Abstract
Titanium carbide quantum dots (Ti3C2 QDs) were synthesized by ammonia-assisted hydrothermal method. We also synthesized potassium permanganate (KMnO4)–functionalized Ti3C2 QDs (Mn-QDs) by modifying Ti3C2 nanosheets with KMnO4 and then cutting the functional nanosheets into Mn-QDs. The Ti3C2 QDs and Mn-QDs were characterized by fluorescence spectroscopy (FL), Fourier transform infrared spectroscopy (FTIR), UV–vis spectrophotometry (UV–vis), X-ray photoelectron spectroscopy (XPS), and transmission electron microscopy (TEM). Furthermore, the modified Mn-QDs have strong luminescence ability and good dispersion stability, which can be used for Cr3+ and Hg2+ double ion detection with enhanced fluorescence specificity. Cr3+/Hg2+ and negatively charged Mn-QDs are bound together by electrostatic interactions. Meanwhile, the surface of Mn-QDs is rich in functional groups, which interacts with Cr3+/Hg2+ to modify the surface traps, leading to defect passivation and exhibiting photoluminescence enhancement. For the dynamic quenching produced by the interaction of Mn-QDs with Hg2+ within 50 μM, it may be caused by the complex formation of Hg2+ trapped by the amino group on the surface of Mn-QDs. The detection limits for Cr3+ and Hg2+ were 0.80 μM and 0.16 μM, respectively. The recoveries of Cr3+ and Hg2+ ions in real water samples were 93.79–105.10% and 93.91–102.05%, respectively, by standard addition recovery test. In this work, the application of Mn-QDs in Cr3+ and Hg2+ ion detection was researched, which opens a new way for its application in the field of detecting heavy metal ions.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, the environmental pollution caused by the discharge of industrial, agricultural, and household wastes has attracted people’s attention. One of them is the water pollution caused by heavy metal ions. Among various heavy metal ions, chromium (III) ion (Cr3+) plays a leading role in glucose metabolism and lipid metabolism, and is one of the essential trace elements for human body [1]. Deficiency of Cr3+ may lead to different health disorders, including disorders of lipid metabolism and diabetes. Likewise, high concentration levels of Cr3+ can also be harmful to our health and negatively affect cellular structure [2]. Another polluting heavy metal ion is mercury (II) ion (Hg2+). Hg2+ readily penetrates the skin of respiratory and gastrointestinal tissues and causes irreversible damage to the central nervous system, leading to renal failure and various cognitive and motor impairments [3]. Meanwhile, the non-biodegradability and bioconcentration of Hg2+ in the environment bring about various serious diseases, including kidney diseases, skin diseases, and genotoxicity [4]. Even when present in trace amounts, it poses a serious biological threat. In consequence, it is necessary to explore an effective and rapid method to detect Cr3+ and Hg2+ in environmental samples. Until now, researchers have developed various methods for the detection of trace metal ions, such as atomic absorption\emission spectrometry [5], electrochemical techniques [6], and fluorescence spectrophotometry [1]. Among them, the fluorescence method has attracted much attention for its unique advantages such as simplicity, fast response, good selectivity, and high sensitivity.
MXene, whose chemical formula is Mn+1Xn, is a two-dimensional (2D) transition metal carbide or nitride, where M is the early transition metal, including Sc, Ti, and V, and X represents C and/or N. MXene is usually obtained by etching the Mn+1AXn phase (where A usually stands for elements III A or IV A), such as the typical material Ti3AlC2. Due to its abundant active sites, good biocompatibility, chemical stability, and surface hydrophilicity, MXene has attracted the attention of many researchers. It shows very good potential for applications in batteries, supercapacitors, photocatalytic applications, sensors, water treatment, and other fields [7,8,9]. The zero-dimensional (0D) MXene QDs derived from 2D MXene nanosheets (NSs) are able to inherit the advantages of MXene NSs in addition to showing unique optical and optoelectronic properties. It has promising applications in bioimaging, biosensing, antioxidant, antibacterial, ion detection, etc. [10,11,12,13]. Potassium permanganate (KMnO4) is a common oxidant with a wide range of applications. After increasing the interlayer spacing by alkalinizing MXene NSs with potassium hydroxide (KOH), KMnO4 was added and metal ions were further inserted into the interlayer to provide tunable properties. Furthermore, MXene can act as a reducing agent and undergo a redox reaction with KMnO4, and the generated manganese dioxide (MnO2) provides more active sites for the material [14, 15].
In the previous literatures, there are a lot of sensing materials for detecting metal ions. For example, Chen Lin et al. studied the organic framework of double layer interpenetrating double emission luminescent metal as Cr3+ ratio sensor [16]. Yi Zhang et al. synthesized CdS/CdS QDs as fluorescent sensors and liposome carriers for signal amplification to detect Hg2+ [17]. However, fluorescence sensors are often designed for one target detection, while those designed for simultaneous detection of two or more metal ions are rarely reported [18,19,20,21]. Besides, there are few reports on nanoprobes for the detection of Cr3+ and Hg2+ ions based on the MXene material field. Therefore, it is still a challenge to explore the relationship between MXene and multi-ion detection of heavy metals.
