Abstract
Magnetite (Fe3O4) nanoparticles were modified with nanocellulose and are showed to be a useful sorbent for magnetic solid-phase extraction of mercury species. Speciation analysis was performed by using gas chromatography coupled to atomic fluorescence detection (GC-pyro-AFS). The magnetic properties of the sorbent make this approach simple and rapid, and the use of a renewable and biodegradable nanomaterial (nanocellulose) makes it environmentally friendly. The factors that affect adsorption (pH value, amount of nanomaterial, time, volume of sample) and desorption (solvent, time) have been optimized. Both desorption and derivatization of mercury species were performed in a single step. This reduces considerably the sample preparation time. Under the optimized conditions, the limits of detection are 4.0 pg mL−1 for monomethylmercury and 5.6 pg mL−1 for inorganic mercury. The repeatability and reproducibility are satisfactory. The method enables inorganic mercury and monomethylmercury to be simultaneously extracted, with preconcentration factors up to 300. The potential interferences of organic matter and/or co-existing ions were also investigated using synthetic waters. The procedure was applied to the analysis of tap water and river water samples with different characteristics from a mercury polluted area (Almadén, Spain). The extraction recoveries ranged from 81 to 98% regardless of the type of water, which demonstrates the applicability of the method. This is the first time that this kind of sorbent is used for trace metal speciation.

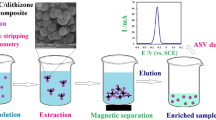
Schematic representation of the new composite material (made of Fe3O4 magnetic nanoparticles and cellulose fibers, MCNPs) for the simultaneous extraction and preconcentration of mercury species taking advantage of the magnetic properties of this eco-friendly sorbent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid phase extraction (SPE) is a widely used technique for preconcentration and clean-up due to its advantages in comparison to other conventional sample preparation techniques such as liquid-liquid extraction [1]. However, SPE presents some operational limitations related to the quality and uniformity of the packing material. The dispersive mode (DSPE) is based on the dispersion of the sorbent in the sample matrix resulting in an extraction of the analytes in the solution and not in a column [2]. In DSPE, column packing and clogging are avoided, but difficulties in the separation of the sorbent still remain. Recent improvements of this technique have come from the incorporation of nanomaterials that allow to overcome part of these drawbacks through the development of new sorbents and working modes. Among the new renewable nanomaterials which have been explored in last years as green alternatives to traditional sorbents, nanocellulose (NC) stands out as an interesting option because of properties such as biocompatibility, biodegradability, or thermal and chemical stability [3, 4]. NC also presents a good surface reactivity producing strong intermolecular and intramolecular hydrogen bonds, which leads to a high extraction efficiency [5]. Regarding the new working modes, an interesting alternative is magnetic solid-phase extraction (MSPE), which is a special kind of DSPE based on the use of solid sorbents with magnetic properties, generally magnetic nanoparticles [6]. Thus, the potential of NC as a nanostructured sorbent can be strongly improved by the coupling of this nanomaterial with magnetic nanoparticles to acquire additional magnetic properties. The combination of SPE with magnetic nanoparticles or composites, i.e. Fe3O4 nanoparticles modified with NC (MCNPs) has become an advantageous approach for sample preparation, as reported in recent works [7, 8]. The use of magnetic nanosorbents simplifies the extraction process and saves time as a result of their easy isolation from the matrix by an external magnetic field [9]. Thus, MCNPs can be considered as an efficient and economic option for preconcentration, clean-up and extraction operations due to the low surface hydrophilic coatings, excellent controlled retention of the target analyte and fast recovery by a magnet [10].
Despite the remarkable advantages of this combination, its applications are still very scarce. Up to now, MCNPs have been mostly used for the adsorption and removal of trace metals, such as Hg(II), Pb (II), Cu(II), Ag(I) or Cr(VI), in some environmental applications [11,12,13,14]. The potential of MCNPs as analytical tools has been reported for the extraction of pesticides in milk samples [15], polychlorinated biphenyls (PCBs) in juice samples [16] and for ions and emerging pollutants in waters [17, 18] but not in trace element speciation. Therefore, the potential of MCNPs as analytical tools should be further explored and extended to other application fields where low concentrations of analytes must be determined and preconcentration is needed, such as trace element speciation analysis. Among the different cases of study within the elemental speciation field, the analysis of mercury is of particular relevance because it is one of the most toxic elements, with severe impact on human health, and it naturally occurs at trace levels in different chemical species, mainly monomethylmercury (MMHg) and inorganic mercury (Hg2+), with a distinct toxicological behaviour.
