant molecule, can significantly inhibit the oxidation of TMB and make the blue color of ox-TMB faded. Based on this theory, a fast cheap colorimetric method was used to determination pyrogallol. Figure 5c shows a typical dose-respond plot of the absorbance at 652 nm and the concentration of PG from 1 to 80 μM. Figure 5d shows the linear range of PG deterimination in the range of 1–10 μM. The detection limit is calculated as 0.179 μM, which was 55 times, 10 times and 2 times lower than that of reported colorimetric sensors based nanozymes including Ch-Ag NPs [36], Cu,N@C-dots [37] and Au-NPs [38], respectively. Our determination limit was even lower than that of some electrochemical assays (Preanodized SPCE [39], CoPc/SPCE [40]) known as the high sensitivity, as well as slightly higher than that of chemiluminescence method (N-CDs[18]), listed in Table 2. Therefore, the constructed colorimetric sensor of PG exhibited a good sensitivity.
The selectivity of this method for H2O2 and PG sensing
The selectivity is important to evaluate the anti-interference performance of the constructed colorimetric sensor. Thereof, different interfering substances including Na+, K+, Ba2+, Suc, Lac, Fru, Arg, Ser, His, UA and Leu were added into the catalytic systems, respectively. From Fig. 6a and 6b, absorbance difference value ΔA (ΔA = Ablank – Asub) of two systems containing H2O2 and PG are much higher than that of other interferents, even though the concentrations of interfering substances are 10 times that of H2O2 and 100 times that of PG, respectively. As seen from Fig. S6, the good selectivity of the colorimetric method in determination of H2O2 and pyrogallol.
Furthermore, the stability of CdV2O6 and PDI-CdV2O6 were investigated by centrifugally recycling and testing the peroxidase-like activity (Fig. S7). The relative activity of PDI-CdV2O6 remains more than 75% after reusing for six times, while CdV2O6 remains less than 40%. It is suggested that the stability of CdV2O6 has been improved by introducing PDI molecules as well as the colorimetric sensing platform based on PDI-CdV2O6 exhibits stable performance.
Determination of H2O2 and PG in real samples
The practicability of the established colorimetric sensors was investigated by detecting for H2O2 in milk and PG in tap water (Table S2 and S3). As can be seen from these Tables, the recovery of H2O2 and PG is 98.90–102.94% and 103.68–105.07%, and the relative standard deviation (RSD) is calculated below 1.79% and 1.34% for H2O2 and PG, respectively. It is indicated that PDI-CdV2O6-based colorimetric sensors of H2O2 and PG have the potential application in determining of real samples.
Conclusions
In a word, the peroxidase-like activity and the robust stability of CdV2O6 have been significantly increased via modification with PDI molecules. PDI-CdV2O6 nanorods rapidly catalyze the oxidation of TMB in the presence of H2O2 only in 90 s. The peroxidase-like activity of PDI-CdV2O6 remains more than 70% catalytic activity over a wide range of 15 to 60 °C, ascribed to the synergetic effect between CdV2O6 and PDI. Based on the excellent catalytic activity of PDI-CdV2O6 nanozyme, a rapid colorimetric sensor for determination of H2O2 and PG has been constructed and has been successfully used in determination of H2O2 in milk and PG in tap water. This constructed colorimetric sensor has the potential application in fields of food, nanotechnology, medicine and environment.
Data Availability
Data openly available in a public repository.