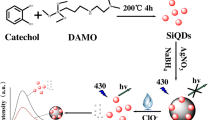
In this paper, Ti3C2 QDs (QDs) were firstly synthesized by ammonia-assisted hydrothermal method. Using ammonia (NH3·H2O) as nitrogen source and KMnO4 as functionalized reagent, KMnO4-functionalized Ti3C2 QDs (Mn-QDs) were synthesized by hydrothermal shearing. The Mn-QDs exhibit bright blue-green under ultraviolet light and have an emission peak at 435 nm. Interestingly, compared with QDs without KMnO4 treatment, Mn-QDs can specifically bind Cr3+ or Hg2+. In the range of 0–2600 μM Cr3+, Mn-QDs showed fluorescence enhancement by fluorescence measurement. After the addition of 0–550 μM Hg2+, Mn-QD fluorescence decreased in the range of 0–50 μM and fluorescence increased in the range of 50 to 350 μM. As a result, we mainly explored a “turn-on” type nanoprobe for Mn-QDs that particularly detects double ions of Cr3+ and Hg2+. To the best of our knowledge, this is the first attempt to functionalize QDs with KMnO4 and apply them to the detection of bimetallic ions. This study opens up a new approach for the functional synthesis of Mn-QD nanomaterials and expands the application of MXene in ion monitoring of environmental pollution.
Experimental section
Chemical and apparatus
Related reagents and instruments have been included in the Electronic Supporting Information.
Preparation of Ti3C2 QDs (QDs)
The synthesis of QDs is an improvement on the previously reported process [22, 23]. Two grams of Ti3AlC2 powder was added to 40 mL 40% HF, stirred at 60 °C for 20 h, washed with pure water, centrifuged at 10,000 rpm for 10 min to collect precipitation, and vacuum dried at 60 °C for 24 h to obtain Ti3C2 MXene powder. Then, 0.25-g powder was placed in 17.5 mL deionized water and ultrasound was performed for 30 min, and 7.5 mL NH3·H2O was added subsequently. The mixture was transferred to a Teflon lined stainless steel autoclave and heated at 120 °C for 6 h. Finally, after the hydrothermal reaction has cooled to room temperature and centrifuged at 14,000 rpm for 30 min, the colorless and clear supernatant was retained and heated at 90 °C for 2 h to remove excess ammonia. QDs were obtained by 100-nm membrane filtration. The prepared colorless and clear solutions of QDs were stored at 4 °C for further characterization and application.
Synthesis of KMnO4-functionalized Ti3C2 QDs (Mn-QDs)
The synthesis procedure of Mn-QDs is shown in Scheme 1. Briefly, the etched Ti3C2 MXene powder was added into 100 mL 3 mM KOH and stirred for 8 h. The turbid solution was centrifuged at 1000 rpm for 10 min, washed for several times, and dried, and the alkalized MXene powder was gained. The treated MXene powder was mixed with 10 mL 0.12 M KMnO4 and 12 mM KCl solution, stirred for 30 min, then transferred to the reaction kettle, and heated at 140 °C for 8 h. The reaction was cooled before performing a series of centrifugation and washing and drying procedures. And KMnO4-functionalized MXene powder was gained. Afterwards, Mn-QDs can be obtained by the above QD synthesis operation. Similarly, the prepared material solution with light yellow was stored at 4 °C for later use. Mn-QD powder was obtained by freeze-drying for the further experimental characterization.
Determination of Cr3+ and Hg2+ ions
In this experiment, we took 10 μL Mn-QDs and added a certain concentration of Cr3+ or Hg2+, mixing with pure water to a total volume of 400 mL. After reaction at room temperature for 10 min, FL measurements were recorded with 246-nm excitation. To test the selectivity of this material, other metal ions including Na+, K+, Al3+, Mg2+, Mn2+, Co2+, Ni2+, Fe3+, Fe2+, Cu2+, and Ag+ were also examined by the same procedure. To determine whether the non-functionalized QDs interact with metal ions, the same series of ions (Na+, K+, Al3+, Mg2+, Mn2+, Hg2+, Co2+, Cr3+, Ni2+, Fe3+, Fe2+, Cu2+, and Ag+) mixed with 100 μL QDs were also performed. The FL (λex = 232 nm) of different ions were measured and compared with the above results. The emission and excitation slit widths of both QDs and Mn-QDs are 5 nm and 10 nm, and the measurement voltage is 700 V.
Fluorescence detection of nanoprobes in real samples
The practicality and feasibility of the Mn-QD nanoprobes for the detection of Cr3+ and Hg2+ in environmental samples were tested using two water samples, Runxi Lake and tap water, collected from the campus of Nanchang University. Prior to analysis, the water sample was treated by centrifugation to remove suspended particles. Then, Mn-QDs and known concentrations of Cr3+ and Hg2+ were added to the sample to determine the FL response of the solution. Calculate the recovery rate using the relationship in Formula 1:
Results and discussion
Material characterization
On the one hand, the morphology, structure, and composition of the material were characterized in detail by TEM, XRD, XPS, and FTIR. The TEM images of the QDs and Mn-QDs are shown in Fig. 1. QDs and Mn-QDs exhibited nearly spherical particles with well monodispersity. The average lateral size of QDs was 1.64 ± 0.30 nm (n = 100), and the particle size distribution was mainly in the range of 1.2 to 2.4 nm. The average size of Mn-QDs was 2.24 ± 0.43 nm (n = 100), and the particle size distribution was mainly in the range of 1.6 to 3.2 nm. The crystallographic features of Mn-QD nanomaterials were further revealed by HRTEM images. Lattice fringes with spacing of 0.26 nm in the inner plane were measured (interpolation in Fig. 1E), corresponding to the (010) facet of Ti3C2 MXene [24, 25], which highlighted the crystalline nature of Mn-QDs.
