Abstract
The authors report on a novel sorbent (thermally treated natural zeolite; clinoptilolite) for use in dispersive micro-solid phase extraction (D-μ-SPE) of polycyclic aromatic hydrocarbons (PAHs) from water samples. The method was applied to the D-μ-SPE of 16 priority PAHs which then were quantified by gas chromatography with mass spectrometric detection (GC-MS). The method was validated in terms of specificity and selectivity, linearity and linear range, accuracy, precision, uncertainty, limits of detection and quantification. Figures of merit include (a) linear analytical ranges between 2.08 and 208 ppb, and (b) detection limits in the range from 0.01 to 0.92 ppb. The method was successfully applied to the determination of PAHs in river waters.

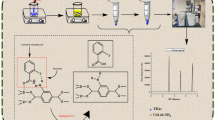
Schematic representation of dispersive micro-solid phase extraction (D-μ-SPE) of trace levels of PAHs in water samples by using thermally treated clinoptilolite as sorbent prior to gas chromatography-mass spectrometry analysis (GC-MS).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The occurrence of micropollutants in the aquatic environment has become a worldwide issue of increasing environmental concern. They are termed as emerging contaminants and consist of a vast and expanding array of substances both of anthropogenic and natural origin. Representative examples include pharmaceuticals, personal care products, steroid hormones, industrial chemicals, and persistent organic pollutants. Analytical control of environmental pollution includes determination of numbers of substances e.g., inorganic compounds, volatile organic compounds, polycyclic aromatic hydrocarbons (PAHs), biphenyls, and some special pollutants like dioxins, pesticides or phenols [1], commonly present in waters at trace concentrations, ranging from a few ng L−1 to several μg L−1.
The United States Environmental Protection Agency and European Union have identified 16 PAHs: (acenaphthene, acenaphthylene, anthracene, benzo[a]anthracene, benzo[a]pyrene, benzo[b]fuoranthene, benzo[g,h,i]perylene, benzo[k]fluoranthene, chrysene, dibenzo[a,h]anthracene, fluoranthene, fluorene, indeno[1,2,3-cd]pyrene, naphthalene, phenanthrene, and pyrene) as priority pollutants because of their wide distribution in the environment and potential risks for human health [2].
Generally, PAHs are hydrophobic with very little solubility in water which decreases with increasing molecular weight or the number of fused aromatic rings. The high molecular weight (HMW) PAHs (≥ 4 fused aromatic rings) are less water-soluble, less volatile and more lipophilic than lower molecular weight (LMW) PAHs (≤ 3 fused aromatic rings) [3].
Trends in sample preparation as the most important step in PAH analysis, are aimed to avoid disadvantages of existing techniques, concerning for example laborious procedure which requires a large amount of solvent and can degrade thermally labile compounds such as Soxhlet [4], and ultrasonic extraction or necessity of expensive equipment like accelerated solvent extraction [5]. The one thing in common of all mentioned extractions is a clean - up step, which is almost always necessary, prolonging the analysis time and potentially compromising its efficiency.
Solid phase extraction (SPE) has been increasingly used as a sample preparation technique, mainly due to the availability of different sorbents, small amounts of used solvents (both ecological and economic beneficial), simple technical procedures, possibility of automation and relatively low price. The classical sorbents in SPE are silica-based and organic polymers. The usage of natural zeolites has increased for SPE applications [6,7,8], because of numerous advantages over the other sorbents. For example, these natural aluminosilicate minerals contain pores and cavities with strictly defined size and shape, providing very effective concentration and separation of organic and inorganic compounds. In addition, zeolites have mechanical strength, good stability in aggressive mediums and under thermal treatment, ability to sorb the trace amounts of analytes, high sorption capacity and selectivity, possibility of easy modification and regeneration, low cost and accessibility [9].
The analysis of micropollutants at ultra - trace level causes the need to develop new extraction methods for sample preparation. In the last two decades, several microextraction methods were successfully applied for sample preparation.
A new, rapid, simple and efficient sample preparation technique was introduced by Anastassiades et al. [10], named QuEChERS and includes dispersive solid phase extraction (dSPE) as a clean - up step, while dispersive micro-solid phase extraction (D-μ-SPE) has been developed as a simple and miniaturized modification of dSPE that can be applied for extraction and enrichment of quinolones [11], tetracyclines [12], organophosphate pesticides [13], PAHs [14], triazines [15], and heavy metal ions [16].
