Abstract
We present a new “off-on” fluorescence probe for detecting hypochlorite (ClO−) based on silicon quantum dots coupled with silver nanoparticles (SiQDs/AgNPs) as nanocomplexes. Via introducing N-[3-(trimethoxysilyl)propyl]ethylenediamine and catechol as initial reactants, silicon quantum dots (SiQDs) with excellent properties were synthesized through a simple hydrothermal method. Transmission electron microscopy, Fourier transform infrared spectroscopy, and X-ray photoelectron spectroscopy were used to characterize the morphology and structure of quantum dots. The fluorescence of SiQDs could be quenched by the silver nanoparticles (AgNPs) by surface plasmon-enhanced energy transfer (SPEET) from SiQDs (donor) to AgNPs (acceptor). The AgNPs could be etched by adding ClO−, thus freeing the SiQDs from the AgNP surfaces and restoring the SiQDs’ fluorescence. The sensing system exhibits many advantages, such as wide linear response range, high sensitivity, and excellent selectivity. Under optimized conditions, wide linear ranges (from 0.1 to 100.0 μM) and low detection limits (0.08 μM) were obtained for ClO−.

Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypochlorite (ClO−) is a strong bactericide and disinfectant. It has been widely used in the purification of sewage, household bleaching, and disinfection of drinking water [1]. However, during the water treatment process, excess ClO− will produce many unwanted by-products, such as trihalomethane (THM) [2, 3], which is harmful to the human body and can cause arteriosclerosis [4], reproductive failure [5], and even cancer [6]. At the same time, low levels of ClO− do not effectively kill pathogenic bacteria and viruses, resulting in many risks. Further, ClO− is a very important active oxygen [7]. This species is endogenous substances that play important roles in pathological and physiological processes. Endogenous ClO− is produced by the reaction between chloride ions and hydrogen peroxide catalyzed by the enzyme myeloperoxidase (MPO) [8]. Abnormal levels of endogenous ClO− are associated with specific diseases such as neuronal deformation, cardiovascular disease, arteriosclerosis, lung injury, and cancer [9, 10]. Therefore, it is necessary to develop probes with selectivity and sensitivity for detecting ClO−.
Up to now, numerous sensing methods have been reported for detection of ClO−, such as electrochemical analysis [11], chemiluminescence [12], fluorometry [13], and colorimetry [14]. Among these methods, fluorescent assays have received considerable attention owing to their low cost, simple operation, and high sensitivity. Most of these fluorescent assays are applied to detect ClO− by employing diverse fluorescent probes such as organic fluorescent probes [15, 16], inorganic semiconductor quantum dots [17], gold/silver nanoclusters [18], carbon quantum dots [19], and amino-functionalized metal-organic frameworks (MOFs) [1]. Unfortunately, there still exist some drawbacks in these fluorescent probes, for example, the complicated synthesis procedure for organic fluorescent probes, high toxicity for inorganic semiconductor quantum dots, high cost for gold/silver nanoclusters, complex modification for carbon quantum dots, and the time-consuming synthesis procedure for MOF material. Especially, most of the existing fluorescent assays are based on the oxidation of ClO− and the fluorescence quenching effect of ClO− on the specific fluorophore. Such fluorescence turn-off sensing systems suffer from high background, low sensitivity, and other potentially interfering substances. Therefore, it is still urgent to establish an “off-on” fluorescent probe for the detection of ClO−.
