Abstract
The preparation of an amino-functionalized hybrid monolithic column (TEOS-co-AEAPTES) via one-pot co-condensation of tetraethoxysilane (TEOS) and N-(β-aminoethyl)-γ-aminopropyltriethoxysilane (AEAPTES) in a capillary is descibed. It was used as solid-phase microextraction (SPME) matrix followed by inductively coupled plasma-mass spectrometry (ICP-MS) for determination of trace metals. Under optimum conditions, the amino-functionalized SPME material can simultaneously retain Cu(II), Zn(II), Au(III), and Pb(II) with adsorption capacities of 148, 60, 81, and 64 μg m−1, respectively. Subsequently, these four metal ions can be quantitatively eluted using 1 mol L−1 HNO3 containing 1% thiourea. The retention mechanism of Cu(II), Zn(II), Au(III), and Pb(II) on the amino-functionalized hybrid monolith was explained as the combination of electrostatic and coordination interactions. With a 10-fold enrichment factor, the calibration curves were established in the range 0.5–100 μg L−1 with linear correlation coefficients above 0.9943 and the limits of quantitation were 0.05 μg L−1 for four target analytes. The limits of detection were 0.006, 0.012, 0.004, and 0.007 μg L−1 for Cu(II), Zn(II), Au(III), and Pb(II), respectively. The protocol was validated by analyzing Certified Reference Materials including standard sediment, soil, and nickel ore, and the results were in good agreement with their certified values. The relative standard deviations of the method were in the range 0.22–17.6%. The recoveries of the four metal ions in spiked samples were in the range 88.0–113.8%. Compared to direct ICP-MS determination, the proposed in-tube SPME procedure can effectively eliminate the interference from complex matrix, especially from those ores with very high content of main metal to improve the accuracy of analysis. Therefore the method is suitable for the simultaneous determination of ultra-trace Cu(II), Zn(II), Au(III), and Pb(II) in environmental and mineral samples.

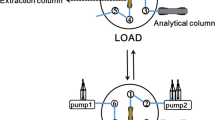
The preparation of the TEOS-co-AEAPTES monolithic column and the SPME procedure of Cu(II), Zn(II), Au(III), and Pb(II)
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metal elements such as copper and zinc are essential and integral trace elements for life [1]. Lead is a highly toxic element which can accumulate in the organism because of biological magnification in the natural environment [2]. Although gold is scarce and stable in nature [3], long-term use of gold-containing products may have a potential impact on human health [4]. Therefore, it is of great significance to analyze these trace metals in environmental samples [5]. Besides, since the existence and level of some characteristic elements, whether macro or trace, reflect important region information of minerals, determination of the abundances of ultra-trace metals in mineral samples is of critical meaning for their source recognition. Because the attention for trace metal elements has been continuously increasing [6], it is quite essential to establish selective, sensitive, and accurate analytical methods for the simultaneous quantification of multiple metals in environmental and mineral samples.
Up to now, there are plenty of techniques for the analysis of metal elements [7,8,9,10]; ICP-MS is most commonly applied in the determination of trace metals in environmental and mineral samples due to its advantages of wide linear range, high sensitivity, and simultaneous detection of multiple elements [10]. However, direct analysis of real samples by ICP-MS still faces some problems. The concentration of some target metals in environmental and mineral samples is very low not to be directly determined, and that is easily contaminated. On the other hand, the analysis of these environmental and mineral samples by ICP-MS suffers from mass spectrum and non-mass spectrum interference resulted from matrix effect [11]. Isobaric overlap, polyatomic or compound ions, refractory oxide ions, and double charge ions all cause mass spectrum interference [12]. For mineral samples, a significant problem to surmount is the severe memory effect that is apparent for high concentration of main metal of ore in the instrument, leading to non-linear calibration graphs, long washout times, decreasing sensitivity with time, and signals dependent on the matrix. Even worse, the metallic mineral matrix not only causes inaccurate measurement results, but also damages mass spectrometer and even causes instrument contamination or flameout [13]. Therefore, efficient sample pretreatment methods are generally required before ICP-MS analysis for real environmental and mineral samples.