Up to now, most MSPE methods applied for the analysis of mercury species in water samples are based on conventional sorbents. Accordingly, they are usually limited by the use of expensive and/or not environmentally friendly raw materials and the need of complex synthesis or functionalization of MNPs [19,20,21,22,23,24]. Furthermore, some of them only enable the selective adsorption and determination of one mercury species [25,26,27,28,29,30]. The development of fast and reliable MSPE methodologies using eco-friendly sorbents and simple analytical tools, which can simultaneously preconcentrate different mercury species, becomes an analytical challenge.
Hence, the aim of this study is to develop and apply, for the first time, an efficient procedure of MSPE based on the use of MCNPs, an environmentally friendly sorbent, for the simultaneous analysis of MMHg and Hg2+ in water samples. The simplification of the sample preparation combining in a single step the preconcentration and the adequation of mercury species for their further analysis by gas chromatography coupled to atomic fluorescence detection (GC-pyro-AFS) will be explored, too.
Experimental
Reagents, standards and materials
All reagents were of analytical grade and low mercury contents in the case of acids. The stock standard solutions (1000 mg L−1) of MMHg and Hg2+ were prepared by dissolving methylmercury chloride (Strem Chemicals, Newburyport, USA, www.strem.com) in methanol and Hg (II) chloride (Merck, Darmstadt, Germany, www.merckgroup.com) in 5% HNO3, respectively. These solutions were stored in amber glass bottles and kept at 4 °C until analysis. The working standard solution for each individual mercury species were prepared daily by diluting the stock solutions with ultrapure water (resistivity ≥18 MΩ cm). Standard solutions of iron and manganese in 2% HNO3 (1000 mg L−1) were purchased from Inorganic Ventures (Lakewood, USA, www.inorganicventures.com).
Iron (II) sulphate heptahydrate, cobalt (II) chloride, potassium nitrate, cellulose microcrystalline (~50 μm particle size) and humic acid sodium salt were purchased from Sigma-Aldrich (Stenheim, Germany, www.sigmaaldrich.com). Sodium chloride was obtained from Panreac (Barcelona, Spain, www.itwreagents.com). Acetic acid and sodium acetate used for the preparation of acetic-acetate 0.1 M buffer (pH 3.9), hydrochloric acid (37%) and solvents (dichloromethane (DCM), acetonitrile (MeCN), isooctane and hexane) were purchased from Scharlab (Barcelona, Spain, www.scharlab.com). Sodium tetraethylborate (NaBEt4, 97%) used as derivatizing reagent was supplied by Acros Organics (Thermo Fisher Scientific, Geel, Belgium, www.acros.com).
Argon C-50 was used as a make-up and sheath gas at the AFS detector, helium C-50 was employed as a carrier gas and nitrogen C-50 was used for evaporation of the organic solvent. All gases were obtained from Carburos Metálicos (Barcelona, Spain, www.carburos.com).
Instruments
Mercury speciation analysis was performed with a non-commercial hyphenated system which consists of a gas chromatograph (GC, Shimadzu GC-2010) coupled to an atomic fluorescence detector (AFS, Millennium Merlin 10,025 P.S. Analytical, United Kingdom) via a pyrolysis unit. Inductively coupled plasma-mass spectrometry (ICP-MS) equipped with a collision cell (Thermo Electron Model XSeries II) was used for the determination of total metal contents.
For sample preparation with the MSPE procedure, a NdFeB magnet (Eclipse Magnetics Ltd., UK), a ZX3 vortex stirrer (Velp Scientifica, Usmate, Italy), an Elmasonic S 30 H ultrasonic bath (Elma, Germany) and a centrifuge from Selecta (Barcelona, Spain) were used. A heating module (Reacti-Therm; Pierce, Rockford, IL, USA) with an evaporating unit was also employed for preconcentration.
Total organic carbon (TOC) was measured with a CHN analyser (micro N/C from Analytic Jena). pH and conductivity were measured in situ with a portable pH-meter (model GLP 20) and a conductimeter (microCM 2200 with temperature measurement capability) supplied by Crison (Barcelona, Spain).