Silicon quantum dots (SiQDs), as a new type of abundant and low-cost fluorescent nanomaterial, have attracted much interest and have been demonstrated to be environmentally friendly fluorescent probes. Furthermore, compared with conventional semiconductor quantum dots, SiQDs are superior in terms of cytotoxicity, photostability, water solubility, and photoluminescence profiles. These interesting advantages enable SiQDs to play a great role in a variety of fields such as bioimaging, biosensors, biological labeling, and catalysis [20,21,22,23,24,25,26]. For instance, Ma et al. [27] reported a water-dispersible and biocompatible SiQDs for selective heparin sensing and cell imaging. Zhang et al. [28] synthesized a highly photoluminescent SiQD nanoprobe for the detection of dopamine. Recently, we have also developed SiQD-based fluorescent sensing assay for the detection of glucose [29] and organophosphorus pesticides [30]. Also, as a new sort of nanomaterial, silver nanoparticles (AgNPs) possess many unique optical properties, such as high extinction coefficient, characteristic surface plasmon resonance absorption, and the size-dependent quantum confinement effects [31]. It has been demonstrated that AgNPs can be used as outstanding quencher for various fluorescence probes, such as QDs [32] and organic dyes [33]. Meanwhile, it has also been reported that AgNP species prepared using NaBH4 as a reducing agent could be etched by ClO− as an oxidant [34].
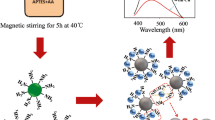
Based on the above statements, in this work, we firstly synthesized SiQDs by hydrothermal heating of an aqueous mixture of catechol and N-[3-(trimethoxysilyl)propyl]-ethylenediamine (DAMO), and sequentially constructed a novel SiQD/AgNP nanocomplex via adding silver nitrate and NaBH4 to a solution of SiQDs (Scheme 1). Finally, we designed successfully an “off-on” fluorescence probe for ClO−. In brief, the fluorescence of SiQDs can be quenched by AgNPs due to their closeness, resulting in surface plasmon–enhanced energy transfer (SPEET) from SiQDs (donor) to AgNPs (acceptor). In the presence of ClO−, AgNPs could be etched by ClO−, owing to its strong oxidizing ability. This results in the release of SiQDs and increased fluorescence, allowing quantitative analysis of ClO−. The proposed “off-on” sensor has a good sensitivity and selectivity toward ClO− in both aqueous solution and real tap water and pool water samples. To our knowledge, this is the first report on the detection of ClO−-based “off-on” fluorescent sensor. Therefore, our study may introduce a new prospect to sense ClO−.
Experimental section
Materials and reagents
N-[3-(Trimethoxysilyl)propyl]-ethylenediamine (DAMO), catechol, silver nitrate (AgNO3), sodium borohydride (NaBH4), sodium hypochlorite (NaClO), and diethyl-p-phenylenediamine sulfate (DPD) were purchased from Aladdin Chemistry Co. Ltd. (Shanghai, China). Other regents were obtained from Sinopharm Chemical Reagent Co.,Ltd (Beijing, China) and used as received. All reagents are of analytical grade and used without any further purification. All solutions were prepared using deionized water in experimental procedures.
Instruments
UV-vis absorption spectra were recorded on a UV-Vis 2600 spectrophotometer (Shimadzu, Japan). Fluorescence measurements were carried out on an F-7000 Spectrofluorometer (Shimadzu, Japan). Particle size distribution was tested by dynamic light scattering (DLS, Mastersizer 3000, Malvern Corp, UK). The experimental parameters were as follows: dispersant, water; scan times, 100; viscosity, 0.8872 cP; temperature, 25 °C; dielectric constant, 78.5; and RI, 1.330. 1.2 mL SiQD sample was transferred into an exclusive vitreous for DLS measurements. The morphology and structure of SiQDs were characterized by Fourier transform infrared spectroscopy (FT-IR; NICOLET 6700, Thermo Fisher Scientific Inc., USA), transmission electron microscopy (TEM; JEM-3010, Joel, Japan), X-ray photoelectron spectroscopy (XPS; K-Alpha 1063, Thermo Fisher Co., UK) and energy-dispersive X-ray spectroscopy (EDS; Tecnai G2 20). The pH measurements were performed with a pH meter PB-10 (Sartorius, Germany). The fluorescence spectra of the resulting solutions were recorded at room temperature (25 °C).