The strongest peak (104) of Ti3AlC2 standard card is 39°, which is the characteristic peak of aluminum (Al) atom [26]. As shown in Fig. 2B, after HF etching, the 39° peak of QDs is significantly reduced or even disappears, indicating that the Al layer is successfully etched off. After the MXene hydrothermal reaction increased the layer spacing, the peaks of 41.7°, 28.6°, and 19° were enlarged and changed to the small angle direction of 40°, 28.1°, and 17.1° [27]. Furthermore, the two intense diffraction peaks located at 17.1° and 34.7°, which correspond to the (004) and (101) peaks of Ti3C2 MXene [28], respectively, indicate that QDs are successfully prepared and still maintain the 2D layered structure of MXene. On the basis of QDs, Mn-QDs exhibited the above phenomenon of weakening or disappearance of the strong peaks. After expanding the layer spacing of MXene by KOH alkalization treatment, the Mn-QDs derived from MXene were further modified with KMnO4, and the insertion of foreign substances caused corresponding changes in the material structure [29,30,31]. The XRD images of the two do not match well, mainly due to the insertion of the exogenous material causes a corresponding change in the material structure. The peak appearing at 24° is TiO2 formed by oxidation of MXene [32], indicating that QDs were successfully functionalized with KMnO4.
FTIR was used to characterize the composition and chemical bond of the material, as shown in Fig. 2A, stretching vibration of hydroxyl (-OH) at 3420 cm−1 and 1401 cm−1, vibration at 3113 cm−1 attributed to amino group (-NH), peak of 1627 cm−1 attributed to carbonyl group (C = O), and 1007 cm−1 band belongs to C-F bond [33,34,35]. In particular, the peaks at 705 cm−1 and 576 cm−1 are related to the vibrations of Ti–O and Ti-C bonds, respectively [36]. It is clear that the wavelengths of the functional groups of QDs and Mn-QDs fluctuate between 3500 and 2800 cm−1. Due to the fact that the addition of KMnO4 may have changed the surface structure of Mn-QDs, thus, changing the wavelengths and intensities of the functional groups also corroborates the change in the structure of their physical phases. The functional groups of QDs and Mn-QDs at 1600 to 600 cm−1 are matched one by one. Beyond that, the existence of bands 576 and 702 is also attributed to Mn–O vibration [37]. In summary, it can be indicated that Mn-QDs have been successfully prepared.
The chemical composition and overall structure of QDs and Mn-QDs were further investigated by XPS. The full-range XPS spectra of QDs showed five significant peaks at 285.2 (C 1 s), 401.8 (N 1 s), 532.2 (O 1 s), 684.9 (F 1 s), and 456.1 eV (Ti 2p), demonstrating the successful synthesis of QDs [12, 24]. The full-range XPS spectra of Mn-QDs showed five significant peaks at 285.0, 407.1, 532.1, 685.1, 455.3, 293.1, and 654.0 eV corresponding to C, N, O, F, Ti, K, and Mn elements [15, 38, 39], further indicating KMnO4 successfully functionalized QDs. The enlarged characteristic spectra of C 1 s, O 1 s, K 2p, and Mn 2p are displayed in Fig. S1, respectively. And the detailed narrow spectrum analysis has been given in the supporting information. On the other hand, the optical properties of QDs and Mn-QDs were characterized. In Fig. 3A, the FL of QDs has two excitation peaks at about 232 nm and 321 nm and one emission peak (black line) at about 413 nm. The FL of Mn-QDs has two excitation peaks at about 246 nm and 314 nm and one emission peak at about 435 nm (red line). An obvious absorption peak located at 209 nm could be observed in the absorption spectrum of Mn-QDs (violet curve) when compared with that of QDs (blue curve), because of the trapping of the excited state energy by the surface states [40]. These changes of optical properties may be related to functionalization, and the embedding of external functional groups improves their optical properties. QDs are colorless and transparent under natural light (first from the left in the inset) and blue under 365-nm UV irradiation (first from the right), and Mn-QDs are a light yellow solution under natural light (second from the left) and blue-green under 365-nm UV light (second from the right). The excellent luminescence of Mn-QDs proves to be an ideal fluorescent probe material for ion detection. On the side, we performed an optimization test regarding the excitation wavelength of Mn-QDs, and as shown in Fig. 3B, the best display wavelength is 245 nm. In detail, the excitation wavelength of our subsequent experiments was concentrated at 246 nm.
Condition optimization and stability performance
To ensure the best FL performance, the synthesis conditions of Mn-QDs were firstly optimized. As shown in Fig. S2, the results showed that the best FL performance of Mn-QDs was achieved when the temperature was 120 °C, the time was 6 h, KMnO4 doping amount was 10 mL, and ammonia/water ratio was 3/7. And then, in order to explore the practical application of Mn-QDs as nanoprobe in ion detection, it is necessary to examine the changes of their FL intensity under different concentrations of NaCl and pH environments. As shown in Fig. S3(A), the material can still maintain the same FL intensity at high and low salt concentrations. Figure S3(B) shows that the FL intensity of the material basically remained unchanged at pH 4–10, but decreased slightly when the pH exceeded 10. Figure S3(C) shows that the FL intensity of the material hardly changed after 55 days of storage, which indicates that the material is stable and can be stored for a long time. Finally, Mn-QDs also appeared outstanding optical stability. In Fig. S3(D), the FL intensity of Mn-QDs did not change significantly under 70-min continuous scanning (λex = 246 nm). This indicates that Mn-QDs have good stability and are expected to be used in environmental applications.