References
Song C, Ding W, Zhao W, Liu H, Wang J, Yao Y, Yao C (2020) High peroxidase-like activity realized by facile synthesis of FeS2 nanoparticles for sensitive colorimetric detection of H2O2 and glutathione. Biosens Bioelectron 151:111983. https://doi.org/10.1016/j.bios.2019.111983
Li N, Liu M, Ma Y, Chang Q, Wang H, Li Y, Zhang H, Liu B, Xue C, Hu S (2021) Molybdenum Selenide/Porous Carbon Nanomaterial Heterostructures with Remarkably Enhanced Light-Boosting Peroxidase-like Activities. ACS Appl Mater Interfaces 13:54274–54283. https://doi.org/10.1021/acsami.1c16569
Li R, Zhou Y, Zou L, Li S, Wang J, Shu C, Wang C, Ge J, Ling L (2017) In situ growth of gold nanoparticles on hydrogen-bond supramolecular structures with high peroxidase-like activity at neutral pH and their application to one-pot blood glucose sensing. Sens Actuators, B Chem 245:656–664. https://doi.org/10.1016/j.snb.2017.01.141
Chen M, Sun L, Ding Y, Shi Z, Liu Q (2017) N, N′-Di-carboxymethyl perylene diimide functionalized magnetic nanocomposites with enhanced peroxidase-like activity for colorimetric sensing of H2O2 and glucose. New J Chem 41:5853–5862. https://doi.org/10.1039/c7nj00292k
Feng L, Zhang L, Chu S, Zhang S, Chen X, Du Z, Gong Y, Wang H (2022) Controllable doping of Fe atoms into MoS2 nanosheets towards peroxidase-like nanozyme with enhanced catalysis for colorimetric analysis of glucose. Appl Surf Sci 583:152496. https://doi.org/10.1016/j.apsusc.2022.152496
Liang Y, Li H, Fan L, Li R, Cui Y, Ji X, Xiao H, Hu J, Wang L (2022) Zwitterionic daptomycin stabilized palladiumnanoparticles with enhanced peroxidase-like properties for glucose detection. Colloids and Surfaces A: Physicochemical and Engineering Aspects 633:127797. https://doi.org/10.1016/j.colsurfa.2021.127797
Wang Y, Liu X, Wang M, Wang X, Ma W, Li J (2021) Facile synthesis of CDs@ZIF-8 nanocomposites as excellentperoxidase mimics for colorimetric detection of H2O2 and glutathione. Sens Actuators B: Chemi 329:129115. https://doi.org/10.1016/j.snb.2020.129115
Li J, Zhao J, Li S, Chen Y, Lv W, Zhang J, Zhang L, Zhang Z, Lu X (2021) Synergistic effect enhances the peroxidase-like activity in platinum nanoparticle-supported metal-organic framework hybrid nanozymes for ultrasensitive detection of glucose. Nano Res 14:4689–4695. https://doi.org/10.1007/s12274-021-3406-z
Nguyen PT, Lee J, Cho A, Kim MS, Choi D, Han JW, Kim MI, Lee J (2022) Rational development of co‐doped mesoporous ceria with high peroxidase‐mimicking activity at neutral ph for paper‐based colorimetric detection of multiple biomarkers. Adv Funct Mater 32:2112428. https://doi.org/10.1002/adfm.202112428
Tran HV, Nguyen ND, Tran CTQ, Tran LT, Le TD, Tran HTT, Piro B, Huynh CD, Nguyen TN, Nguyen NTT, Dang HTM, Nguyen HL, Tran LD, Phan NT (2020) Silver nanoparticles-decorated reduced graphene oxide: A novel peroxidase-like activity nanomaterial for development of a colorimetric glucose biosensor. Arab J Chem 13:6084–6091. https://doi.org/10.1016/j.arabjc.2020.05.008
Wei F, Cui X, Wang Z, Dong C, Li J, Han X (2021) Recoverable peroxidase-like Fe3O4@MoS2-Ag nanozyme with enhanced antibacterial ability. Chem Eng J 408:127240. https://doi.org/10.1016/j.cej.2020.127240
Zhu X, Li H, Zhang D, Chen W, Fu M, Nie S, Gao Y, Liu Q (2019) Novel “On–Off” Colorimetric Sensor for Glutathione Based on Peroxidase Activity of Montmorillonite-Loaded TiO2 Functionalized by Porphyrin Precisely Controlled by Visible Light. ACS Sustainable Chemistry & Engineering 7:18105–18113. https://doi.org/10.1021/acssuschemeng.9b05146
Zhang L, Chen M, Jiang Y, Chen M, Ding Y, Liu Q (2017) A facile preparation of montmorillonite-supported copper sulfide nanocomposites and their application in the detection of H2O2. Sens Actuators, B Chem 239:28–35. https://doi.org/10.1016/j.snb.2016.07.168