Solid-phase extraction (SPE) is one of the frequently used techniques for the preconcentration of trace metal ions [14]. Recently, as a late model solid-phase microextraction extraction (SPME) material, capillary monolithic columns have attracted extensive attention because of their less sample consumption, uniform structure, controllable morphology, convective mass transfer, and excellent adsorption performance [15, 16]. The SPME performed within a capillary tube is also named as in-tube SPME or capillary microextraction (CME) [2]. Monolithic columns include inorganic silica [17], organic polymer [18], and organic-inorganic hybrid monolithic columns [19]. Organic-inorganic hybrid monolithic columns are extensively exploited for separation because of their low-swelling, high mass-exchange, large specific surface area, and high mechanical stability [20]. “One-pot” is a facile preparation procedure for hybrid monolithic column which means functional groups are introduced directly into the monolith in the process of co-condensation of organosilane reagents with tetra-alkoxysilanes. Hybrid monolith prepared via one-pot condensation can make functional groups distributed more evenly and preparation process simpler. Although the application of organic-inorganic hybrid monolithic columns has shown great success in organic and biological analysis fields [21,22,23], only a few works related to them have been conducted in the determination of trace elements and their speciation in environmental samples [24, 25]. Moreover, there are not any reports to utilize hybrid monolithic material as SPME matrix for enriching trace metal elements from high main metal ores prior to ICP-MS determination.
In this present work, organic-inorganic hybrid monolithic column (TEOS-co-AEAPTES) prepared via one-pot process by sol-gel technology in a fused capillary was applied for simultaneous SPME of Cu(II), Zn(II), Au(III), and Pb(II) followed by ICP-MS determination. The adsorption and elution conditions of these four metal ions were optimized comprehensively. The interaction mechanism between target ions and amino-functionalized monolith was explained accordingly. The analytical figures of merits were also concluded to further prove the reliability of the method. Finally, the application potential of TEOS-co-AEAPTES monolith was investigated as SPME absorbent for ICP-MS determination of Cu(II), Zn(II), Au(III), and Pb(II) in environmental waters, sediments, soils, coals, and iron ores.
Materials and methods
Reagents and materials
Tetraethoxysilane (TEOS, 98%) was purchased from Alfa Aesar (Tianjin, China). N-(β-aminoethyl)-γ-aminopropyltriethoxysilane (AEAPTES, 98%) and thiourea (99+%) were purchased from Ourchem (Shanghai, China). Cetyltrimethylammonium bromide (CTAB, 98+%) was purchased from TCI (Tokyo, Japan). Nitric acid was of guaranteed reagent grade and purchased from Merck (Zurich, Switzerland). All other chemicals were at least of analytical reagent grade and used without further purification. Pure water (18.25 MΩ cm) obtained from a Milli-Q water system (Millipore, Bedford, MA, USA) was used throughout the experiment.
Stock solutions (1 mg mL−1) of Cu(II), Zn(II), and Pb(II) were prepared by dissolving appropriate amounts of Cu(NO3)2·2H2O, Zn(NO3)2·6H2O, and Pb(NO3)2 (National Institute of Metrology, Beijing, China), respectively, in 2% (v/v) diluted HNO3. Stock solution (1 mg mL−1) of Au(III) was prepared by diluting 1000 mg mL−1 of HAuCl4 solution (National Research Center for Certified Reference Materials, Beijing, China). Lower concentration standard solutions were prepared daily by appropriate dilutions from their stock solutions. Analytical mixture standard solution (each of 1 mg L−1) containing Cu(II), Zn(II), Au(III), and Pb(II) was prepared by mixing and diluting the above four stock solutions.
Certified Reference Materials of GBW07309 sediment (GSD-9) and GBW07402 soil (GSS-2) were purchased from Institute of Geophysical and Geochemical Prospecting for Certified Reference Materials (Langfang, China). Certified Reference Material of GBW(E)070110 nickel ore (Fe, 46.99%) was purchased from the National Research Center for Certified Reference Materials. Tap water was collected in the Chemical Building in Xianlin Campus of Nanjing University. River water was taken from Yangtze River in Nanjing. Both water samples were stored with 2% (v/v) HNO3. Soil was taken from Gulou Campus of Nanjing University, and sampling of soil is given in the Electronic Supporting Material (ESM). All mineral samples were provided by the Technical Center for Industrial Product and Raw Material Inspection and Testing, Shanghai Customs (Shanghai, China). Water samples were filtered through a 0.45 μm cellulose acetate membrane before analysis. Sediment, soil, coal, and iron ore samples were digested with nitric acid, hydrogen peroxide, and aqua regia before analysis [26]. The detailed procedure is given in the ESM.
Instrumentation
The determination of four target elements including 63Cu, 66Zn, 197Au, and 208Pb was performed on a Perkin-Elmer NexION 350D ICP-MS (Perkin-Elmer SCIEX, Concord, Canada) and the optimum operation conditions are summarized in Table S1. The pH values of solutions were controlled by a FiveEasy Plus pH meter (Mettler-Toledo, Shanghai, China). During the extraction process, a syringe infusion pump (LSP04-1A, LongerPump, Hebei, China) was used to introduce solution into the monolithic column.