D-μ-SPE starts with dispersion of sorbent in sample solution, enables rapid and uniform interaction with all target analytes, leading to enhancement of the overall method precision and shortening of extraction time. Next, isolation of solid sorbent is performed by centrifugation, filtration or using an external magnetic field. D-μ-SPE is based on the SPE methodology, but smaller amount of sorbent (μg or mg range) is applied without conditioning [17]. The solid sorbents used in D-μ-SPE need to meet several requirements. Firstly, they need to have high capacity and large surface area, to guarantee fast, quantitative sorption and elution, and to be characterized by high dispersibility in liquid samples [18]. In comparison to other techniques D-μ-SPE has many advantages, such as simplicity, good recovery, and fast performance, capability of combination with different detection techniques and low consumption of organic solvents [19].
The aim of presented study was to develop novel D-μ-SPE method for GC-MS analysis of PAHs in water samples with purpose to establish rapid, sensitive, accurate, economic and efficient extraction technique as a sample preparation. The first stage was sorbent preparation. The next stage included validation experiments, where specificity and selectivity, linearity and linear range, accuracy, precision, uncertainty, limits of detection and quantification were evaluated to assess the performances of the method, aimed to the analysis of PAHs in river water. To the best of our knowledge, there are no other reports up to date on application of thermally modified clinoptilolite in D-μ-SPE sample preparation for the determination of PAHs in water.
Methods and materials
Chemicals and reagents
Hexane (HPLC grade), Acetonitrile (HPLC grade) - Sigma Aldrich (www.sigmaaldrich.com), PAH mix ampule in acetonitrile 10 μg mL−1 each compound: acenaphthene, acenaphthylene, anthracene, benzo[a]anthracene, benzo[a]pyrene, benzo[b]fuoranthene, benzo[g,h,i]perylene, benzo[k]fluoranthene, chrysene, dibenzo[a,h]anthracene, fluoranthene, fluorene, indeno[1,2,3-cd]pyrene, naphthalene, phenanthrene, and pyrene, Internal standards: perylene d12, phenanthrene d10, acenaphthene d10, Surrogate standard mix: 2-chlorphenol-3,4,5,6-d4, 2,4,6-tribromophenol, 2-fluorobiphenol - Supelco, Bellefonte, Pennsylvania (https://www.laboratoryequipment.com/company-profiles/supelco), Deionized water specific conductivity - 0.05 μS cm−1.
Standard solutions
A standard solution of polycyclic aromatic hydrocarbons containing 16 compounds in total PAH concentration of 16 ppm was prepared in acetonitrile. All standards and working solutions were stored at 4 °C in silanized brown glass bottles with Teflon -lined caps, within the recommended period. A series of standard solutions was prepared by diluting 0–200 μL of the standard solution containing 16 PAHs with hexane, making concentrations from 2.08–208 ppb of each PAH.
Internal standard (ISs) solution mix in dichloromethane, containing equal quantities of perylene d12, phenanthrene d10, acenaphthene d10 was prepared in 30 ppm total concentration. Surrogate standard solution mix in acetonitrile contains 2-chlorphenol-3,4,5,6-d4, 2,4,6-tribromophenol, 2-fluorobiphenol, in equal concentrations, making total concentration of 30 ppm.
The internal standards were added to quantify each PAH, while a surrogate standard was added in order to monitor extraction efficiency. Surrogate standard mix solution (100 μL) in total concentration of 0.75 ppm was added to every tested model sample.
Preparation of model water samples
Deionized water, with verified absence of PAHs was spiked using PAH standard solution mix containing 16 compounds, at three concentrations’ levels (total concentration of PAHs was 0.5, 1.5 and 3 ppm, respectively) and these samples served as model water samples. Surrogate standards in acetonitrile were added in every tested sample and the total concentration of surrogate standards in each model sample was 0.75 ppm. Blanks were prepared following the same procedure without adding PAH 16 standard mixture.
Instrumentation
GS-MS analysis
All extracts were analyzed on a 7890/7000B GC-QQQ-MS system (Agilent Technologies, USA) in the selected ion monitoring (SIM) mode. Analyzed compounds were identified according to qualifier ions and retention times (Table 1). Parameters of gas chromatography and mass spectrometry method of determination are presented in Electronic Supporting Material (Table 1s.ESM).