In order to save the experimental cost, we optimized the usage of Mn-QDs. The solutions of 10, 20, 35, 50, and 65 μL Mn-QDs were taken separately and supplemented with pure water to 400 μL. FL was measured at 246-nm excitation. In Fig. S4, when the amount of Mn-QDs decreased, the emission peak of Mn-QDs appeared blue shift. This may be caused by the reduced content of Mn-QDs in the cuvette. Therefore, from the perspective of saving experimental costs, we selected 10 μL Mn-QDs for the subsequent ion experiments.
Determination of Cr3+ and Hg2+ ions by Mn-QDs
In this study, a novel fluorescence nanoprobe based on Mn-QDs was developed to respond to Cr3+ and Hg2+ ions simultaneously. We first evaluated the feasibility of the experiment. As shown in Fig. S5(A), when Mn-QDs were mixed with 500 μM Cr3+ or Hg2+, the experimental phenomena of FL enhancement and peak blue shift of the mixed solution could be clearly observed, whereas the FL of the mixed solution did not change significantly when 500 μM Cr3+ or Hg2+ was added to QDs. Blank solutions of QDs and Mn-QDs were also measured and were able to exhibit lower FL intensities. Thus, the experiment was judged to be feasible. So as to explore possible practical applications, the effects of 13 common metal ions (Na+, K+, Al3+, Mg2+, Mn2+, Co2+, Hg2+, Ni+, Cr3+, Fe3+, Fe2+, Cu2+, and Ag+ at 300 μM concentrations) on QDs and Mn-QD FL responses were studied. In Fig. S5(B), for Mn-QDs, FL enhancement efficiency of Cr3+ and Hg2+ ions calculated using the formula (F – F0)/F0 was found to reach 1.75 and 0.95, respectively. F0 was the FL intensity of QDs or Mn-QDs, and F was the blank FL intensity of QDs or Mn-QDs reacting with metal ions. Figure S6 shows the fluorescence spectra of QDs and Mn-QDs in a series of different ions. As illustrated in Fig. 5B and Fig. S6, the addition of Cr3+ or Hg2+ has an obviously enhancing effect on the FL response of Mn-QDs; however, the others show minor influence on the response of Mn-QDs. Therefore, Mn-QDs have good selectivity for Cr3+ and Hg2+.
Further analysis was performed for Cr3+ and Hg2+ ions. Figure 4A shows the FL response of Mn-QDs over the 0–2600 μM Cr3+ concentration range. The inset in Fig. 4A shows the photographs under daylight lamp and 365-nm UV lamp regarding the blank Mn-QD solution and the solution after the addition of 500 μM, 1500 μM, and 2600 μM Cr3+. After the addition of Cr3+, Mn-QDs reacted with them resulting in the mixed solution gradually changing its color from light yellow to colorless. And the color of the solution changed from dark blue-green to bright blue under UV lamp. The FL intensity of Mn-QDs increased significantly with the increase of (0–2600 μM) Cr3+ concentration, while the blue-shift phenomenon occurred in the highest site peak. The linear range for Cr3+ is 50–2600 μM with a linear equation of F0/F = 1.4670 – 0.3520 Log [Cr3+] (μM) (R2 = 0.9762, n = 5). The limits of detection (LOD) of the nanoprobe for Cr3+ was 0.80 μM, which was estimated from the formula of LOD = 3σ/K (σ shows the standard deviation of blank signal (n = 5) and K is the slope of the linear equation). The comparison of other methods for the detection of Cr3+ with this experiment is shown in Table 1. Compared with other materials, our work has a good LOD and a wider detection range. Besides, different from the FL enhancement method for Cr3+ detection in this paper, most other detection mechanisms are FL quenching.
A FL emission spectra of Mn-QDs in the presence of Cr3+ ion over 0–2600 μM. The inset shows photographs of Mn-QDs and the solution after the addition of Cr3+ under sunlight and UV lamps. B Plots of the FL versus Cr3+ concentration; the inset is the calibration curve of F0/F and Cr3+ concentrations in the 50 to 2600 μM range. F0 is the FL response of Mn-QDs; F is the FL response of Mn-QDs after the addition of Cr3+. The error bars represent the standard deviation based on five independent measurements
The inset in Fig. 5A shows photographs about the blank Mn-QD solution and the solutions after the addition of 200 μM, 400 μM, and 600 μM Hg2+ under daylight lamp and 365-nm UV lamps. After adding Hg2+, Mn-QDs reacted with them, resulting in the color of the mixed solutions gradually changing from yellow to light yellow. And the color of the solution changed accordingly under the UV lamp. Figure 5A shows that the FL response of Mn-QDs decreases with increasing Hg2+ concentration from 0 to 50 μM and increases from 50 to 550 μM Hg2+. With the increase of Hg2+ concentration, the FL intensity of Mn-QDs decreases, while the peak undergoes a blue-shift phenomenon. When the Hg2+ concentration reached 65 μM, the FL intensity gradually showed an increasing trend. The top left inset in Fig. 5B shows the linear plot of the FL quenching of the Mn-QD solution after the addition of 0–50 μM Hg2+. The linear equation for this interval is F = 1212.58 – 5.5320 [Hg2+] (μM) (R2 = 0.9837, n = 5). According to the illustration in the lower right corner, the linear range for Hg2+ is 50–550 μM and the linear equation is F0/F = 2.7399 – 0.8152 Log [Hg2+] (μM) (R2 = 0.9948, n = 5). The LOD was 0.16 μM by calculating the 3 σ/K formula. The comparison of other methods for the detection of Hg2+ with this experiment is shown in Table 2. Compared to most of the fluorescence-off assays in the table, the FL “turn-on” type of this experiment has a wider detection range and good detection limits.