Scanning electron microscopy (SEM) images were recorded with a Hitachi S-3400 II SEM (Hitachi, Tokyo, Japan). Fourier-transform infrared (FT-IR) spectra were collected on a NEXUS 870 FT-IR spectrometer (Nicolet, USA) using KBr pellets. Elemental analysis (EA) of the monolith was carried out using an Elementar Vario EL II elemental analyzer (Elementar, German) with oxygen as the combustion gas. Pore size distribution was investigated by a mercury intrusion porosimeter (Poremaster GT-60, Quantachrome, USA). The surface area was calculated using mercury intrusion porosimetry methods.
Preparation of TEOS-co-AEAPTES hybrid monolithic column
The TEOS-co-AEAPTES monolithic capillary was prepared by the procedures reported previously [27, 28] with minor modifications, as detailed in the ESM.
SPME and analysis procedure
The TEOS-co-AEAPTES monolithic capillary was used as SPME matrix during Cu(II), Zn(II), Au(III), and Pb(II) enrichment procedure (Scheme 1) prior to ICP-MS detection. The pH of sample solution was adjusted to 4.5 with HNO3 or NH3·H2O. In the extraction step, 3 mL solution (pH 4.5) was allowed to pump through the TEOS-co-AEAPTES column at a flow rate of 150 μL min−1. Subsequently, 300 μL of mixture of 1 mol L−1 HNO3 with 1% (m/v) thiourea was used as eluent to syringe the analytes retained on the monolithic capillary at the same flow rate. Both effluents obtained during the loading and elution processes were collected and four analytes were measured simultaneously by ICP-MS. Purified water was adjusted to pH 4.5 as the blank sample.
Results and discussion
Choice of materials and metal ions
The extraction efficiency of SPE/SPME depends mainly on the properties of the adsorption materials. According to the Lewis acid-base theory, there is a high affinity between transition metal ions as Lewis acid and ligands containing amino as Lewis base. Therefore, extraction matrices with amino-containing functional group have high adsorption efficiency towards metal ions [28, 29]. Inspired by this knowledge, three amino-functional monomers including aminopropyltriethoxysilane (APTES), AEAPTES, and [(2-aminoethylamino)ethylamino]propyltrimethoxysilane (AAAPTES) were considered in this study. It was found that AEAPTES had the best solubility in the solvent system of the experiment. The obtained monolith prepared by AEAPTES was uniform and porous, which was suitable for the extraction of metal ions. The octanol-water partition coefficients (logP) of APTES, AEAPTES, and AAAPTES calculated by ACD ChemSketch were − 0.22 ± 0.44, − 0.71 ± 0.56, and 1.07 ± 0.55, respectively. Therefore, AEAPTES was the most hydrophilic among the three amino-functional monomers, which supported the experimental results. As mentioned, hybrid monolith prepared via one-pot condensation can make functional groups distributed more evenly and preparation process simpler. Consequently, TEOS-co-AEAPTES hybrid monolithic column prepared via one-pot method was employed to investigate the extraction of trace metal ions.
The existence and level of some characteristic elements including Cu(II), Zn(II), Au(III), and Pb(II) prevalent in environmental and mineral samples can reflect important region information. The extraction feasibility of the prepared TEOS-co-AEAPTES hybrid monolithic column towards the characteristic metal ions in environmental and mineral samples was investigated. It was found that Cu(II), Zn(II), Au(III), and Pb(II) can be quantitatively adsorbed in the same weakly acidic condition (pH 4.0–6.0). Therefore, these four ions were selected as the target analytes in this study. The preparation of the TEOS-co-AEAPTES monolithic column and the SPME procedure of Cu(II), Zn(II), Au(III), and Pb(II) under optimal conditions are shown in Scheme S1 in the ESM.
Characterization of hybrid monolithic column
As the SEM photographs at different magnifications showed, the TEOS-co-AEAPTES skeletons formed were homogeneous and also attached to the inner surface of the fused silica capillary tightly. Even better, no apparent voids along the inner wall of capillary could be observed (Fig. 1a, b). The monolith possessed continuous macroporous structure and interconnecting spheres with uniformly small size of particles and rough surface (Fig. 1c, d). Therefore, the well-controlled skeleton of the monolith was beneficial to the formation of high surface area, low back-pressure, and high permeability. The diameter size of the pore of the TEOS-co-AEAPTES monolith was analyzed by mercury intrusion porosimetry to be 3 μm (Fig. S1), and the average surface area was calculated 15 m2 g−1. In addition, in order to examine the mechanical stability of the monolithic capillary, the pressure variation of the column was recorded at different flow rates of methanol (Fig. S2). The pressure was linearly increased in the tested flow rate range, which revealed that the column had low back-pressure and excellent mechanical strength.