Synthesis and activation of magnetic cellulose nanoparticles
The protocol for synthesis of MCNPs was adapted from some previously described ones in the literature [17, 31] and it is carefully detailed in the Electronic Supplementary Material.
For activation of the nanomaterial, 25 mg of MCNPs were vortexed for 1 min with 1 mL of ultrapure water three times and then two times with 1 mL of MeCN as explained in a previous work [17]. The solvents were discarded after each step.
MSPE procedure and analysis of mercury species
For adsorption of mercury species, 10 mL of sample or standard was added to 25 mg of activated MCNPs in a 15 mL vial and vortexed for 25 min. The mixture was exposed to a strong magnet for 2 min. After that time, the solution became limpid and the supernatant solution was completely decanted.
For desorption and ethylation of target analytes, 1 mL of acetic-acetate buffer (0.1 M, pH 3.9), 1 mL of hexane and 0.25 mL of NaBEt4 (6%) were added to the vial containing the MCNPs with the adsorbed mercury species and sonicated for 15 min. The liquid phase was centrifuged at 600 g for 5 min. The organic layer containing the ethylated derivatives of mercury species was stored at −20 °C until analysis. The organic extract was further concentrated up to 30 times by evaporation with nitrogen stream to achieve a volume of 25–30 μL. Finally, the organic layer was injected into the GC-pyro-AFS where the analytes were separated in less than 5 min. The optimal conditions for this hyphenated system have already been described in the literature [32].
Sampling, preservation and preparation of water samples
Water samples from the Valdeazogues River basin in the Almadén mining district (Ciudad Real, Spain), placed around the major mercury mines worldwide, were collected from three sites (ALM-1 to ALM-3). Global positioning system (GPS) coordinates of sampling points are indicated in Table 1. Tap water was sampled from our lab after letting flow for 10 min. In all cases, the bottles were rinsed three times with the water before they were filled up. The samples were transported to the lab in a cooler and then they were filtered through Millipore nylon membrane filters (0.45 μm), acidified with 0.5% glacial acetic acid for stabilization of mercury species and stored in clean glass bottles at 4 °C before use.
Water samples were characterized by determination of different physicochemical parameters. Firstly, pH and conductivity were measured in situ. TOC were analysed in aliquots of the samples taken before filtration and acidification. Finally, total concentrations of mercury, iron and manganese were determined by ICP-MS in the filtered and acidified waters.
Results and discussion
Optimization of the MSPE conditions
The parameters that affect the adsorption, including the sample pH, the amount of sorbent material, the sample volume or the adsorption time, and several conditions influencing the desorption were optimized. The adsorption efficiency (AE), calculated as the difference between the initial concentration of mercury species in the sample and the remaining content after the adsorption, was the selected factor for the study of the adsorption step. The extraction recovery (ER), calculated as the ratio of the desorbed mercury species concentration to the absorbed mercury species, was selected to assess the overall MSPE procedure. Both ratios were expressed as a percentage.
Adsorption
The initial conditions were 10 mg of MCNPs, 5 mL of sample containing 20 μg L−1 of MMHg and Hg2+, and 30 min of vortex agitation. The magnetic nanomaterials were separated rapidly from the solution by the external magnet and the supernatant was decanted. To evaluate the efficiency of the adsorption onto the MCNPs, 1 mL of the decanted solution obtained after the adsorption step was added 5 mL of acetic-acetate buffer (0.1 M, pH 3.9), 1 mL of hexane and 0.25 mL of NaBEt4 (6%). Then, the mixture was manually shaken for 5 min and centrifuged for 5 min at 600 g. The organic layer was stored at −20 °C until analysis by GC-pyro-AFS.
The first parameter to be optimized was the pH. This is a critical parameter in MSPE because mercury species in natural water samples are stabilized after sampling by addition of an acid preservative. For that reason, the effect of pH on the adsorption of analytes was investigated over the pH range of 1–6. Typically, strong acids such as nitric or hydrochloric acids are used for this purpose. However, the lowest pH reached using both strong acids showed unsatisfactory results (the AE were between 60 and 70% for both species at pH of 1 and below 80% at pH of 2). Accordingly, as the subsequent adequation of mercury species for analysis should be performed in an acetic-acetate buffered medium, it was considered that acetic acid, which has also been proposed as an appropriate agent for stabilization and preservation of mercury species in waters [33], would be the best option to adjust the pH without the addition of new ions to the sample. The AE of both species was around 95% at pH 3, and then dropped gradually to 74% and 77% for MMHg and Hg2+, respectively, when pH values increased up to 6. Thus, pH 3 adjusted with a 0.5% CH3COOH solution was selected for the simultaneous adsorption of the two target mercury species.