Quantitative analysis was performed using quantifier ions (same as qualifier ions) corresponding to each PAH and corresponding retention time (Table 1). Acquisition data were processed using Mass Hunter QQQ Quantitative Analysis software (Agilent Technologies, USA);
Scanning electronic microscopy of the thermally treated clinoptilolite has been performed using an SEM - JSM 5300 JEOL instrument. Accelerating voltage was 0.5–30 kV, resolution 4.5 nm, magnification × 15–20,000; The attenuated total reflection (ATR) technique was applied to acquire Fourier-transform infrared (FTIR) spectra by using an instrument from Thermo Nicolet (model 6700).
Sorbent preparation
Zeolitic material(grain size 0.063–0.1 mm) containing over the 90% clinoptilolite, obtained from the mine Zlatokop (South Serbia), was washed with deionized water to remove impurities, dried and thermally treated in Annealing furnace for the 3 h at temperatures of 120 °C (CM1), 300 °C (CM2), 400 °C (CM3), 500 °C (CM4), 600 °C (CM5), and 700 °C (CM6). Clinoptilolite used in this study consisted of SiO2 (62.28%), Al2O3 (12.33%), Fe2O3 (3.20%), CaO (6.65%), MgO (1.18%), Na2O (1.46%) and K2O (0.85%) [20].
Dispersive micro - solid phase extraction
Dispersive micro-solid phase extraction (D-μ-SPE), as a new type of solid phase extraction, was used to achieve specific selectivity and high extraction efficiency in the extraction/clean up protocol. The extractant used for the D-μ-SPE was hexane, while disperser was solvent system acetonitrile-water (1:4 v/v). Model samples (400 μL, containing three levels of total PAHs concentration 0.5, 1.5 and 3 ppm) were transferred into microextraction tubes, which contained 460 mg of the tested sorbent. After shaking (1 min) and centrifugation (5 min) water was removed via micropipette and 500 μL of extractant and 100 μL of disperser was added to the solid residue. After shaking for 5 min and centrifugation (15 min), 400 μL of extract was transferred to GC vial [21]. Then, 200 μL of internal standard mix was added and extracts were analyzed by gas chromatography - mass spectrometry. All experiments were done in triplicate.
River water analysis
The sampling locations were chosen according to the assumed PAHs pollution of river Nisava, Serbia in vicinity of two most important industrial centers situated at Nisava’s river banks- city of Pirot and Nis. Samples were collected at four locations: industrial areas Rzana and Zukovo which are up- and down – stream from Pirot, (43°10′N 22°36′E) and urban settlements Brzi Brod (up-stream) and the city center of Nis (down-stream) (43°19′09″N 21°53′46″E) and presented in Fig. 1s (ESM).
All water samples were collected in 500 mL dark glass bottles with Teflon caps and stored at 4 °C prior to extraction (normally within 48 h). Before analysis, water samples were filtered through 0.45 μm sieve to remove suspended particles [22]. Real water samples were analyzed in the same way as model samples.
Results and discussion
Characterization of thermally treated clinoptilolite
The FTIR spectrum of the thermally treated clinoptilolite at 300 °C is shown in Fig. 1. (wavelength region is between 500 and 4500 cm−1). The characteristic wide band between 2900 and 3750 cm−1 is attributed to the existence of OH group stretching vibrations, originating from Al-Al-OH and Al-Ca-OH groups, as well as from traces of adsorbed water. Band at 3388.6 cm−1 is attributed to the characteristic hydrogen-bonded OH to oxygen ions (broad band), the sharp band typical of isolated OH stretching vibration of water at∼ 3620 cm−1, and usual bending vibration of H2O is observed at 1627.9 cm−1. The intensity of these bands decreases with thermal treatment, possibly due to loss of OH groups and adsorbed water (ESM 1, Fig. 2–13 s). Since the band in question is wide, it covers carbon-hydrogen stretching vibration we expected to notice for clinoptilolite modifications bounded to PAHs (ESM1, Fig. 2s, 4s, 6s, 8 s, 10s, and 12 s). Other bands appear at 1019.9, 673.6, and 598.0 cm−1. The 1019.9 cm−1 band corresponds to asymmetric stretching vibration of internal T-O bonds in TO4 tetrahedra (T = Si and Al). The bands at 673.6 and 598.0 cm−1 are assigned to stretching vibration of O-T-O groups and the bending vibrations of T-O bonds, respectively [23]. There are no significant changes in these bonds wave numbers, with increased temperature (ESM1, Fig. 2s, 4s, 6s, 8 s, 10s, and 12 s). Moderate increase of wave number of asymmetric stretching vibration of internal T-O bonds in TO4 tetrahedra for clinoptilolite modifications with adsorbed PAHs (ESM1, Fig. 3s, 5s, 7 s, 9 s, 11 s, and 13 s) can be attributed to linkage of PAHs to T (Si or Al).