Preparation of SiQDs and SiQDs/AgNPs
The synthetic procedure of SiQDs has been previously reported by Han et al. [35]. In a typical experimental procedure, the SiQDs were prepared by adding 2.0 mL of DAMO to 10.0 mL of deionized water with stirring. Then, 11.0 mg of catechol was added to the above solution by stirring for 1 min. The mixture solution was then transferred into a Teflon-lined autoclave and processed under 200 °C for 4 h. Then, the as-prepared SiQDs were cooled to room temperature and purified through a dialysis tube (1000 Da, molecular weight cutoff) for about 6 h. The obtained SiQDs were stored at 4 °C for further use. For synthesis of SiQDs/AgNPs, 0.1 mM AgNO3 was added into the SiQD solution to give a final concentration of 50-fold diluted SiQDs, and then 0.1 mM fresh NaBH4 solution was added, followed by vigorous shaking for 10 min. The solution altered from yellow to brown, suggesting the formation of SiQDs/AgNPs.
Fluorescence assay of ClO−
In a typical process of detecting ClO−, 50.0 μL ClO− with different concentrations (0.1 to 200.0 μM) and 100.0 μL of phosphate buffer (10.0 mM, pH = 7.0) were sequentially added into a 2.0-mL plastic centrifuge tube. Then, 250.0 μL of SiQDs/AgNPs was added to the reaction mixture. The mixture was diluted to 500.0 μL with water and mixed thoroughly. After 15 min, the fluorescence spectrum of the resulting solutions was recorded using excitation at 430 nm. Each experiment was repeated three times.
Analysis of real sample
Tap water (from Lab), swimming pool water (from Jiangsu University gymnasium swimming pool), and wastewater (collected from Jingkou district sewage treatment plant) samples were used. The collected water samples were centrifuged and filtered before detection. To ensure the accuracy of the results, the water samples were analyzed as soon as collected in consideration of their poor stability. Moreover, standard addition experiments were carried out to evaluate the reliability of the method.
Results and discussion
Characterization of SiQDs
The TEM images and the size distributions of the obtained SiQDs are shown in Fig. S1a-b (see Electronic Supplementary Material, ESM). It is noted that the produced SiQDs were uniform in size with an average diameter of 3.3 nm. The diameter measured by DLS confirms the small size of the SiQDs with an average diameter of ∼ 4.85 nm (with PDI = 0.08) in Fig. S1c (see ESM). The EDS pattern of SiQDs indicated that SiQDs contain Si, C, O, and N elements (ESM Fig. S1d). FT-IR was used to perform the presence of various functional groups in SiQDs (ESM Fig. S1e), and the absorption peaks at 3371 and 3290 cm−1 could be assigned to the stretching vibrations of O–H and N–H [36] respectively, while the relatively strong peak at 1635 cm−1 could be ascribed to the bending vibration of N–H [37]. Meanwhile, the broad peak at 777 cm−1 suggested the existence of N–H wagging vibration [38]. Moreover, the secondary amine exhibited a peak at 696 cm-1, and a peak belonging to Si–O stretching was at 923 cm−1. These results demonstrated that the SiQDs contain amount of hydroxyl and amino groups which could greatly enhance solubility and stability of the SiQDs. Importantly, these functional groups can be used as binding sites for silver ions.
XPS measurements were performed to further study the surface composition of the obtained SiQDs (ESM Fig. S2). It is noted from ESM Fig. S2a that the peaks of C 1s, N 1s, O 1s, Si 2p, and Si 2s are located at 283.7, 399.1, 532.1, 101.3, and 152.1 eV. The high-resolution XPS spectrum of N 1s (ESM Fig. S2b) presented three fitted peaks centered at 397.8 eV (N–Si), 398.9 eV (C–N–C), and 400.5 eV (N–(C)3), indicating that N exists in three forms. Two peaks at 530.6 and 531.8 eV were ascribed to Si–O in the XPS spectrum of O 1s (ESM Fig. S2c), and the peak at 532.3 eV attributed to C–O [39]. The binding energy of Si 2p (ESM Fig. S2d) was observed at 102.4, 101.7, and 101.1 eV, which were connected with Si–O, Si–N, and Si–C groups, respectively [40].