Liu Q, Yang Y, Li H, Zhu R, Shao Q, Yang S, Xu J (2015) NiO nanoparticles modified with 5,10,15,20-tetrakis(4-carboxyl pheyl)-porphyrin: promising peroxidase mimetics for H2O2 and glucose detection. Biosens Bioelectron 64:147–153. https://doi.org/10.1016/j.bios.2014.08.062
Lyu H, Yin D, Zhu B, Lu G, Liu Q-Y, Zhang X, Zhang X (2020) Metal-Free 2(3),9(10),16(17),23(24)-Octamethoxyphthalocyanine-Modified Uniform CoSn(OH)6 Nanocubes: Enhanced Peroxidase-like Activity, Catalytic Mechanism, and Fast Colorimetric Sensing for Cholesterol. ACS Sustainable Chemistry & Engineering 8:9404–9414. https://doi.org/10.1021/acssuschemeng.0c02151
Ding K, Wang Y, Shan T, Xu J, Bao Q, Liu F, Zhong H (2020) Propeller-like acceptors with difluoride perylene diimides for organic solar cells. Organic Electronics 78:105569. https://doi.org/10.1016/j.orgel.2019.105569
Wurthner F, Saha-Moller CR, Fimmel B, Ogi S, Leowanawat P, Schmidt D (2016) Perylene Bisimide Dye Assemblies as Archetype Functional Supramolecular Materials. Chem Rev 116:962–1052. https://doi.org/10.1021/acs.chemrev.5b00188
Shah SN, Li H, Linv JM (2016) vEnhancement of periodate-hydrogen peroxide chemiluminescence by nitrogen doped carbon dots and its application for the determination of pyrogallol and gallic acid. Talanta 153:23–30. https://doi.org/10.1016/j.talanta.2016.02.056
Li Z, Yang Y, Zeng Y, Wang J, Liu H, Guo L, Li L (2017) Novel imidazole fluorescent poly(ionic liquid) nanoparticles for selective and sensitive determination of pyrogallol. Talanta 174:198–205. https://doi.org/10.1016/j.talanta.2017.06.007
Vafaei A, Bin Mohamad J, Karimi E (2019) HPLC profiling of phenolics and flavonoids of Adonidia merrillii fruits and their antioxidant and cytotoxic properties. Nat Prod Res 33:2531–2535. https://doi.org/10.1080/14786419.2018.1448810
Raghu P, Madhusudana Reddy T, Reddaiah K, Jaidev LR, Narasimha G (2013) A novel electrochemical biosensor based on horseradish peroxidase immobilized on Ag-nanoparticles/poly(l-arginine) modified carbon paste electrode toward the determination of pyrogallol/hydroquinone. Enzyme Microb Technol 52:377–385. https://doi.org/10.1016/j.enzmictec.2013.02.010
Mostafa IM, Gilani M, Chen Y, Lou B, Li J, Xu G (2021) Lucigenin-pyrogallol chemiluminescence for the multiple detection of pyrogallol, cobalt ion, and tyrosinase. J Food Drug Anal 29:510–520. https://doi.org/10.38212/2224-6614.3361
Gebers J, Rolland D, Marty R, Suarez S, Cervini L, Scopelliti R, Brauer JC, Frauenrathv H (2015) Solubility and crystallizability: facile access to functionalized pi-conjugated compounds with chlorendylimide protecting groups. Chemistry 21:1542–1553. https://doi.org/10.1002/chem.201403623
Li D, Bai X, Pan C, Zhu Y (2013) Investigations on the Phase Transition between CdV2O6 and Cd2V2O7 and Their Photocatalytic Performances. Eur J Inorg Chem 2013:3070–3075. https://doi.org/10.1002/ejic.201300020
Jia R, Wang Y, Wang C, Ling Y, Yu Y, Zhang B (2020) Boosting Selective Nitrate Electroreduction to Ammonium by Constructing Oxygen Vacancies in TiO2. ACS Catal 10:3533–3540. https://doi.org/10.1021/acscatal.9b05260
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S, Yan X (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2:577–583. https://doi.org/10.1038/nnano.2007.260
Xiang Z, Wang Y, Ju P, Zhang D (2015) Optical determination of hydrogen peroxide by exploiting the peroxidase-like activity of AgVO3 nanobelts. Microchim Acta 183:457–463. https://doi.org/10.1007/s00604-015-1670-x
Zhu Q, Yang J, Peng Z, He Z, Chen W, Tang H, Li Y (2021) Selective detection of glutathione by flower-like NiV2O6 with only peroxidase-like activity at neutral pH. Talanta. 234:122645. https://doi.org/10.1016/j.talanta.2021.122645