FT-IR spectrum of the organic-inorganic hybrid monolith is shown in Fig. 2. The absorption bands around 1058 cm−1 corresponded to the stretching vibrations of Si-O-Si groups. The strong peaks around 1472 and 1638 cm−1 resulted from the C-N and N-H stretching vibrations, respectively. The appearances of adsorption bands around 2925 and 3375 cm−1 were ascribed to the vibration of C-H and O-H, respectively. All above indicated that the amino-silane group from AEAPTES had been incorporated into the monolith. Moreover, thermal analysis including TGA and DSC proved the incorporation of AEAPTES in the matrix (Fig. S3). TGA showed that the weight loss process could be divided into three stages, and the proportion of each stage was approximately 7.5%:7.5%:10%, which was consistent with the decomposition of AEAPTES. There were two sharp peaks in the DSC curve between 200 and 400 °C, which was related to the decomposition of amino groups. In addition, EA data showed the presence of carbon and nitrogen elements derived from organosilane reagents (Table S2). In conclusion, all of the characterization results proved that the amino-functionalized monolithic capillary had been successfully synthesized.
Optimization of analysis procedure
Optimization of adsorption condition
The pH of aqueous solution plays a significant role in adsorption process [21, 24]. In this work, the effect of the pH of 1 mL aqueous solution on the adsorption percentages of 30 μg L−1 Cu(II), Zn(II), Au(III), and Pb(II) on the TEOS-co-AEAPTES monolithic column was investigated with pH varying in the range of 1.0–8.0. It could be observed in Fig. 3 that in the relatively wide pH range of 3.0–8.0, four studied metals were quantitatively adsorbed by TEOS-co-AEAPTES monolithic column. At pH below 3.0, the amino-functionalized monolith had a positively charged surface due to protonation of amino groups (NH3+), leading to electrostatic repulsion between amino and metal ions including Cu2+, Zn2+ and Pb2+. On the contrary, NH3+ on the monolith had certain electrostatic attraction towards AuCl4−, making partial gold ions absorb on the column. When the pH increased from 3.0 to 8.0, the functional groups on the monolith changed from NH3+ to NH2, the electrostatic attraction interaction gradually disappeared, and instead, the coordination interaction between NH2 and Cu2+, Zn2+, and Pb2+ dominated. In addition, there was a strong affinity between NH2 and Au3+, so NH2 could replace Cl− and coordinate with Au3+, resulting in the adsorption of Au3+ on the AEAPTES monolith. Since the metal ions might be hydrolyzed at the higher pH than 6.0 [30], solution pH was adjusted to 4.5 for the further experiments.
Other adsorption conditions including the sample flow rate and solution volume [2, 24] were investigated with the results shown in Fig. S4 and S5, respectively. In short, the following experimental conditions were found to give optimal results: (a) Sample pH value 4.5; (b)Flow rate 150 μL min−1; (c) Solution volume 3 mL.
Optimization of elution condition
Strong acids were selected as the eluents at the beginning in view of the result of dependence of adsorption rate on pH (Fig. 3). The result in Fig. 4a showed that either 1 mol L−1 HNO3 or HCl can elute Cu(II), Zn(II), and Pb(II) successfully, except Au(III). Due to the electrostatic attraction interaction between AuCl4− and the protonated amino groups at very low pH, it was difficult to release Au(III) from amino-functionalized monolith utilizing acids only. Therefore, thiourea was added to the eluent according to its good affinity to Au(III) [31]. As could be seen, the desorption effects of all four metal ions were excellent when 1% (m/v) thiourea was added in the eluent. In Fig. 4b, there was no obvious difference in the desorption effect of Cu(II), Zn(II), Au(III), and Pb(II) while changing the acid concentration. All these ions could be quantitatively eluted when the concentration of thiourea varying from 1% to 5% with HNO3/HCl concentration fixing at 1 mol L−1 (Fig. 4c). Consequently, 1 mol L−1 HNO3 with 1% thiourea was employed for the elution of all target ions in the following experiments. The effect of elution volume on desorption rate of target ions was also optimized. A total of 1500 μL eluent (1 mol L−1 HNO3 with 1% thiourea) was continuously used to desorb Cu(II), Zn(II), Au(III), and Pb(II) from the TEOS-co-AEAPTES monolithic column and the effluent was collected each 300 μL. The result in Fig. 4d distinctly indicated that the initial 300 μL eluents were plentiful for desorbing all target ions from the column. Comprehensive considering the experimental efficiency and enrichment factor, the eluent being as less as better, the elution volume was ultimately set to 300 μL when the sample consumption was 3 mL with the enrichment of 10 times for target ions. The desorption rates of Cu(II), Zn(II), Au(III), and Pb(II) did not change obviously during experiment of elution flow rate varying in the range of 20–300 μL min−1 (Fig. S6). The result was similar to the flow rate experiment of adsorption. As a result, the elution flow rate we employed in this research work was determined as 150 μL min−1.