Fluorescence and UV-vis absorption spectra were used to study the optical properties of the synthesized SiQDs. As seen from Fig. 1a, the SiQD aqueous solution shows obvious typical UV-vis absorbance band at 430 nm compared with DAMO and catechol. And the SiQD solution was yellowish, transparent, and clear under sunlight; meanwhile, it could emit bright green light under UV light excitation (insert in Fig. 1b), suggesting that the obtained SiQDs have excellent fluorescence property. As shown in Fig. 1b, the fluorescence emission spectra of SiQDs exhibited a typical excitation-dependent feature and the emission peak gradually shifts to longer wavelength with the increase of the excitation wavelength, with an optimum excitation at 430 nm and emission maximum at 520 nm. Besides, the fluorescence of SiQDs showed remarkable stability. It was noted from Fig. S3a (see ESM) that the normalized fluorescence almost did not change when the pH values ranged from 4 to 12, indicating that the SiQDs have excellent stability in acid and alkali. As such, the normalized fluorescence of SiQDs did not change obviously under continuous irradiation (ESM Fig. S3b), suggesting that their photostability is very outstanding. In addition, the obtained SiQD solution could keep stable for several months (ESM Fig. S3c), and the normalized fluorescence changed less than 5% in NaCl solution with high concentration (1.5 M) (ESM Fig. S3d). These results demonstrated that the SiQDs are excellent candidates as a powerful reagent and probe in biological applications.
a The absorbance of SiQDs, catechol, and DAMO. SiQDs, catechol, and DAMO were 0.05 mg/mL in 0.01 M PBS of pH 7.0. b The fluorescence spectra of SiQDs, the excitation wavelengths are 350, 370, 390, 410, 430, 450, 470, 490, and 510 nm, respectively. Insert in b shows the photograph of SiQD solution under visible and UV light (365 nm) respectively
Fluorescence “off-on” strategy for ClO− detection
The working principle of this “off-on” fluorescence probe is illustrated in Scheme 1. Firstly, the added Ag+ can interact with the surface of the SiQDs through the amino, carboxyl, and other functional groups. Next, SiQD/AgNP nanocomplexes were formed by the use of NaBH4 to reduce a mixture of Ag+ and SiQDs. As a consequence, the AgNPs can effectively quench the fluorescence of SiQDs due to SPEET. When ClO− is added to the solution, it can etch AgNPs to form silver ions, thereby releasing SiQDs and regenerating the fluorescence of SiQDs. Therefore, the “off-on” fluorescence probe was established and it can be used for the quantitative assay of ClO−.
The optical properties of SiQDs and SiQDs/AgNPs were studied to prove the mechanism of AgNPs quenching the fluorescence of SiQDs. It can be seen in Fig. 2a that the absorption spectrum of SiQDs/AgNPs (centered at 416 nm) and the excitation spectrum of SiQDs (centered at 413 nm) have a large overlap. This is a necessary condition for SPEET which was reported in previous paper [32]. Thus, SPEET from SiQDs to AgNPs can be described as the mechanism of AgNPs quenching SiQDs. When ClO− is added to the SiQD/AgNP solution, the absorbance at 416 nm is reduced because AgNPs are etched. To further confirm the mechanism, TEM assays were performed. As shown in Fig. 3a, SiQDs are uniform in size with an average diameter of 3 nm. When used for the synthesis of SiQDs/AgNPs, SiQDs can combine well with AgNPs with the diameter of around 10 nm (Fig. 3b). Consequently, the fluorescence of SiQDs can be obviously quenched. When ClO− is added to the SiQD/AgNP solution, free SiQDs reappear in TEM images of the reaction system because AgNPs are etched (Fig. 3c). As a result, enhanced fluorescence was detected in the presence of ClO− (blue line, Fig. 2b). These results are consistent with the spectrum and also demonstrate our assumption about the detection mechanism. Before and after adding ClO−, the fluorescence intensity of SiQDs did not change. This result eliminates the interference of ClO− which enhances the fluorescence of SiQDs (ESM Fig. S4).