Su L, Feng J, Zhou X, Ren C, Li H, Chen X (2012) Colorimetric detection of urine glucose based ZnFe2O4 magnetic nanoparticles. Anal Chem 84:5753–5758. https://doi.org/10.1021/ac300939z
Zhang CY, Zhang H, Yang FQ (2021) Enhanced peroxidase-like activity of copper phosphate modified by hydrophilic phytic-acid and its application in colorimetric detection of hydrogen peroxide. Microchem J168:106489. https://doi.org/10.1016/j.microc.2021.106489
He Y, Li N, Lian L, Yang Z, Liu Z, Liu Q, Zhang X, Zhang X (2020) Colorimetric ascorbic acid sensing from a synergetic catalytic strategy based on 5,10,15,20-tetra(4-pyridyl)-21H,23H-porphyrin functionalized CuS nanohexahedrons with the enhanced peroxidase-like activity. Colloids and Surfaces A: Physicochemical and Engineering Aspects 598:124855. https://doi.org/10.1016/j.colsurfa.2020.124855
Achari DS, Santhosh C, Deivasegamani R, Nivetha R, Bhatnagar A, Jeong SK, Grace AN (2017) A non-enzymatic sensor for hydrogen peroxide based on the use of α-Fe2O3 nanoparticles deposited on the surface of NiO nanosheets. Microchimica Acta 184:3223–3229. https://doi.org/10.1007/s00604-017-2335-8
Guan J, Peng J, Jin X (2015) Synthesis of copper sulfide nanorods as peroxidase mimics for the colorimetric detection of hydrogen peroxide. Anal Methods 7:5454–5461. https://doi.org/10.1039/c5ay00895f
Rivero PJ, Ibañez E, Goicoechea J, Urrutia A, Matias IR, Arregui FJ (2017) A self-referenced optical colorimetric sensor based on silver and gold nanoparticles for quantitative determination of hydrogen peroxide. Sens Actuators, B Chem 251:624–631. https://doi.org/10.1016/j.snb.2017.05.110
Cui M, Zhou J, Zhao Y, Song Q (2017) Facile synthesis of iridium nanoparticles with superior peroxidase-like activity for colorimetric determination of H2O2 and xanthine. Sens Actuators, B Chem 243:203–210. https://doi.org/10.1016/j.snb.2016.11.145
Chen Z, Zhang X, Cao H, Huang Y (2013) Chitosan-capped silver nanoparticles as a highly selective colorimetric probe for visual detection of aromatic ortho-trihydroxy phenols. Analyst 138:2343–2349. https://doi.org/10.1039/c3an36905f
Ali HRH, Hassan AI, Hassan YF, El-Wekil MM (2019) Colorimetric and fluorimetric (dual-mode) nanoprobe for the determination of pyrogallol based on the complexation with copper(II)- and nitrogen-doped carbon dots. Mikrochim Acta 186:850. https://doi.org/10.1007/s00604-019-3892-9
Nezhad MR, Alimohammadi M, Tashkhourian J, Razavian SM (2008) Optical detection of phenolic compounds based on the surface plasmon resonance band of Au nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc 71:199–203. https://doi.org/10.1016/j.saa.2007.12.003
Feng P-S, Wang S-M, Su W-Y, Cheng S-H (2012) Electrochemical Oxidation and Sensitive Determination of Pyrogallol at Preanodized Screen-Printed Carbon Electrodes. J Chin Chem Soc 59:231–238. https://doi.org/10.1002/jccs.201100384
Matemadombo F, Apetrei C, Nyokong T, Rodríguez-Méndez ML, de Saja JA (2012) Comparison of carbon screen-printed and disk electrodes in the detection of antioxidants using CoPc derivatives. Sens Actuators, B Chem 166–167:457–466. https://doi.org/10.1016/j.snb.2012.02.088
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21971152), Shandong Key Laboratory of Biochemical Analysis (SKLBA2207) and the Project of Shandong Province Higher Educational Young Innovative Talent Introduction and Cultivation Team [Nanozymes Biomedical Innovation Team].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Hao, P., Wang, L. et al. N,N-dicarboxymethyl Perylene-diimide-modified CdV2O6 Nanorods for Colorimetric Sensing of H2O2 and Pyrogallol. Microchim Acta 190, 270 (2023). https://doi.org/10.1007/s00604-023-05846-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05846-4