The adsorption time was also investigated to seek the minimum time that is enough to get quantitative results securely. Thus, times from 5 to 30 min of vortex agitation were studied keeping constant the other conditions. As it is shown in Fig. 1a, the AE of MMHg and Hg2+ increased apparently with the increasing of adsorption time and achieved the maximum value from 20 min with no further significant change in the studied range. However, the selected time was 25 min to ensure a security margin in which quantitative adsorption is obtained.
The optimum amount of MCNPs for the quantitative adsorption of mercury species was evaluated from 5 to 40 mg. Effective adsorption of MMHg and Hg2+ was found from 10 mg onwards, as it is shown in Fig. 1b, which demonstrates the potential of MCNPs as sorbent even when a small amount is used. Therefore, 10 mg was chosen because it allows the quantitative adsorption of both mercury species with a minimum consumption of material.
In order to achieve high preconcentration factors, a large volume of sample is required. To study the effect of sample volume on adsorption of the target mercury species, water solutions (5, 10, and 15 mL) containing MMHg and Hg2+ each at 20 μg L−1 were prepared and subjected to the procedure. The results exhibited that the AE had no significant change for both species in the range of 5–10 mL (≥ 90%) but it decreased when the sample volume increased to 15 mL (~ 80%). Hence, sample volume of 10 mL was fixed for subsequent experiments.
Simultaneous desorption and derivatization
The analysis of mercury species based on a chromatographic separation by GC requires a previous transformation of the ionic mercury species into their volatile derivatives, which is usually performed through derivatization. This process involves two steps: 1) the reaction with the derivatizing reagent (ethylation, in this case) in which the derivatives of mercury species are generated, and 2) the extraction of derivatized species into an organic solvent, which will be injected in the GC. The incorporation of this additional step after the desorption of mercury species from the sorbent would make the whole sample preparation more tedious and time consuming. For that reason, it would be most interesting to develop a strategy which includes both desorption and derivatization in a single step.
Therefore, preliminary experiments were devoted to the selection of a suitable reagent for the simultaneous desorption and extraction of derivatized mercury species. The derivatization was carried out by ethylation with NaBEt4 (6%) in acetic-acetate buffer at pH 3.9. Several reagents (hexane, DCM and isooctane), previously reported as adequate for the extraction of mercury species after derivatization, were tested. It was observed that the use of DCM caused the loss of magnetic properties of MCNPs, which led to difficulties in the separation of MCNPs and the organic layer using the magnet. In contrast, both isooctane and hexane allowed an easy removal of the organic phase, even though the desorption ability of hexane was higher than that of isooctane with ER values ranging 25–32% for isooctane and 39–44% for hexane. Accordingly, hexane was chosen as an adequate solvent for the simultaneous desorption of the ethylated mercury species. Regarding the volume of organic solvent, the minimum volume that can be handled comfortably was 1 mL, so this was set for all the experiments. Therefore, the derivatization and extraction were performed by using 1 mL of acetic-acetate buffer (0.1 M, pH 3.9), 0.25 mL of NaBEt4 (6%) and 1 mL of hexane.
The parameters affecting desorption were initially studied under the previously optimized adsorption conditions. Thus, to assess the ER, 5 mL of a MMHg and Hg2+ standard solution (pH 3) at 20 μg L−1 were adsorbed onto 10 mg of MCNPs by vortexing for 25 min. Then, the MCNPs with the adsorbed mercury species were separated by the magnet and the reagents needed for ethylation and desorption were added. Thus, the target mercury species were simultaneously derivatized and desorbed in hexane as previously described after 10 min of sonication. Working under these conditions, the ERs were not quantitative, so some parameters were re-evaluated focusing now in the desorption step.
New experiments were carried out with a sample volume of 10 mL increasing the amount of sorbent from 10 to 25 mg, and it was observed that the ER of both mercury species went up to 90%. Therefore, 25 mg of the sorbent was chosen as the optimum amount of MCNPs for volumes of sample equal or higher than 10 mL.