a Absorption spectra of SiQDs/AgNPs before (green) and after (purple) adding ClO−. Red line represents the excitation spectrum of SiQDs at 416-nm emission. b Emission spectra of SiQDs/AgNPs before (green line) and after (blue line) adding ClO−. Red line represents the emission spectrum of free SiQDs
Optimization of assay parameters
In order to enhance the sensitivity of the reaction system toward ClO−, we optimized the conditions including the silver ion concentration, pH value, and reacting time. Since the concentration of silver ion directly affects the synthesis of AgNPs, which are responsible for the background signal and the required amount of ClO− for recovery of fluorescence of SiQDs, we studied the effect of silver ion concentration on the detection of ClO− by fluorescent probes. As shown in Fig. S5a and S5b (see ESM), by monitoring the fluorescence ratio (F/F0), where F and F0 are the fluorescence intensities at 520 nm in the presence and absence of ClO−, respectively, we finally select 0.1 mM silver ion to provide excellent SiQDs/AgNPs to obtain the best fluorescence ratio. Another key factor for the sensing system is pH value. As indicated in Fig. S6a-b (see ESM), the highest fluorescence ratio was obtained in the pH = 7.0. Therefore, the final selection of pH = 7.0 was the best pH value for the fluorescence probe. To understand the response rate of this probe to ClO−, the fluorescence signal of this probe detection ClO− was measured at four time points (0, 10, 15, 20 min). As shown in Fig. S7b (see ESM), the fluorescence ratio can increase with time and reach the highest value at 15 min. Therefore, incubation time of 15 min was adopted for the subsequent fluorescent measurements.
Sensitivity of SiQDs/AgNPs for ClO− detection
Under the optimal experimental conditions discussed above, the linear response of the reaction system to ClO− concentration ranging from 0.1 to 200.0 μM was investigated. As shown in Fig. 4a, the fluorescence of SiQDs at 520 nm was gradually increased with increasing ClO− concentration from 0.1 to 200.0 μM, indicating that the correlation between ClO− concentration and restored fluorescence is dose-dependent. Figure 4b shows the calibration curves based on the fluorescence enhancement (F/F0 − 1) versus ClO− concentration. The linear equation is F/F0 − 1 = 0.014 [ClO−] + 0.0032 with a correlation coefficient R2 of 0.9972. The limit of detection for ClO− was calculated to be 0.08 μM (3σ/slope, where σ is the standard deviation of the blank samples), Furthermore, a comparison between other reported methods and the proposed method for ClO− determination is summarized in Table 1. It was noted that the developed sensing system in this work reveals remarkable sensitivity.
Selectivity of SiQDs/AgNPs for ClO− detection
The selectivity of the proposed sensor for ClO− was then investigated by analyzing various possible interferences including common substances (H2O2, ClO2, ClO4−, Co2+, Br−, NO3−, CH3CO2−, Cl−, SO42−, S2−, SO32−, CO32−, PO43−, NH4+, Cu2+, Ca2+, Mg2+, Zn2+, and Fe3+) and heavy metal ions (Cd2+, Cr3+, Ag+, Pb2+, and Hg2+). As shown in Fig. 5, it is noted that almost all these interfering substances have nearly no interference to the fluorescence response of 60.0 μM ClO− even at a rather high concentration (interferences were 200.0 μM). This result suggests that the present sensing system exhibits good specificity for ClO− against other common interferences. The observed high sensitivity and specificity are due to the fact of strong oxidation and etching properties of ClO− onto AgNPs. However, an obvious interference can be observed from H2O2 when its concentration is much larger than that of ClO− (60.0 μM), so this point should be taken into account for real applications.