Effect of eluent on the desorption rates of Cu(II), Zn(II), Au(III), and Pb(II). a Effect of eluent composition; b Effect of nitric acid or hydrochloric acid concentration (0.5, 1, and 1.5 mol L−1 HNO3/HCl with 1% thiourea); c Effect of thiourea concentration (1 mol L−1 HNO3/HCl with 1%, 3%, and 5% thiourea); d Effect of elution times, 300 μL eluent (1 mol L−1 HNO3 with 1% thiourea) each time. Concentration of each metal ions in sample solution, 30 μg L−1; Sample volume, 3 mL; Solution pH, 4.5; Eluent volume of a, b, and c, 1 mL
Adsorption capacity of TEOS-co-AEAPTES monolithic column
Adsorption capacity is a significant indicator to evaluate the performance of SPME material. The breakthrough curve experiment recommended by Hu et al. [32] was carried out in this present work. In order to investigate the maximum adsorption capacity of TEOS-co-AEAPTES monolithic column for four target ions, 15 mL solutions containing each target metal ion (1 mg L−1, pH 4.5) were continuously pumped through the 5-cm length monolithic column at 150 μL min−1, respectively. Then, the concentration of target ion in the effluent was determined by ICP-MS. When the adsorption of an ion by the monolithic column was saturated, the concentration of the ion in the effluent suddenly increased and approached the initial concentration. The breakthrough curves were shown in Fig. S7 and the maximum adsorption capacities of the monolithic column for Cu(II), Zn(II), Au(III), and Pb(II) were 148, 60, 81, and 64 μg m−1 with the relative standard deviations (RSDs) of 1.0%, 2.2%, 1.6%, and 1.1%, respectively, which were much higher than subsequent application needs.
The reproducibility and reusability of TEOS-co-AEAPTES monolithic column
The reproducibility of TEOS-co-AEAPTES monolithic columns was evaluated by investigating the recoveries of 30 μg L−1 solution containing four target metal ions under the optimized conditions with six segments of monolithic capillary prepared in the same batch and different batches. The results in Table S3 indicated that the intra-batch RSDs of the preparation reproducibility of monolithic column ranged from 1.8% (Cu(II)) to 7.3% (Zn(II)) and the inter-batch RSDs ranged from 2.4% (Pb(II)) to 8.1% (Zn(II)). Furthermore, the prepared monolithic column can be reused for six times at least because the recoveries of Cu(II), Zn(II), Au(III), and Pb(II) did not decrease obviously during six successive repeating experiments with the RSDs of 4.0%, 8.1%, 0.47%, and 1.8%, respectively (Fig. S8). In addition, the stability of the columns stored for different duration (freshly prepared, stored for 1 week, 2 weeks, and 4 weeks) was also evaluated under the same conditions, and the RSDs (n = 4) of the recovery rates ranged from 4.8% (Cu(II)) to 7.7% (Zn(II)). The experiments verified excellent repeatability and stability of the amino-functionalized hybrid monolithic capillary.
Interference study
The possible coexisting ions in environmental and mineral samples may compete with the analytes for the active sites of the amino-functionalized monolith, especially the ore samples that were rich in a variety of high level main metals. Thus, the interference effects of coexisting ions were investigated as follows. 3 mL aqueous solution containing Cu(II), Zn(II), Au(III), and Pb(II) each at 5 μg L−1 with a certain concentration of coexisting ion was subjected to the general SPME-ICP-MS procedure. The results in Table 1 indicated that the recoveries of four target ions were in the range of 84.3–108.9% in the presence of 25 mg L−1 of Na+, 25 mg L−1 of K+, 10 mg L−1 of Fe3+, 1 mg L−1 of Ca2+, Al3+, Mg2+, Ni2+, and Mn2+. In addition, main anions such as SO42−, NO3−, and Cl− with the concentration of 10 mg L−1 and PO43− of 1 mg L−1 did not change the recoveries obviously. It demonstrated that the prepared TEOS-co-AEAPTES monolithic column had a relatively good tolerance to the coexisting interference and maintained adsorption capacity of target ions at a high concentration of interfering ions. According to the main components of the real samples that had been digested already, the presence of major interfering ions and their concentrations were included in this interference study. Furthermore, the important interference components in the real samples were focused. For example, the concentration of Fe3+ in the SPME effluent was 18.3% of the initial concentration, which indicated that the matrix interference could be effectively reduced by the TEOS-co-AEAPTES monolithic column. Therefore, the SPME-ICP-MS protocol had an excellent anti-interference ability for the determination of ultra-trace Cu(II), Zn(II), Au(III), and Pb(II) in environmental and mineral samples of complex matrices.