To improve the ER for the two analytes, the desorption–sonication time was assessed from 5 to 20 min. The best extraction recovery was obtained for 15 min (Fig. 2). This is due to the complete mass transfer of both mercury species from the sorbent towards the organic solvent reached at that time.
Therefore, it was proved that working under the optimal experimental conditions, a simultaneous desorption and derivatization of both mercury species can be achieved, which simplifies considerably the sample preparation step and it is one of the main operational advantages of the present method.
Analytical characterization
The analytical performance of the procedure was evaluated. The limits of detection (LOD) and quantification (LOQ) were established as the sample concentration that caused a peak with a height 3-fold and 10-fold the base line noise level, respectively. The procedural LODs were 4.0 pg mL−1 and 5.6 pg mL−1, while LOQs were 13.3 pg mL−1 and 18.6 pg mL−1 for MMHg and Hg2+, respectively.
Precision was evaluated as the relative standard deviation (% RSD) of replicate measurements of intra-day (n = 4) and inter-day assays (n = 4). Intra-assay precision was calculated after injections during the same day of samples from four independent experiments, whereas inter-assay precision was measured on 4 consecutive days. The % RSD values for MMHg and Hg2+ at 1 μg L−1 and 10 μg L−1, respectively, were 4.8 and 3.1% for intra-day precision, and 6.7 and 4.8% for inter-day precision, demonstrating the adequate repeatability and reproducibility.
Batch to batch reproducibility was also investigated by using MCNP composite sorbents corresponding to three independent synthesis. These different nanomaterials were used for the extraction of standards of MMHg and Hg2+ at 20 μg L−1 with comparable ER values for both mercury species (% RSD was lower than 5%). It was also demonstrated that there were no statistically significant differences between the recoveries using the different batches (p = 0.083 > 0.05, Student’s t test).
To evaluate the selectivity of the method, the potential interference of co-existing ions and organic matter was also investigated. For this purpose, 10 mL of the water containing 1.0 μg L−1 of MMHg and 10 μg L−1 of Hg 2+ were spiked with different amounts of Fe3+, Mn2+ and humic acid (as simulator of natural organic matter) and then subjected to the whole analytical procedure. As it is shown in Table 2, the recoveries in presence of the other metal ions were in the range of 86.0–98.5% for MMHg and 83.5–92.0% for Hg2+, which means that the interferences from these potential co-existing metal ions are negligible. Although a slight decreasing trend in recoveries with increasing concentrations of humic acid was observed (Table 2), recoveries higher than 80% were found even at high concentration (100 mg L−1) of humic acid, which proved that this new approach for mercury speciation was applicable to the analysis of environmental water samples with complex matrices.
Reusability assessment
The potential reuse of the nanocomposite is an important factor for the assessment of the performance of the sorbent. The reusability of the MCNPs was studied by performing several adsorption and desorption cycles repeatedly under the optimized experimental conditions. The adsorption of Hg2+ was quantitative up to the fifth cycle and dropped to 75% in the sixth one, whereas a slight decrease on MMHg adsorption was already observed after reusing the MCNPs five times and this decreasing trend continued in the sixth cycle. Nevertheless, considering the whole MSPE procedure, it was found that the MCNPs can be reused up to four times with quantitative recoveries of mercury species in the range of 86–95%. ERs below 80% (76–78%) were reported for both species after reusing the MCNPs five times.
Analysis of real samples
The method was applied to water samples with different matrices. Thus, three river water samples collected from the Almadén mining district (Ciudad Real, Spain) and tap water were analyzed. The main features of these samples are summarized in Table 1. The pH values in river waters were between 7.4 and 8.3, whereas the pH of tap water was slightly more acidic. As for conductivity, ALM-1 showed a value almost 2-fold higher (~500 μS/cm) than those presented by the rest of the samples (200–300 μS/cm). Regarding the organic matter, there were no large differences in TOC levels found in Almadén waters with values ranging from 34 to 41 mg/L, whereas the concentration in tap water was considerably lower (<10 mg/L). The most remarkable differences in the physicochemical characteristics of the studied water samples were reported in metal ions contents with variable concentrations of Fe and Mn for each sample.
The natural concentrations of mercury species were in all cases below the corresponding LOD. Thus, for the evaluation of the MSPE procedure, filtered water samples were analyzed in duplicate, both directly and after being spiked with mercury species at two levels of concentration (0.1 and 1 μg L−1 for MMHg and 1 and 10 μg L−1 for Hg2+).