Real samples detection
Based on the excellent characteristics of the proposed method, the application of SiQD/AgNP nanocomplex as an “off-on” fluorescent probe for detecting ClO− in swimming pool water, tap water, and sewage water was put into practice. The ClO− concentrations were calculated using a calibration equation. The results given in Table 2 show that the recoveries for sample determination were in the range of 95.9–106.0%. Meanwhile, the results obtained by this method are consistent with the results of the standard DPD method. These results indicated that the proposed method has potential applications in real sample analysis for ClO−.
Conclusions
In summary, a novel SiQD/AgNP nanocomplex using SiQDs bound to AgNPs was designed for the first time and used successfully to construct “off-on” fluorescent sensor for ClO−. Since energy transfers between SiQDs as donors and AgNPs as acceptors, the fluorescence of SiQDs is quenched. AgNPs are etched by ClO−, leading to a fluorescence enhancement response to the ClO− concentration. Therefore, an “off-on” fluorescence sensor approach with low background and detection limit and high sensitivity and selectivity based on a SiQD/AgNP nanocomplex has been developed for ClO−. Meanwhile, the accurate quantitative assay of ClO− in tap water and swimming pool water indicates the potential for the practical application. It is believed that this study could give a new sight for developing “off-on” SiQD-based sensors and detecting ClO− in the water quality monitoring.
References
Lu T, Zhang L, Sun M, Deng D, Su Y, Yi L. Amino-functionalized metal-organic frameworks nanoplates-based energy transfer probe for highly selective fluorescence detection of free chlorine. Anal Chem. 2016;88(6):3413.
Rook JJ. Formation of haloforms during chlorination of natural water. Acta Polytech Hung. 2002;42(2):234–43.
Bellar TA, Lichtenberg JJ, Kroner RC. The occurrence of organohalides in chlorinated drinking waters. J Am Water Works Assoc. 1974;66(12):703–6.
Hanson SJ, Punzalan RC, Greenup RA, Liu H, Sato TT, Havens PL. Incidence and risk factors for venous thromboembolism in critically ill children after trauma. J Pediatr Surg. 2010;45(9):1920–1.
Hinckley AF, Bachand AM, Reif JS. Late pregnancy exposures to disinfection by-products and growth-related birth outcomes. Environ Health Perspect. 2005;113(12):1808–13.
Emmanuel E, Keck G, Blanchard JM, Vermande P, Perrodin Y. Toxicological effects of disinfections using sodium hypochlorite on aquatic organisms and its contribution to AOX formation in hospital wastewater. Environ Int. 2004;30(7):891–900.
Manish M, Rizwan SM, Khiem T, RS P, MA B. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20(7):1126–67.
Goiffon RJ, Martinez SC, Piwnicaworms D. A rapid bioluminescence assay for measuring myeloperoxidase activity in human plasma. Nat Commun. 2015;6:6271.
Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158(3):879–91.
Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94(1):437–44.
Pan S, Deen MJ, Ghosh R. Low-cost graphite-based free chlorine sensor. Anal Chem. 2015;87(21):10734–7.
Tang Y, Su Y, Yang N, Zhang L, Lv Y. Carbon nitride quantum dots: a novel chemiluminescence system for selective detection of free chlorine in water. Anal Chem. 2014;86(9):4528.
Wang Y, Zhang P, Lu Q, Wang Y, Fu W, Tan Q, et al. Water-soluble MoS2 quantum dots are a viable fluorescent probe for hypochlorite. Mikrochim Acta. 2018;185(4):233.
Walekar LS, Pawar SP, Gore AH, Suryawanshi VD, Undare SS, Anbhule PV, et al. Surfactant stabilized AgNPs as a colorimetric probe for simple and selective detection of hypochlorite anion (ClO−) in aqueous solution: environmental sample analysis. Colloid Surface A. 2016;491:78–85.
Li K, Hou JT, Yang J, Yu XQ. A tumor-specific and mitochondria-targeted fluorescent probe for real-time sensing of hypochlorite in living cells. Chem Commun. 2017;53(40):5539–41.