A FL emission spectra of Mn-QDs in the presence of Hg2+ ion over 0–550 μM. The inset shows photographs of Mn-QDs and the solution after the addition of Hg2+ under sunlight and UV lamps. B Plots of the FL versus Hg2+ concentration. The inner illustration in the upper left corner is a linear FL quenching diagram of 0–50 μM Hg2+. Another inset is linear relationship between F0/F and Hg2+ concentrations in the range 50 to 550 μM. F0 is the FL response of Mn-QDs and F is that of Mn-QDs with Hg2+. The error bars are the same as those in Fig. 4
In this experiment, with the increase of the concentration of Cr3+ and Hg2+, the maximum emission peak of Mn-QDs gradually shifted blue. Through zeta potential analysis, we can judge the binding between metal ions and Mn-QDs through electrostatic interaction, and the DLS data showed that average hydrodynamic radius of ultra-small Mn-QDs was also increased upon the addition of Cr3+ and Hg2+ ions (Supporting Information of Fig. S7A and S8). The strong interaction between Cr3+/Hg2+ ion and Mn-QDs with abundant functional groups on the surface leads to the formation of ground state complex [53]. Furthermore, the average fluorescence decay time of Mn-QDs is 4.77 ns; however, it decreases to 3.57 and 1.71 ns by the addition of Cr3+ and Hg2+ ions (Supporting Information of Fig. S7D), which further confirms the formation of complexes between Mn-QDs and Cr3+/Hg2+.
When appropriate metal ions are added to the functionalized Mn-QDs, the D-orbital electrons of the existing metal ions can repel these trapped conduction band electrons, which is caused by the binding between the surface functional groups and the metal ions [54]. This association of metal ions promotes the complexation of electron–hole pairs, which restores the fluorescence intensity of the functionalized Mn-QDs. This is why the fluorescence intensity of each quantum dot increases with increasing metal ion concentration and shifts in wavelength.
Investigation into the response mechanism for Cr3+ and Hg2+
To understand the fluorescence-on detection of Cr3+ and Hg2+ ions based on Mn-QD sensing nanoprobes, we performed a preliminary study of the response mechanism using zeta potential, fluorescence lifetime measurements, and absorption spectroscopy. As shown in Fig. S7(A), the zeta potential of Mn-QDs was − 47.9 mV. After mixing Mn-QDs with Cr3+ or Hg2+ solutions, the zeta potential increased to − 36.8 mV and − 13.65 mV, respectively. This indicates that there is an electrostatic interaction between Mn-QDs and Cr3+/Hg2+ [55]. This also demonstrates that Mn-QDs and Cr3+/Hg2+ can bind together. Furthermore, the small size and high quantum confinement effects of Mn-QDs lead to the formation of unsaturated bonds at their edges and surfaces, which exerted a higher chance to attach with other atoms [56].
The enhancement mechanism may be caused by passivation, absorption, and modification of the surface of Mn-QDs. The surface of Mn-QDs is rich in functional groups, which interact with Cr3+/Hg2+ to modify the surface traps, resulting in passivation of defects and exhibiting photoluminescence enhancement [57, 58]. In addition, due to the similarity of Al atoms, the alkali metal ions (K element) may have the ability to inhibit the passivation defects generated during the preparation process, resulting in the FL enhancement of Mn-QDs [59].
To investigate the FL quenching mechanism of Mn-QDs and Hg2+, the Stern–Volmer equation (F0/F = 1 + Ksv [Q], where Ksv represents the quenching constant and [Q] represents the concentration of the quenching agent) was used to determine the quenching mechanism [60]. As shown in Fig. S7 (B), a linear relationship between the F0/F ratio and concentration of Hg2+ (10–50 μM) indicates that there is a dynamic quenching between Hg2+ and Mn-QDs. Meanwhile, the UV–vis of Mn-QDs showed some changes of absorption peaks after the addition of Hg2+, which indicated that the FL quenching might be caused by the capture of Hg2+ by the amino groups on the surface of Mn-QDs to form Mn-QDs@Hg2+ complex [13, 61, 62]. The average fluorescence lifetime of QDs and Mn-QDs is 3.68 ns and 4.77 ns, respectively. Due to the fact that the functionalization of KMnO4 may change the properties of the surface chemical states of QDs, the lifetime of Mn-QDs is prolonged [63]. After the addition of Hg2+, the fluorescence lifetime of Mn-QDs decreased significantly to 1.71 ns. Besides, the value of τ0/τ for static quenching (average fluorescence lifetime τ0: QDs; τ: Mn-QDs@Hg2+) should be 1, while F0/F = τ0/τ should be valid for dynamic quenching [64]. It is calculated that τ0/τ = 4.77/1.71 = 2.79. Therefore, the type of FL quenching of Mn-QDs induced by Hg2+ was dynamic quenching, which was consistent with the conclusion obtained from the study of the Stern–Volmer equation.