Duplicate aliquots of 10 mL of each real water sample were submitted to analysis. The extraction recoveries, summarized in Table 1, revealed that the mean ERs for MMHg and Hg2+ were quantitative in all cases with values ranging from 81 to 98%.
Figure 3 illustrates chromatograms obtained from an Almadén water sample (ALM-3) spiked at a concentration level of 0.1 and 1 μg L−1 for MMHg and Hg2+, respectively, before and after the application of the MSPE, demonstrating the high efficiency for preconcentration of mercury species of the method.
Comparison with other methods
A comparison with other MSPE procedures previously used for mercury speciation in water samples is summarized in Table 3. The present work offers important advantages related to: (i) the use of a biodegradable and environmentally friendly nanocomposite as sorbent; (ii) the easy synthesis of MCNPs without the need for an additional modification or functionalization; (iii) the possibility of simultaneous extraction of MMHg and Hg2+; (iv) the high preconcentration efficiency for both mercury species; and (v) a considerable save of time in the sample preparation step due to the simultaneous desorption and derivatization of mercury species in a single step.
It is remarkable that this new approach is the only one using a sorbent based on the combination of magnetic nanoparticles with a renewable and eco-friendly nanomaterial such as nanocellulose for MSPE, as opposed to the SiO2 based nanoparticles that are used in most works [19–21; 25–29]. In addition, most magnetic sorbents including SiO2 are core-shell type, which tends to make synthesis and further functionalization longer and more complicated [34].
Regarding the selectivity of the MSPE procedures, the present method allows the simultaneous extraction and preconcentration of the two main mercury species (MMHg and Hg2+), which had been achieved previously only in a few cases [19,20,21].
It can also be observed that the lowest LODs are reached by methods based on the coupling of a separation technique with ICP-MS detection [19,20,21, 26]. Nevertheless, the LODs achieved in our work, with spectroscopic detection, are still in the low pg mL−1 range.
Conclusions
MCNPs have been used in MSPE for the first time for trace element speciation analysis. The present MSPE–GC-pyro-AFS method allows the simultaneous extraction and preconcentration of MMHg and Hg2+ and it was applied to water samples of different characteristics. Desorption and derivatization were achieved in one step, which greatly simplifies the sample preparation process. The use of MCNPs, an eco-friendly and reusable sorbent that can be easily synthetized, is an important advantage in relation to other MSPE methods. Overall, this new method is an interesting and advantageous alternative for mercury speciation analysis in water samples.
References
Płotka-Wasylka J, Szczepańska N, de la Guardia M, Namieśnik J (2016) Modern trends in solid phase extraction: new sorbent media. TrAC - Trends Anal Chem 77:23–43. https://doi.org/10.1016/j.trac.2015.10.010
Buszewski B, Szultka M (2012) Past, present, and future of solid phase extraction: a review. Crit Rev Anal Chem 42:198–213. https://doi.org/10.1080/07373937.2011.645413
Zhang S, Fu R, Wang S, Gu Y, Chen S (2017) Novel nanocellulose/conducting polymer composite nanorod films with improved electrochromic performances. Mater Lett 202:127–130. https://doi.org/10.1016/j.matlet.2017.05.044
Dufresne A (2013) Nanocellulose: a new ageless bionanomaterial. Mater Today 16:220–227. https://doi.org/10.1016/j.mattod.2013.06.004
Ruiz-Palomero C, Soriano ML, Valcárcel M (2017) Nanocellulose as analyte and analytical tool: opportunities and challenges. TrAC - Trends Anal Chem 87:1–18. https://doi.org/10.1016/j.trac.2016.11.007
Chisvert A, Cárdenas S, Lucena R (2019) Dispersive micro-solid phase extraction. TrAC Trends Anal Chem 112:226–233. https://doi.org/10.1016/J.TRAC.2018.12.005