Yan L, Hu C, Li J. A fluorescence turn-on probe for rapid monitoring of hypochlorite based on coumarin Schiff base. Anal Bioanal Chem. 2018;410:7457.
Hosseini MS, Hashemipour Z, Hosseini N. Synthesis of l-tyrosine-capped ZnSe quantum dots and its application to hypochlorite determination in water. Int J Environ Anal Chem. 2016;96(10):1–14.
Park S, Choi S, Yu J. DNA-encapsulated silver nanodots as ratiometric luminescent probes for hypochlorite detection. Nanoscale Res Lett. 2014;9(1):129.
Zhan Y, Luo F, Guo L, Qiu B, Lin Y, Li J, et al. Preparation of an efficient ratiometric fluorescent nanoprobe (m-CDs@[Ru(bpy)3](2+)) for visual and specific detection of hypochlorite on site and in living cells. ACS Sens. 2017;2(11):1684–91.
Zhang J, Yu SH. Highly photoluminescent silicon nanocrystals for rapid, label-free and recyclable detection of mercuric ions. Nanoscale. 2014;6(8):4096–101.
Zhang XD, Chen XK, Yang JJ, Jia HR, Li YH, Chen Z, et al. Quaternized silicon nanoparticles with polarity-sensitive fluorescence for selectively imaging and killing gram-positive bacteria. Adv Funct Mater. 2016;26(33):5958–70.
Zhong Y, Peng F, Bao F, Wang S, Ji X, Yang L, et al. Large-scale aqueous synthesis of fluorescent and biocompatible silicon nanoparticles and their use as highly photostable biological probes. J Am Chem Soc. 2013;135(22):8350–6.
Zhong Y, Sun X, Wang S, Peng F, Bao F, Su Y, et al. Facile, large-quantity synthesis of stable, tunable-color silicon nanoparticles and their application for long-term cellular imaging. ACS Nano. 2015;9(6):5958–67.
Yan Y, Zhang K, Yu H, Zhu H, Sun M, Hayat T, et al. Sensitive detection of sulfide based on the self-assembly of fluorescent silver nanoclusters on the surface of silica nanospheres. Talanta. 2017;174:387–93.
Qu F, Xia W, Xia L, You J, Han W. A ratiometric detection of heparin with high sensitivity based on aggregation-enhanced emission of gold nanoclusters triggered by silicon nanoparticles. Talanta. 2019;193:37–43.
Zhang Y, Ning X, Mao G, Ji X, He Z. Fluorescence turn-on detection of target sequence DNA based on silicon nanodot-mediated quenching. Anal Bioanal Chem. 2018;410(13):3209–16.
Ma SD, Chen YL, Feng J, Liu JJ, Zuo XW, Chen XG. One-step synthesis of water-dispersible and biocompatible silicon nanoparticles for selective heparin sensing and cell imaging. Anal Chem. 2016;88(21):10474–81.
Zhang X, Chen X, Kai S, Wang HY, Yang J, Wu FG, et al. Highly sensitive and selective detection of dopamine using one-pot synthesized highly photoluminescent silicon nanoparticles. Anal Chem. 2015;87(6):3360–5.
Yi Y, Deng J, Zhang Y, Li H, Yao S. Label-free Si quantum dots as photoluminescence probes for glucose detection. Chem Commun. 2013;49(6):612–4.
Yi Y, Zhu G, Liu C, Huang Y, Zhang Y, Li H, et al. A label-free silicon quantum dots-based photoluminescence sensor for ultrasensitive detection of pesticides. Anal Chem. 2013;85(23):11464–70.
Chen S, Quan Y, Yu Y-L, Wang J-H. Graphene quantum dot/silver nanoparticle hybrids with oxidase activities for antibacterial application. ACS Biomater-Sci Eng. 2017;3(3):313–21.