Fluorescence detection of Cr3+/Hg2+ ions in real samples
The practicality and reliability of the Mn-QDs probe in detecting environmental samples were tested using samples from Runxi Lake and tap water of Nanchang University. In the present experiments, the Cr3+/Hg2+ solution with different concentrations was dropped into the pretreated real samples by standard addition method and then analyzed via the proposed approach. The specific procedure is that when a certain concentration of Cr3+ or Hg2+ ions was added to the Runxi Lake and tap water samples, their signals are measured by fluorescence spectrometry. Their recovery values can then be calculated by the corresponding linear equations. The measured results are summarized in Table S1 and Table S2. It can be clearly seen that the recoveries of Cr3+ and Hg2+ in water samples were 93.79–105.10% and 93.91–102.05%, respectively, with the relative standard deviations (RSD) of 0.86–3.32% and 0.66–5.00%. The results indicate that the method has great potential for the analysis of Cr3+ and Hg2+ ions in real samples.
Conclusion
In summary, Mn-QDs were prepared from KMnO4-functionalized MXene NSs by a hydrothermal reaction. The Mn-QDs displayed well-monodispersed near-spherical particles with average size of 2.24 nm and exhibited brightly blue-green emission and good long-term storage stability. In addition, Mn-QDs were used as a simple and reliable turn-on fluorescent nanoprobe for the sensitive and simultaneous detection of Cr3+ and Hg2+ ions. The surface trap of Mn-QDs was modified by binding Cr3+/Hg2+ through electrostatic interaction, which led to the passivation of surface defects and enhanced photoluminescence. This work not only leads to the development of a sensitive fluorescence assay for the detection of heavy metal ions, but also opens up a new and exciting channel for functionalized applications of MXene.
References
Dey G, Venkateswarulu M, Vivekananthan V et al (2016) Sub-picomolar recognition of Cr3+ through bioinspired organic–inorganic ensemble utilization. ACS sensors 1(6):663–669
Saha S, Mahato P, Reddy GU et al (2012) Recognition of Hg2+ and Cr3+ in physiological conditions by a rhodamine derivative and its application as a reagent for cell-imaging studies. Inorg Chem 51(1):336–345
Hu PP, Liu N, Wu KY et al (2018) Successive and specific detection of Hg2+ and I- by a DNA@MOF biosensor: experimental and simulation studies. Inorg Chem 57(14):8382–8389
Pang X, Bai H, Zhao H et al (2021) Biothiol-functionalized cuprous oxide sensor for dual-mode sensitive Hg2+ detection. ACS Appl Mater Interfaces 13(39):46980–46989
Borowska M, Jankowski K (2020) Sensitive determination of bioaccessible mercury in complex matrix samples by combined photochemical vapor generation and solid phase microextraction coupled with microwave induced plasma optical emission spectrometry. Talanta 219:121162
Yu J, Zhang X, Zhao M et al (2021) Fabrication of the Ni-based composite wires for electrochemical detection of copper(II) ions. Anal Chim Acta 1143:45–52
Qin R, Shan G, Hu M et al (2021) Two-dimensional transition metal carbides and/or nitrides (MXenes) and their applications in sensors. Materials Today Physics 21:100527
Meng W, Liu X, Song H et al (2021) Advances and challenges in 2D MXenes: from structures to energy storage and conversions. Nano Today 40:101273
Yu L, Chang J, Zhuang X et al (2022) Two-dimensional cobalt-doped Ti3C2 MXene nanozyme-mediated homogeneous electrochemical strategy for pesticides assay based on in situ generation of electroactive substances. Anal Chem 94(8):3669–3676
Lu S, Sui L, Liu Y et al (2019) White photoluminescent Ti3C2 MXene quantum dots with two-photon fluorescence. Adv Sci (Weinh) 6(9):1801470
Xue Q, Zhang H, Zhu M, et al (2017) Photoluminescent Ti3C2 MXene quantum dots for multicolor cellular imaging. Adv Mater Weinheim 29:15
Wang L, Zhang N, Li Y et al (2021) Mechanism of nitrogen-doped Ti3C2 quantum dots for free-radical scavenging and the ultrasensitive H2O2 detection performance. ACS Appl Mater Interfaces 13(36):42442–42450
Luo W, Liu H, Liu X et al (2021) Biocompatibility nanoprobe of MXene N-Ti3C2 quantum dot/Fe3+ for detection and fluorescence imaging of glutathione in living cells. Colloids Surf, B 201:111631
Pandey RP, Rasool K, Madhavan VE et al (2018) Ultrahigh-flux and fouling-resistant membranes based on layered silver MXene Ti3C2Tx nanosheets. J Mater Chem A Mater Energy Sustain 6(8):3522–3533
Zhu X, Cao Z, Wang W et al (2021) Superior-performance aqueous zinc-ion batteries based on the in situ growth of MnO2 nanosheets on V2CTX MXene. ACS Nano 15(2):2971–2983