Giakisikli G, Anthemidis AN (2013) Magnetic materials as sorbents for metal/metalloid preconcentration and/or separation. A review. Anal Chim Acta 789:1–16. https://doi.org/10.1016/j.aca.2013.04.021
Faraji M (2016) Recent analytical applications of magnetic nanoparticles. Nano Res 1:264–290. https://doi.org/10.1016/j.hrtlng.2006.08.005
Xie L, Jiang R, Zhu F, Liu H, Ouyang G (2014) Application of functionalized magnetic nanoparticles in sample preparation. Anal Bioanal Chem 406:377–399. https://doi.org/10.1007/s00216-013-7302-6
Chang PR, Yu J, Ma X, Anderson DP (2011) Polysaccharides as stabilizers for the synthesis of magnetic nanoparticles. Carbohydr Polym 83:640–644. https://doi.org/10.1016/j.carbpol.2010.08.027
Donia AM, Atia AA, Abouzayed FI (2012) Preparation and characterization of nano-magnetic cellulose with fast kinetic properties towards the adsorption of some metal ions. Chem Eng J 191:22–30. https://doi.org/10.1016/J.CEJ.2011.08.034
Sun X, Yang L, Li Q, Zhao J, Li X, Wang X, Liu H (2014) Amino-functionalized magnetic cellulose nanocomposite as adsorbent for removal of Cr(VI): synthesis and adsorption studies. Chem Eng J 241:175–183. https://doi.org/10.1016/J.CEJ.2013.12.051
Zarei S, Niad M, Raanaei H (2018) The removal of mercury ion pollution by using Fe3O4-nanocellulose: synthesis, characterizations and DFT studies. J Hazard Mater 25:22060–22074. https://doi.org/10.1016/j.jhazmat.2017.10.009
Wei J, Yang Z, Sun Y, Wang C, Fan J, Kang G, Zhang R, Dong X, Li Y (2019) Nanocellulose-based magnetic hybrid aerogel for adsorption of heavy metal ions from water. J Mater Sci 54:6709–6718. https://doi.org/10.1007/s10853-019-03322-0
Adelantado C, Ríos Á, Zougagh M (2018) Magnetic nanocellulose hybrid nanoparticles and ionic liquid for extraction of neonicotinoid insecticides from milk samples prior to determination by liquid chromatography-mass spectrometry. Food Addit Contam Part A 35:1755–1766. https://doi.org/10.1080/19440049.2018.1492156
Abujaber F, Guzmán Bernardo FJ, Rodríguez Martín-Doimeadios RC (2019) Magnetic cellulose nanoparticles as sorbents for stir bar-sorptive dispersive microextraction of polychlorinated biphenyls in juice samples. Talanta 201:266–270. https://doi.org/10.1016/J.TALANTA.2019.04.005
Abujaber F, Zougagh M, Jodeh S, Ríos Á, Guzmán Bernardo FJ, Rodríguez Martín-Doimeadios RC (2018) Magnetic cellulose nanoparticles coated with ionic liquid as a new material for the simple and fast monitoring of emerging pollutants in waters by magnetic solid phase extraction. Microchem J 137:490–495. https://doi.org/10.1016/j.microc.2017.12.007
Karimi MA, Ghasemi MH, Aghagoli MJ, Beyki MH (2016) Preconcentration of cobalt ions by a melamine-modified cellulose@MWCNT nanohybrid. Microchim Acta 183:2949–2955. https://doi.org/10.1007/s00604-016-1943-z
Ma S, He M, Chen B, Deng W, Zheng Q, Hu B (2016) Magnetic solid phase extraction coupled with inductively coupled plasma mass spectrometry for the speciation of mercury in environmental water and human hair samples. Talanta 146:93–99. https://doi.org/10.1016/j.talanta.2015.08.036
Zhang S, Luo H, Zhang Y, Li X, Liu J, Xu Q, Wang Z (2016) In situ rapid magnetic solid-phase extraction coupled with HPLC-ICP-MS for mercury speciation in environmental water. Microchem J 126:25–31. https://doi.org/10.1016/j.microc.2015.11.040
Zhu S, Chen B, He M, Huang T, Hu B (2017) Speciation of mercury in water and fish samples by HPLC-ICP-MS after magnetic solid phase extraction. Talanta 171:213–219. https://doi.org/10.1016/j.talanta.2017.04.068
Lopez-Garcia I, Vicente-Martinez Y, Hernandez-Cordoba M (2015) Determination of ultratraces of mercury species using separation with magnetic core-modified silver nanoparticles and electrothermal atomic absorption spectrometry. J Anal At Spectrom 30:1980–1987. https://doi.org/10.1039/C5JA00213C