The morphological features of the thermally treated clinoptilolite are visible in Fig. 2 which show the surface of clinoptilolite, imaged by SEM at different magnifications. The SEM micrographs of thermally treated clinoptilolite demonstrate that the clinoptilolite has a lamellar structure, but heterogeneity of clinoptilolite grains is clearly presented (Fig. 2).
Method validation
In order to develop the D-μ-SPE procedure with novel sorbents followed by GC-MS, for an effective and reproducible detection and quantification of low concentrations of PAHs in water, several parameters such as selectivity, linearity and linear range, accuracy, precision, uncertainty, limits of detection and quantification were determined.
Selectivity
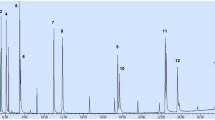
of the method required absence of peaks in the region of the retention times for the analyzed compounds, and this was followed as a necessary condition [24]. The obtained chromatogram (Fig. 3) verifies an appropriate chromatographic separation. All peaks of the compounds with the same mass were separated with good resolution, without any interference, which allows their quantification.
Regarding numerous applications of the sorbent, it is reasonable to expect bounding of many various compounds, usually present in the real samples that at first sight may compromise selectivity of the method. But, using predefined combination of extractants/sorbents/dispersers the high selectivity can be achieved. Parameters of GC-MS analysis in great extent provide method selectivity as well.
As selectivity criteria, the retention times of the 16 priority PAHs under investigation were determined. For this determination, three replicates of the standard solution were used. The selectivity proved good separation of all PAHs in GC (retention times) or MS (monitored m/z).
The calibration
plots were constructed with matrix-matched standards, that is, the analysis was carried out by spiking water matrix samples with different amounts of standards using deuterated PAH-Mix as internal standards and treated by D-μ-SPE procedure. Calibration plots were constructed using the least squares linear regression model, plotting the peak area ratios of the different compounds and respective internal standard versus the concentration of each analyte under study. Standard calibration plots were prepared using eight calibration points for PAHs concentrations (2.08, 4.17, 20.8, 41.7, 83.3, 125, 167, and 208 ppb of each PAH).
Accuracy
was evaluated using recovery for each PAH for three spiking concentration levels and six differently modified clinoptilolite sorbents. Model samples were prepared in triplicate.
In this analysis, it is necessary to consider easily volatile compounds which require using appropriate extractant and disperser. To provide good extraction efficiency PAHs were extracted with the hexane/acetonitrile mixture (1:4 v/v). As a disperser small amount of the acetonitrile in water (1:4 v/v) must be added into hexane to enhance the extraction of PAHs.
The accuracy of the method was estimated as PAHs recovery from spiked model water samples. Water samples were spiked in 0.5, 1.5 and 3 ppm total concentration.
Recovery values were the best for sorbents modified on 300 o C (CM2) and 400 °C (CM3) compared to the rest of used clinoptilolite modifications (Table 3s. ESM). Thus, optimal temperature for thermal treatment of clinoptilolite is from 300 to 400 °C. Since both modifications exerted similar recovery values, lower temperature modification (300 °C) was chosen, because the treatment at lower temperature is time and money more beneficial. Recoveries for CM2 modification range between 63.5 ± 0.4% for pyrene to 132.0 ± 0.3% for fluoranthene and most of PAHs were in recommended range.
Extraction efficiency was monitored using surrogate standards. PAH surrogate standards are chemically similar to target PAHs and they behave in similar manner throughout the sample preparation and analysis procedures. PAH surrogate standard mix (containing 2-chlorphenol-3,4,5,6-d4, 2,4,6-tribromophenol and 2-fluorobiphenol) was added to the model samples and total concentration of surrogate standard was 0.75 ppm. To validate an extraction method with novel sorbents it is necessary to ensure that chosen method for sample extraction will provide acceptable extraction efficiency for the target PAHs in water matrix. For this reason, every model sample was spiked with surrogate standard mix at 0.75 ppm total concentration level. Surrogate recoveries are presented in Fig.4.
For three different surrogate standard compounds and six different sorbent modifications average recovery values varied between 26.6 ± 0.4 for 2,4,6 -tribromophenol and 129.1 ± 0.6% for 2 -fluorobiphenyl. Recoveries for 2-chlorphenol - 3,4,5,6 - d4, 2,4,6 - tribromophenol and 2-fluorobiphenyl were in the recommended range for the extraction protocol where CM2 and CM3 modifications were applied.