Ma JL, Yin BC, Wu X, Ye BC. Simple and cost-effective glucose detection based on carbon nanodots supported on silver nanoparticles. Anal Chem. 2017;89(2):1323–8.
Li J, Li Y, Shahzad SA, Chen J, Chen Y, Wang Y, et al. Fluorescence turn-on detection of glucose via the Ag nanoparticle mediated release of a perylene probe. Chem Commun. 2015;51(29):6354–6.
Sasikumar T, Ilanchelian M. Colorimetric detection of hypochlorite based on the morphological changes of silver nanoprisms to spherical nanoparticles. Anal Methods. 2017;9(21):3151–8.
Han Y, Chen Y, Feng J, Liu J, Ma S, Chen X. One-pot synthesis of fluorescent silicon nanoparticles for sensitive and selective determination of 2,4,6-trinitrophenol in aqueous solution. Anal Chem. 2017;89(5):3001–8.
Feng J, Chen Y, Han Y, Liu J, Ren C, Chen X. Fluorescent carbon nanoparticles: a low-temperature trypsin-assisted preparation and Fe(3+) sensing. Anal Chim Acta. 2016;926:107–17.
Ou S-H, Pan L-S, Jow J-J, Chen H-R, Ling T-R. Molecularly imprinted electrochemical sensor, formed on Ag screen-printed electrodes, for the enantioselective recognition of D and L phenylalanine. Biosens Bioelectron. 2018;105:143–50.
Liu X, Yan Z, Sun Y, Ren J, Qu X. A label-free ratiometric electrochemical DNA sensor for monitoring intracellular redox homeostasis. Chem Commun. 2017;53(46):6215–8.
Zhu X, Zhao T, Nie Z, Liu Y, Yao S. Non-redox modulated fluorescence strategy for sensitive and selective ascorbic acid detection with highly photoluminescent nitrogen-doped carbon nanoparticles via solid-state synthesis. Anal Chem. 2015;87(16):8524–30.
Yu Y, Tao Y, Yang B, Wu D, Qin Y, Kong Y. Smart chiral sensing platform with alterable enantioselectivity. Anal Chem. 2017;89(23):12930–7.
Dong Y, Li G, Zhou N, Wang R, Chi Y, Chen G. Graphene quantum dot as a green and facile sensor for free chlorine in drinking water. Anal Chem. 2012;84(19):8378–82.
Hu Y, Yang J, Jia L, Yu J-S. Ethanol in aqueous hydrogen peroxide solution: hydrothermal synthesis of highly photoluminescent carbon dots as multifunctional nanosensors. Carbon. 2015;93:999–1007.
Tang Q, Yang T, Huang Y. Copper nanocluster-based fluorescent probe for hypochlorite. Microchim Acta. 2015;182(13–14):2337–43.
Huang Y, Zhang P, Gao M, Zeng F, Qin A, Wu S, et al. Ratiometric detection and imaging of endogenous hypochlorite in live cells and in vivo achieved by using an aggregation induced emission (AIE)-based nanoprobe. Chem Commun. 2016;52(45):7288–91.
Xue M, Zhang L, Zou M, Lan C, Zhan Z, Zhao S. Nitrogen and sulfur co-doped carbon dots: a facile and green fluorescence probe for free chlorine. Sensors Actuators B Chem. 2015;219:50–6.
Funding
This study received support from the National Natural Science Foundation of China (21607061), the Opening Project of State Key Laboratory of Chemo/Biosensing and Chemometrics of Hunan University (2016017), the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Collaborative Innovation Center of Technology and Material of Water Treatment, and the Program of Young Backbone Teachers in Jiangsu University (2015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1552 kb)
Rights and permissions
About this article
Cite this article
Liu, L., Zhu, G., Zeng, W. et al. Highly sensitive and selective “off-on” fluorescent sensing platform for ClO− in water based on silicon quantum dots coupled with nanosilver. Anal Bioanal Chem 411, 1561–1568 (2019). https://doi.org/10.1007/s00216-019-01597-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01597-5