Faraji M, Yamini Y, Rezaee M (2010) Extraction of trace amounts of mercury with sodium dodecyle sulphate-coated magnetite nanoparticles and its determination by flow injection inductively coupled plasma-optical emission spectrometry. Talanta 81:831–836. https://doi.org/10.1016/j.talanta.2010.01.023
Li G, Liu M, Zhang Z, Geng C, Wu Z, Zhao X (2014) Extraction of methylmercury and ethylmercury from aqueous solution using surface sulfhydryl-functionalized magnetic mesoporous silica nanoparticles. J Colloid Interface Sci 424:124–131. https://doi.org/10.1016/j.jcis.2014.03.026
Corps Ricardo AI, Sánchez-Cachero A, Jiménez-Moreno M, Guzmán Bernardo FJ, Rodríguez Martín-Doimeadios RC, Ríos Á (2017) Carbon nanotubes magnetic hybrid nanocomposites for a rapid and selective preconcentration and clean-up of mercury species in water samples. Talanta 179:442–447. https://doi.org/10.1016/j.talanta.2017.11.024
Jiang W, Jin X, Yu X, Wu W, Xu LJ, Fu FF (2017) Ion-imprinted magnetic nanoparticles for specific separation and concentration of ultra-trace methyl mercury from aqueous sample. J Chromatogr A 1496:167–173. https://doi.org/10.1016/j.chroma.2017.03.049
Xiang G, Li L, Jiang X, He L, Fan L (2013) Thiol-modified magnetic silica sorbent for the determination of trace mercury in environmental water samples coupled with cold vapor atomic absorption spectrometry. Anal Lett 46:706–716. https://doi.org/10.1080/00032719.2012.726679
Zhai Y, Duan S, He Q et al (2010) Solid phase extraction and preconcentration of trace mercury(II) from aqueous solution using magnetic nanoparticles doped with 1,5-diphenylcarbazide. Microchim Acta 169:353–360. https://doi.org/10.1007/s00604-010-0363-8
Zhang W, Sun C, Yang X (2014) Magnetic solid-phase extraction combined with in situ slurry cold vapor generation atomic fluorescence spectrometry for preconcentration and determination of ultratrace mercury. Anal Methods 6:2876–2882. https://doi.org/10.1039/C3AY42118J
Ziaei E, Mehdinia A, Jabbari A (2014) A novel hierarchical nanobiocomposite of graphene oxide-magnetic chitosan grafted with mercapto as a solid phase extraction sorbent for the determination of mercury ions in environmental water samples. Anal Chim Acta 850:49–56. https://doi.org/10.1016/j.aca.2014.08.048
Li W, Zhao X, Liu S (2013) Preparation of entangled nanocellulose fibers from APMP and its magnetic functional property as matrix. Carbohydr Polym 94:278–285. https://doi.org/10.1016/j.carbpol.2013.01.052
Jiménez-Moreno M, Lominchar MÁ, Sierra MJ, Millán R, Martín-Doimeadios RCR (2018) Fast method for the simultaneous determination of monomethylmercury and inorganic mercury in rice and aquatic plants. Talanta 176:102–107. https://doi.org/10.1016/j.talanta.2017.08.015
Terán-Baamonde J, Bouchet S, Tessier E, Amouroux D (2018) Development of a large volume injection method using a programmed temperature vaporization injector – gas chromatography hyphenated to ICP-MS for the simultaneous determination of mercury, tin and lead species at ultra-trace levels in natural waters. J Chromatogr A 1547:77–85. https://doi.org/10.1016/J.CHROMA.2018.02.056
Casado-Carmona FA, Alcudia-León MC, Lucena R, Cárdenas S, Valcárcel M (2016) Magnetic nanoparticles coated with ionic liquid for the extraction of endocrine disrupting compounds from waters. Microchem J 128:347–353. https://doi.org/10.1016/j.microc.2016.05.011
Acknowledgements
The authors thank the Spanish Ministry of Economy and Competitiveness for the financial support (Project CTQ2016-78793-P) to this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 33 kb)
Rights and permissions
About this article
Cite this article
Abujaber, F., Jiménez-Moreno, M., Guzmán Bernardo, F.J. et al. Simultaneous extraction and preconcentration of monomethylmercury and inorganic mercury using magnetic cellulose nanoparticles. Microchim Acta 186, 400 (2019). https://doi.org/10.1007/s00604-019-3492-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3492-8