The method precision was determined through repeatability studies and was expressed as relative standard deviation (RSD), also known as coefficient of variation. The average of the results was used to estimate the precision of the method. The RSD was determined by analyzing one sample on the same day, same instrument and by the same analyst under identical conditions [25].
As a criterion of evaluation, the RSD was considered as a function of the concentration range of the studied PAH analytes. Trace analysis methods generally accept values for RSD up to 20% [26].
Precision of the procedure was considered satisfactory because the RSD values were found to be lower than 6.5% for all investigated compounds (Table 1). These values are in accordance with previously reported study of Nuhu et al., 2012 [27] for the determination of PAHs in water samples by GC-MS where one of D-μ-SPE techniques was employed for sample preparation.
According to the Eurachem Guide [28], uncertainty (U) is defined as “a parameter, associated with the result of a measurement that characterizes the dispersion of the values that could reasonably be attributed to the measurand”. In practice, information on uncertainty is needed in a test report whenever (1) it is relevant to the validity or application of the test results, (2) a client requires so for particular purposes and (3) the uncertainty may affect compliance to a specification limit. Especially the last requirement for uncertainty declaration is important in PAH analysis, the results of which are connected to the decision-making process. In analytical chemistry, an expanded uncertainty (U) is commonly used rather than standard uncertainty. Its value defines an interval within which the value of the measurand lies with a known level of confidence. U is obtained by multiplying the combined standard uncertainty (u) by a coverage factor (k) [29].
Where y(x1, x2, …)is function of several independent variables, x1, x2, …, ci– sensitivity coefficient, u (xi) - standard uncertainty of measurement expressed through standard deviation:
Expanded uncertainty vales of the method varied between 0.02 for naphthalene to 0.93% for phenanthrene (Table 1).
The limit of detection (LOD) and limit of quantification (LOQ)
were estimated based on the signal of the background noise measured from the chromatograms of the standard at the lowest calibration level. The LOD was calculated to be three times higher than the level of noise, while the LOQ was equal to ten times the noise level [30]. Experimental LOD based on the calibration plot parameters varied from 0.01for naphthalene to 0.92 ppb for chrysene (Table 1). LOQ values varied from 0.02 ppb for naphthalene to 2.24 ppb for benzo[g,h,i]perylene (Table 1).
To estimate analytical performances of the proposed method, its characteristics (extraction time, LOD, and precision) were compared to similar methods for PAHs determination and overview is presented in Table 2.
The proposed method has the shortest extraction time, moderate LOD and RSD, but used sorbent requires simple techniques of preparation and its price is low, which makes it more convenient for routine application. Possibility of its regeneration is also under consideration, dominantly from environmental reasons. The only defiance of the proposed sorbent is its availability on the market. After comparison of the results for extraction efficiencies and recoveries for D-μ-SPE, and comprehensive consideration of all tested sorbents, modification of clinoptilolite treated at 300 °C was chosen for D-μ-SPE of PAHs in river water.
River water analysis
Validated method employing sorbent with optimal characteristics is applied for analysis of four water samples, collected in different urban and rural areas near cities Nis and Pirot. Preparation of real water samples was carried out using previously described protocol, with hexane as extractant and small amount of acetonitrile in water (1:4 v/v) as a disperser and clinoptilolite modification (CM2) as sorbent in a dispersive micro-solid phase extraction/cleanup step followed with gas chromatography mass spectrometry. The concentrations (ppb) and distribution of PAHs along the sites of Nisava River are presented in Table 3. Total concentrations of 16 PAHs ranged from 155 ± 1 to 260 ± 2 ppb which is higher than EPA guideline limits of 0.05 ppb for uncontaminated water [36]. Concentrations above this level indicate contamination by PAHs mainly through industrial point sources, urban runoff and atmospheric deposition.
According to the results, the concentration of low molecular weight (2–3 ring) PAHs (naphthalene, acenaphthylene, acenaphthene, fluorene, phenanthrene and anthracene) was lower than high molecular weight (4–6 rings) PAHs (fluoranthene, pyrene, chrysene, benzo[a]anthracene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, indeno[1,2,3-cd]pyrene, dibenzo[a,h]anthracene and benzo[g,h,i]perylene) (Fig. 5).