Lin C, Wang M, Tang J et al (2021) A two-fold interpenetrated dual-emitting luminescent metal-organic framework as a ratiometric sensor for chromium(III). Inorg Chem 60(21):16803–16809
Zhang Y, Xiao JY, Zhu Y et al (2020) Fluorescence sensor based on biosynthetic CdSe/CdS quantum dots and liposome carrier signal amplification for MERCURY DEtection. Anal Chem 92(5):3990–3997
Kaur N, Kaur P, Singh K (2016) Ferrocene-BODIPY Push–Pull dyad: a common platform for the sensing of Hg2+ and Cr3+. Sensors Actuators B Chem 229:499–505
Yang Y, Feng Y, Wang Y et al (2017) A novel ratiometric fluorescent probe for selective detection of Hg2+, Cr3+ and Al3+ and its bioimaging application in living cells. Sens Actuators, B Chem 253:1055–1062
Mahato P, Saha S, Suresh E et al (2012) Ratiometric detection of Cr3+ and Hg2+ by a naphthalimide-rhodamine based fluorescent probe. Inorg Chem 51(3):1769–1777
Zhan L, Yang T, Zhen S J, et al (2017) Cytosine triphosphate-capped silver nanoparticles as a platform for visual and colorimetric determination of mercury(II) and chromium(III). Mikrochimica Acta 184(9)3171–3178
Ai F, Fu C, Cheng G et al (2021) Amino-functionalized Ti3C2 MXene quantum dots as photoluminescent sensors for diagnosing histidine in human serum. ACS applied nano materials 4(8):8192–8199
Fu C, Ai F, Huang J et al (2022) Eu doped Ti3C2 quantum dots to form a ratiometric fluorescence platform for visual and quantitative point-of-care testing of tetracycline derivatives. Spectrochim Acta A Mol Biomol Spectrosc 272
X Zhou Q, Wang J, Li B, et al (2020) Dual emission reverse change ratio photoluminescence sensor based on a probe of nitrogen doped Ti3C2 quantum dots@DAP to detect H2O2 and xanthine. Anal Chem 92(11):7770. Washington
Zeng Z, Yan Y, Chen J, et al (2019) Boosting the photocatalytic ability of Cu2O nanowires for CO2 conversion by MXene quantum dots. Adv Funct Mater 29:2
Li Y, Ding L, Guo Y et al (2019) Boosting the photocatalytic ability of g-C3N4 for hydrogen production by Ti3C2 MXene quantum dots. ACS Appl Mater Interfaces 11(44):41440–41447
Lai C, An Z, Yi H et al (2021) Enhanced visible-light-driven photocatalytic activity of bismuth oxide via the decoration of titanium carbide quantum dots. J Colloid Interface Sci 600:161–173
Yang Y, Zeng Z, Zeng G et al (2019) Ti3C2 Mxene/porous g-C3N4 interfacial Schottky junction for boosting spatial charge separation in photocatalytic H2O2 production. Appl Catal B 258:117956
Lukatskaya MR, Mashtalir O, Ren CE et al (2013) Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 341(6153):1502–1505
Wang C, Xie H, Chen S et al (2018) Atomic cobalt covalently engineered interlayers for superior lithium-ion storage. Adv Mater 30(32):e1802525
Wu J, Yang Q, Li Q et al (2021) Two-dimensional MnO2 nanozyme-mediated homogeneous electrochemical detection of organophosphate pesticides without the interference of H2O2 and color. Anal Chem 93(8):4084–4091
Li Y, Xu D, Zhang D et al (2019) Study on MnO2/MXene–Ti3C2 composite materials as cathode materials for magnesium batteries. RSC Adv 9(58):33572–33577
Guan Q, Ma J, Yang W et al (2019) Highly fluorescent Ti3C2 MXene quantum dots for macrophage labeling and Cu2+ ion sensing. Nanoscale 11(30):14123–14133
Yang F, Ge Y, Yin T et al (2020) Ti3C2Tx MXene quantum dots with enhanced stability for Ultrafast Photonics. ACS applied nano materials 3(12):11850–11860
Yang EJ, Jeon OS, Yang JU et al (2020) Room temperature manufacturing photoluminescent graphene quantum dots based on MXene. Carbon (New York) 167:863–869
Liu M, He Y, Zhou J et al (2020) A “naked-eye” colorimetric and ratiometric fluorescence probe for uric acid based on Ti3C2 MXene quantum dots. Anal Chim Acta 1103:134–142
Aadil M, Mahmood M, Warsi MF et al (2021) Fabrication of MnO2 nanowires and their nanohybrid with flat conductive matrix for the treatment of industrial effluents. FlatChem 30:100316
Huang D, Wu Y, Ai F, et al (2021) Fluorescent nitrogen-doped Ti3C2 MXene quantum dots as a unique on off on nanoprobe for chrominum VI and ascorbic acid based on inner filter effect. Sensors Actuators B Chem 342:130074
Gao M, Song Y, Liu Y et al (2021) Controlled fabrication of Au@MnO2 core/shell assembled nanosheets by localized surface plasmon resonance. Appl Surf Sci 537:147912
Jiang X, Wang H, Shen Y, et al (2022) Nitrogen-doped Ti3C2 MXene quantum dots as novel high-efficiency electrochemiluminescent emitters for sensitive mucin 1 detection. Sensors Actuators B Chem 350:130891
Desai ML, Jha S, Basu H et al (2020) Simple hydrothermal approach for synthesis of fluorescent molybdenum disulfide quantum dots: sensing of Cr3+ ion and cellular imaging. Mater Sci Eng C Mater Biol Appl 111:110778