Analytical performance of SPME-ICP-MS method
The analytical performance of the developed TEOS-co-AEAPTES monolith based SPME-ICP-MS method was evaluated under the optimized conditions. The calibration curves established in the range of 0.5–100 μg L−1 for Cu(II), Zn(II), Au(III), and Pb(II) equations were Y = 10.245X − 0.1289, Y = 9.3065X − 0.2218, Y = 9.7406X + 0.3544, and Y = 9.8710X + 0.2647, respectively with r2 of 0.9972, 0.9992, 0.9958, and 0.9943, and the limits of quantitation (LOQs) were 0.05 μg L−1 for these ions. The limits of detection (LODs, defined as 3-fold the standard deviation of blank signal intensity) of Cu(II), Zn(II), Au(III), and Pb(II), with 10-fold enrichment factor, were 0.006, 0.012, 0.004, and 0.007 μg L−1, respectively for water samples, and 0.008, 0.016, 0.005, and 0.009 μg g−1, respectively for sediment, soil, coal, and ore samples. Therefore, this protocol is quite suitable for ultra-trace metal analysis of environmental and mineral samples.
For comparison, the analytical performance of the method and other related approaches reported in the literature for Cu(II), Zn(II), Au(III), and Pb(II) analysis is listed in Table 2. The proposed method has relatively lower LODs for all target ions and lower than most methods reported. The method can analyze four target ions at the same time with high analysis efficiency, while the work about analysis of Au(III) based on similar materials has been rarely reported. In addition, the proposed method can be applied to more kinds of real samples with complex matrices. Although the monolith did not have the ability to extract a certain metal ion specifically like ion-imprinted materials, and anti-interference test should be carefully carried out, the proposed method can eliminate the interference from those ores with extremely high content of main metal, which is very meaningful for accurate analysis of multiple characteristic trace ions in environmental and mineral samples.
The accuracy of the proposed method was verified by determining trace Cu(II), Zn(II), Au(III), and Pb(II) in Certified Reference Materials of GSD-9 (Sediment), GSS-2 (Soil) and GBW(E)070110 (Nickel ore). The results summarized in Table S4 indicated that the determined values of these target metals were in accordance with the certified values, which further proved the reliability of this protocol. However, if the nickel ore was directly determined by ICP-MS after digestion and simple dilution without SPME pretreatment, the results of four target elements greatly differed from the certified values (Table S5). This is likely attributed to the strong interference of high concentration of main metal ions in the sample solution. It was interestingly found that most of the interfering ions in the ore could be eliminated by the SPME procedure with the prepared amino-functionalized hybrid monolith capillary. For example, 82.4% Fe3+ and 78.6% Ni2+ in the digestion solution were removed after SPME by detecting them in the effluent. Therefore, this TEOS-co-AEAPTES monolithic column can not only effectively achieve the preconcentration of target ions but also powerfully reduce the interference from the main metals in ores.
Furthermore, the proposed method was applied to the analysis of environmental water, soil, coal, and iron ore samples from different sources. The determination results for Cu(II), Zn(II), Au(III), and Pb(II) were also listed in Table 3, indicating good precision of the method with the RSDs of 0.22–17.6%. The recoveries of four metal ions spiked in these environmental and mineral samples were summarized in Table S6. The recoveries of four target ions in all spiked samples were obtained 88.0–113.8%, demonstrating that the proposed SPME-ICP-MS protocol could be used in the simultaneous determination of ultra-trace elements in real environmental and mineral samples.
Conclusions
TEOS-co-AEAPTES hybrid capillary monolithic column prepared via one-pot was employed for the SPME of Cu(II), Zn(II), Au(III), and Pb(II) in environmental and mineral samples. The adsorption mechanism of four metal ions was explained as the combination of electrostatic and coordination interactions based on the transformation of NH2 and NH3+ at different pH. Notably to Au(III), there exists a competing coordination between amino group and chloride ion under pH 3.0–5.0. The purposed SPME-ICP-MS method possessed strong anti-interference ability, simple preparation and reusability, low sample consumption, and high efficiency of analysis. Although the proposed method was limited in specificity, it can effectively reduce miscellaneous matrix interference in complex samples, when simultaneous multi-element analysis was performed. To our knowledge, this work is the first time to apply organic-inorganic hybrid monolithic capillary for the determination of ultra-trace elements in complex environmental and mineral samples, especially in the ores with very high content of main metal.