All 16 priority PAHs according US EPA were detected in analyzed samples. Benzo[a]pyrene, indeno[1,2,3-cd]pyrene, dibenzo[a,h]anthracene (5–6 rings) had the highest concentration on all sampling locations. Their presence in river indicates pyrogenic origin and combustion of fossil fuels. Predominant amount of benzo[a]pyrene (Fig. 6) was detected in all collected samples (47.3 ± 0.1, 70.6 ± 0.0, 88.8 ± 0.0 and 92.7 ± 0.0 ppb, respectively). Benzo[a]pyrene is the only polycyclic aromatic hydrocarbon with enough toxicological evidence to allow the setting of a guideline for PAH analysis [37].
Downstream from Pirot to Nis increased concentration of benzo[a]pyrene was registered, pointing to enlarged PAHs pollution of Nisava River.
Moreover, dibenzo[a,h]anthracene (32.3 ± 0.0, 54.1 ± 0.1, 43.3 ± 0.1 and 70.2 ± 0.0 ppb, respectively) and indeno[1,2,3-cd]pyrene (20.2 ± 0.1, 28.1 ± 0.0, 26.4 ± 0.2 and 33.5 ± 0.4 ppb, respectively) were found in high concentrations in all collected samples. Low molecular weights (LMW) PAHs were detected in lower concentrations, which can be explained with their volatile nature. Sample 4, from urban area of Nis downtown, showed the highest total concentration of all detected PAHs. This is expected due to intensive traffic pollution, but also because of PAHs afflux from the river up-stream. The high total PAHs concentration was observed in sample 2, down-stream from the Tigar Tyres Industry (Pirot). Lower than expected were concentrations of the investigated PAHs in sample 3, despite the long line of probable emission of PAHs (traffic, heating plants and households) and can be explained by the retention of PAHs on the solids in down-stream of the river.
Conclusion
Thermally treated natural zeolite, clinoptilolite sorbents were tested in a dispersive micro - solid phase extraction of 16 priority PAHs from water, followed by GC-MS. Due to the best analytical and economic characteristics, as optimal sorbent was chosen clinoptilolite prepared at 300 °C. Its structural characterization was done (SEM, FTIR) and it was applied in dispersive micro solid phase extraction of PAHs in river water. The combination of D-μ-SPE and GC-MS was proven as selective and sensitive in analysis of the trace levels of PAHs in water. Easy identification and quantification of individual PAH compounds enabled proposal of the new sample preparation protocol (10 min per sample including weighing of the sorbent and solvent mixtures preparation). The method demonstrated great applicability for routine PAHs analysis in water, where low values of limit of detection and quantification and high extraction efficiency confirmed method’s benefits. Another advantage of the validated method is low cost, environmental friendly (using of minimal volume of solvents) and convenient cheap materials in handling through sample preparation.
The results of analytical procedures used in presented article are accurate, readable, contemporaneous, original, reliable and reproducible. Analysis of real samples performed in accordance with the described protocol for sample preparation and instrumental analysis, revealed concerning concentrations of PAHs in river water.
References
Biernat JF, Makuch B (2000) Sorbents for Preconcentration of phenols from polluted waters. Pol J Environ Stud 9(2):71–75
Buco S, Moragues M, Doumenq P, Noor A, Mille G (2004) Analysis of polycyclic aromatic hydrocarbons in contaminated soil by curie point pyrolysis coupled to gas chromatography-mass spectrometry, an alternative to conventional methods. J Chromatogr A 1026(1–2):223–229. https://doi.org/10.1016/j.chroma.2003.11.065
Wild SR, Jones KC (1995) Polynuclear aromatic hydrocarbons in the United Kingdom environment: a preliminary source inventory and budget. Environ Pollut 88:91–108
Castro M, Luque D, Priego-Capote F (2009) Soxhlet extraction: past and present panacea. J Chromatogr A 1217(16):2383–2389. https://doi.org/10.1016/j.chroma.11.027.