Guo J, Ye S, Li H et al (2021) Novel fluorescent probes based on nitrogen-sulfur Co-doped carbon dots for chromium ion detection. New J Chem 45(1):4828–4834
Lu H, Xu S, Liu J (2019) One pot generation of blue and red carbon dots in one binary solvent system for dual channel detection of Cr3+ and Pb2+ based on ion imprinted fluorescence polymers. ACS Sens 4(7):1917–1924
Jin H, Xu J, Zhang L et al (2018) Multi-responsive luminescent sensor based on Zn (II) metal-organic framework for selective sensing of Cr(III), Cr(VI) ions and p-nitrotolune. J Solid State Chem 268:168–174
Wang C, Xu J, Li H et al (2020) Tunable multicolour S/N Co-doped carbon quantum dots synthesized from waste foam and application to detection of Cr3+ ions. Luminescence (Chichester, England) 35(8):1373–1383
Li D, Li C, Qi H et al (2016) Rhodamine-based chemosensor for fluorescence determination of trivalent chromium ion in living cells. Sens Actuators, B Chem 223:705–712
Chu H, Yao D, Chen J et al (2020) Double-emission ratiometric fluorescent sensors composed of rare-earth-doped ZnS quantum dots for Hg2+ detection. ACS Omega 5(16):9558–9565
Lu L, Han X, Lin J et al (2021) Ultrasensitive fluorometric biosensor based on Ti3C2 MXenes with Hg2+-triggered exonuclease III-assisted recycling amplification. Analyst 146(8):2664–2669
Cao B, Yuan C, Liu B et al (2013) Ratiometric fluorescence detection of mercuric ion based on the nanohybrid of fluorescence carbon dots and quantum dots. Anal Chim Acta 786:146–152
Zhang KX, Yu YX, Sun SQ (2013) Facile synthesis L-cysteine capped CdS: Eu quantum dots and their Hg2+ sensitive properties. Appl Surf Sci 276:333–339
Li H, Wang W, Wang Z et al (2021) Analyte-enhanced photocatalytic activity of CdSe/ZnS quantum dots for paper-based colorimetric sensing of Hg2+ under visible light. Microchem J 164:106037
Wen X, Fan Z (2017) A novel ‘turn-on’ fluorescence probe with aggregation-induced emission for the selective detection and bioimaging of Hg2+ in live cells. Sens Actuators, B Chem 247:655–663
Desai ML, Basu H, Singhal RK et al (2019) Ultra-small two dimensional MXene nanosheets for selective and sensitive fluorescence detection of Ag+ and Mn2+ ions. Colloids Surf, A 565:70–77
Lin T, Dhenadhayalan N, Lee H et al (2019) Fluorescence turn-on chemosensors based on surface-functionalized MoS2 quantum dots. Sens Actuators, B Chem 281:659–669
Chen X, Sun X, Xu W et al (2018) Ratiometric photoluminescence sensing based on Ti3C2 MXene quantum dots as an intracellular pH sensor. Nanoscale 10(3):1111–1118
Wang H, Zhao R, Hu H et al (2020) 0D/2D Heterojunctions of Ti3C2 MXene QDs/SiC as an efficient and robust photocatalyst for boosting the visible photocatalytic NO pollutant removal ability. ACS Appl Mater Interfaces 12(36):40176–40185
Wang H, Zhu W, Fang M et al (2016) Turn-on fluorescence probe for high sensitive and selective detection of Ag+ by L-glutathione capped CdTe quantum dots in aqueous medium. J Lumin 180:14–19
Ge J, Li W, He X et al (2020) Charge behavior modulation by titanium-carbide quantum dots and nanosheets for efficient perovskite solar cells. Materials today energy 18:100562
Zhang S, Qi Z, Li Y (2022) Alkali preparation and ions detection of Ti3C2 quantum dots. Ceram Int 48(15):21118–21124
Zhong Y, Yi T (2019) MoS2 quantum dots as a unique fluorescent “turn-off-on” probe for the simple and rapid determination of adenosine triphosphate. Journal Of Materials Chemistry B 7(15):2549–2556
Feng Y, Zhou F, Deng Q et al (2020) Solvothermal synthesis of in situ nitrogen-doped Ti3C2 MXene fluorescent quantum dots for selective Cu2+ detection. Ceram Int 46(6):8320–8327
Wan M, Zhou J, Yang H, et al (2022) Covalently N-doped MXene quantum dots for highly stable fluorescent Cu2+ ion sensor. ACS Appl Nano Mater
Xu Q, Ding L, Wen Y, et al (2018) High photoluminescence quantum yield of 18.7% by using nitrogen- doped Ti3C2 MXene quantum dots. J Mater Chem C 6(24)6360–6369
Behera PK, Mukherjee T, Mishra AK (1995) Simultaneous presence of static and dynamic component in the fluorescence quenching for substituted naphthalene—CCl4 system. J Lumin 65(3):131–136
Funding
This work was financially supported by the Natural Science Foundation of Jiangxi Province (20212BAB206093) and the National Natural Science Foundation of China (nos. 22064012, 21907048, 31960207, and 81860610).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, X., Shi, Z., Fu, C. et al. A novel fluorescent nanoprobe based on potassium permanganate–functionalized Ti3C2 QDs for the unique “turn-on” dual detection of Cr3+ and Hg2+ ions. Microchim Acta 190, 153 (2023). https://doi.org/10.1007/s00604-023-05710-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05710-5