References
Wong CK, Pak AP (2004) Acute and subchronic toxicity of the heavy metals copper, chromium, nickel, and zinc, individually and in mixture, to the freshwater copepod Mesocyclops pehpeiensis. Bull Environ Contam Toxicol 73:190–196
Zhang L, Chen BB, Peng HY, He M, Hu B (2011) Aminopropyltriethoxysilane-silica hybrid monolithic capillary microextraction combined with inductively coupled plasma mass spectrometry for the determination of trace elements in biological samples. J Sep Sci 34:2247–2254
Medveď J, Bujdoš M, Matúš P, Kubová J (2004) Determination of trace amounts of gold in acid-attacked environmental samples by atomic absorption spectrometry with electrothermal atomization after preconcentration. Anal Bioanal Chem 379:60–65
Chen ZN, Chen BB, He M, Wang H, Hu B (2019) A porous organic polymer with magnetic nanoparticles on a chip array for preconcentration of platinum(IV), gold(III) and bismuth(III) prior to their on-line quantitation by ICP-MS. Microchim Acta 186:107
Yu J, Zhu SW, Lu QF, Zhang ZC, Zhang XM, Wang X, Yang W (2018) High sensitive determination of Pb and Zn in refined copper ores samples using liquid cathode glow discharge-atomic emission spectrometry. Spectrosc Spect Anal 38:3550–3557
Yang H, He L, Long Y, Li H, Pan S, Liu H, Hu X (2018) Fluorescent carbon dots synthesized by microwave-assisted pyrolysis for chromium(VI) and ascorbic acid sensing and logic gate operation. Spectrochim Acta A 205:12–20
Zhou SY, Song N, Liu SX, Chen DX, Jia Q, Yang YW (2014) Separation and preconcentration of gold and palladium ions with a carboxylated pillar arene derived sorbent prior to their determination by flow injection FAAS. Microchim Acta 181:1551–1556
Chen AH, Chen SL, Wu YH, Shao Y, Zhang CL (2014) Direct and sequential determination of six metal elements in cooking wine by HR-CS GFAAS. Adv Mater Res 1033-1034:658–662
Tang R, Li TP, Gu XS, Li YJ, Yang Y (2010) Studies on six heavy metal elements dissolution characteristics of Andrographis herb by ICP-OES. Spectrosc Spect Anal 30:528–531
Heitland P, Köster HD (2004) Fast, simple and reliable routine determination of 23 elements in urine by ICP-MS. J Anal At Spectrom 19:1552–1558
Huang CY, Beauchemin D (2003) Direct multielemental analysis of human serum by ICP-MS with on-line standard addition using flow injection. J Anal At Spectrom 18:951
Burney D, Neal CR (2019) Method for quantifying and removing polyatomic interferences on a suite of moderately volatile elements (Zn, Se, Rb, Ag, Cd, In, Sb, Tl, Pb, and Bi) during solution-mode ICP-MS. J Anal At Spectrom 34:1856–1864
Harrington CF, Merson SA, D’Silva TM (2004) Method to reduce the memory effect of mercury in the analysis of fish tissue using inductively coupled plasma mass spectrometry. Anal Chim Acta 505:247–254
Chen ZN, Chen BB, He M, Hu B (2019) Magnetic metal-organic framework composites for dual-column solid-phase microextraction combined with ICP-MS for speciation of trace levels of arsenic. Microchim Acta 187:48
Xu L, Shi ZG, Feng YQ (2011) Porous monoliths: sorbents for miniaturized extraction in biological analysis. Anal Bioanal Chem 399:3345–3357
Xi Y, Du YX, Sun XD, Zhao SY, Feng ZJ, Chen C, Ding W (2019) A monolithic capillary modified with a copoplymer prepared from the ionic liquid 1-vinyl-3-octylimidazolium bromide and styrene for electrochromatography of alkylbenzenes, polycyclic aromatic hydrocarbons, proteins and amino acids. Microchim Acta 187:67
Alzahrani E, Fallatah AM (2019) Fabrication of inorganic monolith coated with gold nanoparticles for protein purification. Int J Electrochem Sci 14:1293–1309
Vyviurska O, Lv YQ, Mann BF, Svec F (2018) Comparison of commercial organic polymer-based and silica-based monolithic columns using mixtures of analytes differing in size and chemistry. J Sep Sci 41:1558–1566
Wang Z, Zhao JC, Lian HZ, Chen HY (2015) Aptamer-based organic-silica hybrid affinity monolith prepared via “thiol-ene” click reaction for extraction of thrombin. Talanta 138:52–58
Wang KY, Chen YZ, Yang HH, Li Y, Nie LH, Yao SZ (2012) Modification of VTMS hybrid monolith via thiol-ene click chemistry for capillary electrochromatography. Talanta 91:52–59