Lau EV, Gan S, Ng HK (2010) Extraction techniques for polycyclic aromatic hydrocarbons in soils. Int J Anal Chem 2010:1–9. https://doi.org/10.1155/2010/398381
Faghihian H, Kabiri-Tadi M (2010) A novel solid-phase extraction method for separation and preconcentration of zirconium. Microchim Acta 168:147–152. https://doi.org/10.1007/s00604-009-0273-9
Yang XQ, Yang CX, Yan XP (2013) Zeolite imidazolate framework-8 as sorbent for on-line solid-phase extraction coupled with high-performance liquid chromatography for the determination of tetracyclines in water and milk samples. J Chromatogr A1304:28–33. https://doi.org/10.1016/j.chroma.2013.06.064
Ghazaghi M, Shirkhanloo H, Mousavi HZ, Rashidi AM (2015) Ultrasound-assisted dispersive solid phase extraction of cadmium(II) and lead(II) using a hybrid nanoadsorbent composed of graphene and the zeolite clinoptilolite. Microchim Acta 182(7–8):1263–1272
Vasylechko VO, Gryshchouk GV, Zakordonskiy VP, Vyviurska O, Pashuk AV (2015) A solid-phase extraction method using Transcarpathian clinoptilolite for preconcentration of trace amounts of terbium in water samples. Chem Cent J 9:45. https://doi.org/10.1186/s13065-015-0118-z
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC 86(2):412–431
Tsai WH, Chuang HY, Chen HH, Huang JJ, Chen HC, Cheng SH, Huang TC (2009) Application of dispersive liquid-liquid microextraction and dispersive micro-solid-phase extraction for the determination of quinolones in swine muscle by high-performance liquid chromatography with diode-array detection. Anal Chim Acta 656(1–2):56–62. https://doi.org/10.1016/j.aca.2009.10.008
Tsai WH, Huang TC, Huang JJ, Hsue YH, Chuang HY (2009) Dispersive solid-phase microextraction method for sample extraction in the analysis of four tetracyclines in water and milk samples by high-performance liquid chromatography with diode-array detection. J Chromatogr A 1216(12):2263–2269. https://doi.org/10.1016/j.chroma.2009.01.034
Galán-Cano F, Lucena R, Cárdenas S, Valcárcel M (2013) Dispersive micro-solid phase extraction with ionic liquid-modified silica for the determination of organophosphate pesticides in water by ultra performance liquid chromatography. Microchem J 106:311–317. https://doi.org/10.1016/j.microc.2012.08.016
Jiménez-Soto JM, Cárdenas S, Valcárcel M (2012) Dispersive micro solid-phase extraction of triazines from waters using oxidized single-walled carbon nanohorns as sorbent. J Chromatogr A 1245:17–23. https://doi.org/10.1016/j.chroma.2012.05.016
Reyes-Gallardo EM, Lucena R, Cárdenas S, Valcárcel M (2014) Magnetic nanoparticles-nylon 6 composite for the dispersive micro solid phase extraction of selected polycyclic aromatic hydrocarbons from water samples. J Chromatogr A 1345:43–49. https://doi.org/10.1016/j.chroma.2014.04.033
Kocot K, Sitko R (2014) Trace and ultra trace determination of heavy metal ions by energy-dispersive X-ray fluorescence spectrometry using graphene as solid sorbent in dispersive micro solid-phase extraction. Spectrochim Acta B 94–95:7–13. https://doi.org/10.1016/j.sab.2014.02.003
Jiang X, Wu M, Wu W, Cheng J, Zhou H, Cheng M (2014) A novel dispersive micro-solid phase extraction method combined with gas chromatography for analysis of organochlorine pesticides in aqueous samples. Anal Method 6:9712–9717. https://doi.org/10.1039/C4AY02302A
Asgharinezhad AA, Ebrahimzadeh H, Mirbabaei F, Mollazadeh N, Shekari N (2014) Dispersive micro-solid-phase extraction of benzodiazepines from biological fluids based on polyaniline/magnetic nanoparticles composite. Anal Chim Acta 844:80–89
Płotka-Wasylka J, Szczepańska N, Guardia M, Namieśnik J (2015) Miniaturized solid-phase extraction techniques. TRAC – Trends Anal Chem 73:19–38. https://doi.org/10.1016/j.trac.2015.04.026
Sekulic Z, Dakovic A, Kragovic M, Markovic M, Ivosevic B, Kolonja B (2013) Quality of zeolite from Vranjska Banja deposit according to size classes. Chem Ind 67:663–669
Stankov Jovanović V, Mitić V, Ilić M, Jovanović S, Ćirić S, Stojanović G (2018) Application of dispersive micro solid phase extraction as a sample preparation technique for GC-MS analysis of PAHs in water. XXIII Symposium on Biotechnology with International Participation 364–369.