Wang M, Ma HF, Chi Q, Li Q, Li M, Zhang HJ, Li CY, Fang HF (2019) A monolithic copolymer prepared from N-(4-vinyl)-benzyl iminodiacetic acid, divinylbenzene and N,N'-methylene bisacrylamide for preconcentration of cadmium(II) and cobalt(II) from biological samples prior to their determination by ICP-MS. Microchim Acta 186:537
Liu XL, Suo FY, He M, Chen BB, Hu B (2017) Imidazole functionalized organic monoliths for capillary microextraction of Co(II), Ni(II) and Cd(II) from urine prior to on-line ICP-MS detection. Microchim Acta 184:927–934
Weller A, Carrasco-Correa EJ, Belenguer-Sapina C, de los Reyes Mauri-Aucejo A, Amoros P, Herrero-Martinez JM (2017) Organo-silica hybrid capillary monolithic column with mesoporous silica particles for separation of small aromatic molecules. Microchim Acta 184:3799–3808
Zhao LY, Fei JJ, Lian HZ, Mao L, Cui XB (2019) Simultaneous speciation analysis of chromium and antimony by novel carboxyl-functionalized hybrid monolithic column solid phase microextraction coupled with ICP-MS. J Anal At Spectrom 34:1693–1700
Zhao LY, Zhu QY, Mao L, Chen YJ, Lian HZ, Hu X (2019) Preparation of thiol- and amine-bifunctionalized hybrid monolithic column via “one-pot” and applications in speciation of inorganic arsenic. Talanta 192:339–346
Santoro A, Held A, Linsinger TPJ, Perez A, Ricci M (2017) Comparison of total and aqua regia extractability of heavy metals in sewage sludge: the case study of a certified reference material. Trends Anal Chem 89:34–40
Yu SB, Geng J, Zhou P, Wang J, Feng AR, Chen XD, Tong H, Hu JM (2008) Application of a new hybrid organic-inorganic monolithic column for efficient deoxyribonucleic acid purification. Anal Chim Acta 611:173–181
Li P, Zhang XQ, Chen YJ, Lian HZ, Hu X (2014) A sequential solid phase microextraction system coupled with inductively coupled plasma mass spectrometry for speciation of inorganic arsenic. Anal Methods 6:4205–4211
Chen S, Hayakawa S, Shirosaki Y, Fujii E, Kawabata K, Tsuru K, Osaka A (2009) Sol-gel synthesis and microstructure analysis of amino-modified hybrid silica nanoparticles from aminopropyltriethoxysilane and tetraethoxysilane. J Am Ceram Soc 92(9):2074–2082
Wang LY, Li JH, Wang JN, Guo XT, Wang XY, Choo J, Chen LX (2019) Green multi-functional monomer based ion imprinted polymers for selective removal of copper ions from aqueous solution. J Colloid Interf Sci 541:376–386
Calle IDL, Cabaleiro N, Costas M, Pena F, Gil S, Lavilla I, Bendicho C (2011) Ultrasound-assisted extraction of gold and silver from environmental samples using different extractants followed by electrothermal-atomic absorption spectrometry. Microchem J 97:93–100
Hu WL, Hu B, Jiang ZC (2006) On-line preconcentration and separation of Co, Ni and Cd via capillary microextraction on ordered mesoporous alumina coating and determination by inductively plasma mass spectrometry (ICP-MS). Anal Chim Acta 572:55–62
Zheng F, Hu B (2007) Preparation of a high pH-resistant AAPTS-silica coating and its application to capillary microextraction (CME) of Cu, Zn, Ni, Hg and Cd from biological samples followed by on-line ICP-MS detection. Anal Chim Acta 605:1–10
Rahmi D, Zhu YB, Fujimori E, Umemura T, Haraguchi H (2007) Multielement determination of trace metals in seawater by ICP-MS with aid of down-sized chelating resin-packed minicolumn for preconcentration. Talanta 72:600–606
Yin J, Jiang ZC, Chang G, Hu B (2005) Simultaneous on-line preconcentration and determination of trace metals in environmental samples by flow injection combined with inductively coupled plasma mass spectrometry using a nanometer-sized alumina packed micro-column. Anal Chim Acta 540:333–339
Funding
This work was supported by the National Key R&D Program of China (2018YFF0215400), the National Natural Science Foundation of China (21577057, 91643105, 21874065), the Natural Science Foundation of Jiangsu Province (BK20171335), and the Scientific Research Projects of the General Administration of Customs (2019HK074).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 3.47 mb)
Rights and permissions
About this article
Cite this article
Fei, Jj., Zhao, Ly., Wu, Xh. et al. In-tube solid-phase microextraction with a hybrid monolithic column for the preconcentration of ultra-trace metals prior to simultaneous determination by ICP-MS. Microchim Acta 187, 356 (2020). https://doi.org/10.1007/s00604-020-04329-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04329-0