Joil JC, Henry XC, Marilda F, Gisele MH (2012) Persistent toxic substances in surface water of Todos Os Santos Bay, Brazil. Resour Environ 2(4):141–149
Tanaka H, Yamasaki N, Muratani M, Hino R (2003) Structure and formation process of (K, Na)- clinoptilolite. Mater Res Bull 38(4):713–722. https://doi.org/10.1016/S0025-5408(03)00006-0
Lanças FM (2004) Validação de métodos cromatográficos de análise, 1st edition, Rima, São Carlos, SP1–62
Stankov Jovanović V, Mitić V, Ćirić S, Ilić M, Nikolic J, Dimitrijević M, Stojanović G (2017) Optimized ultrasonic extraction for the determination of polyaromatic hydrocarbons by gas chromatography-mass spectrometry. Anal Lett 50(15):2491–2504. https://doi.org/10.1080/00032719.2017.1293677
Brito NM, Amarante OPJ, Polese L, Santos TCR, Ribeiro ML (2002) Avaliação da exatidão e da precisão de métodos de análise de resíduos de pesticidas medianteensaios de recuperação, Pestic. Rev Ecotoxicol Meio Ambient 12:155–168
Nuhu AA, Basheer C, Shaikh AA, Al-Arfaj AR (2012) Determination of polycyclic aromatic hydrocarbons in water using Nanoporous material prepared from waste avian egg Shell. J Nanomater 2012:1–7. https://doi.org/10.1155/2012/305691
Thompson M, Ellison SLR, Wood R (2002) Harmonized guidelines for single laboratory validation of methods of analysis (IUPAC Technical Report). Pure ApplChem 74:835–855
Jánská M, Tomaniová M, Hajšlová J, Kocourek V (2006) Optimization of the procedure for the determination of polycyclic aromatic hydrocarbons and their derivatives in fish tissue: estimation of measurements uncertainty. Food Addit Contam 23(3):309–325. https://doi.org/10.1080/02652030500401207
Cvetkovic J, Violeta M, Stankov Jovanovic V, Dimitrijevic M, Petrovic G, Nikolic-Mandic S, Stojanovic G (2016) Optimization of the QuEChERS extraction procedure for the determination of polycyclic aromatic hydrocarbons in soil by gas chromatography- mass spectrometry. Anal Method 8(7):1711–1720. https://doi.org/10.1039/c5ay03248b
Fan J, Dong ZL, Qi ML, Fu RN, Qu LT (2013) Monolithic graphene fibers for solid-phase microextraction. J Chromatogr A 1320:27–32
Zhang X, Zang XH, Wang JT, Wang C, Wu QH, Wang Z (2015) Porous carbon derived from aluminum-based metal organic framework as a fiber coating for the solid-phase microextraction of polycyclic aromatic hydrocarbons from water and soil. Microchim Acta 182(13):2353–2359
Guo L, Lee HK (2011) Development of multiwalled carbon nanotubes based micro-solid-phase extraction for the determination of trace levels of sixteen polycyclic aromatic hydrocarbons in environmental water samples. J Chromatogr A 1218:9321–9327. https://doi.org/10.1016/j.chroma.2011.10.066
Gallardo EMR, Lucena R, Cárdenas S, Valcárcel M (2014) Magnetic nanoparticles-nylon 6 composite for the dispersive micro solid phase extraction of selected polycyclic aromatic hydrocarbons from water samples. J Chromatogr A 1345:43–49. https://doi.org/10.1016/j.chroma.2014.04.033
Abboud AS, Sanagi MM, Ibrahim WAW, Keyon ASA, Aboul-Enein HY (2018) Calcium alginate-caged multiwalled carbon nanotubes dispersive micro solid phase extraction combined with gas chromatography-flame ionization detection for the determination of polycyclic aromatic hydrocarbons in water samples. J Chromatogr Sci 56(2):177–186. https://doi.org/10.1093/chromsci/bmx095
US Environmental Protection Agency (1984) Federal Register, Rules and Regulations, Polynuclear Aromatic Hydrocarbons 49
Moret S, Purcaro G, Conte IS (2005) Polycyclic aromatic hydrocarbons in vegetable oils from canned foods. Eur J Lipid Sci Technol 107:488–496
Acknowledgments
The research was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia [Project Grant Numbers OI172051 and OI172047].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests. The manuscript has not been published elsewhere and it has not been submitted simultaneously for publication elsewhere.
Electronic supplementary material
ESM 1
(DOCX 2276 kb)
Rights and permissions
About this article
Cite this article
Ćirić, S., Mitić, V., Jovanović, S. et al. Dispersive micro-solid phase extraction of 16 priority polycyclic aromatic hydrocarbons from water by using thermally treated clinoptilolite, and their quantification by GC-MS. Microchim Acta 185, 556 (2018). https://doi.org/10.1007/s00604-018-3091-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-3091-0