Abstract
This work assesses the use of modified natural clinoptilolite as an adsorptive material for separation and preconcentration of trace amounts of zirconium ions. A simple, rapid and economical method was developed for the preconcentration of trace amounts of zirconium in aqueous medium using 1-(2-pyridylazo)-2-naphthol as a complexing agent. Effect of sample pH, flow rate of sample and elution solutions, breakthrough volume and interference of several ions were studied. Determination of zirconium was made by ICP-AES technique. The sorption was quantitative in the pH range from 3.0 to 4.0, whereas quantitative desorption occurred instantaneously with 2 mol L−1 hydrochloric acid. Linearity was maintained between 0.05 and 9.0 μg mL−1. Relative standard deviations range from ±0.9% to ±2.3% (n = 5). The detection limit was 0.1 ng mL−1. Because of good recovery (>97%), this method is suitable for preconcentration and determination of zirconium in effluents containing trace amount of zirconium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zirconium is extensively used in chemical industries where corrosive agents are employed, because it is exceptionally resistant to corrosion by many common acids, alkalis and also other agents. It is also used in vacuum tubes, steels as an alloying agents, surgical appliances, photoflash bulbs, explosive primers, rayon spinnerets, lamp filaments, etc. Zirconium is superconductive at low temperatures and is used to make superconductive magnets which offer a possibility of realizing direct large-scale generation of electric power [1].
Solid-phase extraction (SPE) is one of the most important methods for separation and preconcentration of trace elements in samples [2–4]. Solid-phase extraction of heavy metal ions from aqueous solutions are preferentially used as compared to other preconcentration techniques, mainly due to its simplicity, low cost, contaminant free and adaptation to flow injection techniques [5–7]. Many different types of adsorbents such as thiol cotton [8] activated carbon [9], cellulose [10], solvent-impregnated resins [11], microcrystalline naphthalene [12], Amberlite XAD-2 resin [13], alumina [14], silica [15], agar [16], clays [17] and zeolite [18, 19] have been used for SPE.
Zeolites are porous crystalline aluminosilicates having a uniform pore structure framework comprising [SiO4]4− and [AlO4]5− tetrahedral units. T atoms (Si, Al) are joined by an oxygen bridge, thus resulting in the general framework formula (AlO2)x(SiO2)n−x, where n is the number of tetrahedrals per unit cell, and x < n/2. Since aluminum is trivalent, every AlO2 unit carries a negative charge, which is compensated by a positive charge associated with a cation [20, 21]. Due to their unique structure, zeolites and modified zeolites have been utilized for the pollution control of heavy metal ions [22] and organic pollutants [23], separation processes [24], solid-phase ion exchange [25] and ion selective electrode [26, 27].
One of the reagents that have been widely used as analytical reagent is 1-(2-pyridylazo)-2-naphtol (PAN) which reacts with many metallic ions, producing colored complexes that are usually insoluble in water but soluble in organic reagents, like chloroform, benzene and carbon tetrachloride [28]. Cheng and Bray [29] first utilized PAN as an analytical reagent and it has recently been immobilized on solid supports [30], ion exchange resins [31], polyurethane foam [32] and naphthalene [33].
In this work, PAN is immobilized on hexadecyltrimethyleammonium-coated clinoptilolite. Then, the sorbent is used for separation and preconcentration of trace amounts of zirconium in aqueous samples prior to their determinations by ICP-AES.
Experimental
Apparatus and reagents
All chemical reagents used in this study were of analytical reagent grade (AR Grade). All solutions were prepared in double distilled water. Solutions of zirconium were prepared by dissolving known quantities of zirconium tetrachloride (ZrCl4) in distilled water. Working solutions were prepared daily from the stock solution by appropriate dilution with distilled water. A 0.05% solution of PAN in ethanol was prepared. Natural clinoptilolite was collected from Semnan deposits in Iran. It was crushed and pulverized in mortar and sieved to particle sizes of 100–224 µm. The powder was refluxed in water to remove soluble salts, then washed and dried at 110°C. The powder was stored in a desiccator over saturated NaCl solution in order to maintain a constant vapor pressure during the whole period of the experiments. Integra-XL ICP-AES from GBC Company was used for zirconium measurement. A Metrohm model 691 pH meter with a combined glass electrode was employed for measuring the pH of the solutions. FTIR spectra of the samples were obtained by a Nicolet Impact 400D Model spectrophotometer (Nicolet Impact, Madison, USA) using the KBr pressed disk technique. For KBr pellet, 1 mg of zeolite and 100 mg of KBr were weighted, ground in an agate mortar, and pressed. Spectra were recorded in the wave number range from 400 to 4,000 cm−1. Thermogravimetric analysis was performed using Mettler, TG-50 thermal analyzer from ambient temperature to 900°C at a heating rate of 10°C min−1 in air condition. The flow rate of influents for column experiments was adjusted with PLG-peristaltic pump manufactured by Desaga (Germany).
Sample preparation
The sample taken from Zayanderood river or tap water was filtered through a 0.45 µm filter paper and transferred to a 100 mL polyethylene bottle. pH of the sample was adjusted to pH = 4.0 with 0.1 mol L−1 HCl or 0.1 mol L−1 NaOH.
Preparation of modified clinoptilolite
Surfactant modification of clinoptilolite
Natural zeolite was characterized by X-ray diffraction and thermal methods of analysis. X-ray diffraction pattern was taken by a Bruker, D8ADVANCE X-ray diffractometer using Cu Kα radiation. Na, K, Ca, Si, and Al contents of the sample were determined by a Bruker, S4PIONEER X-ray fluorescence spectrometer [34].
Na-form of clinoptilolite was used for surfactant modification. 5.0 g of the purified zeolite was shaken with 100 mL solution of 1 N NH4Cl at 60°C for 72 h. The solid (NH +4 -form) was filtered, washed and dried at 110°C. Then NH +4 -form was calcinated at 450°C for 2 h to prepare H-form. Na form was prepared by shaking 5.0 g of H-form of zeolite with 100 mL of 1 mol L−1 solution of NaNO3 at 60°C for 72 h. The solid was separated, washed with distilled water, dried at 110°C and stored in the desiccator. Hexadecyltrimethyle ammonium bromide (HDTMABr) was used for modifying the zeolite surface. Modified zeolite was prepared by shaking 5.0 g of Na-form of clinoptilolite with 100 mL of 50 mmol L−1 HDTMABr at 30°C for 24 h and then the adsorbent was washed with water and dried at room temperature overnight.
Preparation of PAN-coated clinoptilolite column
HDTMABr coated clinoptilolite (1.0 g) was added to a funnel-tipped glass tube (10 × 1 cm) as a column. Then 10 mL of 0.05% PAN solution in ethanol was passed through the column at a flow rate of 0.2 mL min−1. Excess reagent was washed thoroughly with distilled water.
FTIR and thermoanalysis characterization
The FTIR spectra, TG and DTG curves of the zeolite are presented in Electronic Supplementary Section Fig. S1 and S2.
General procedure
Batch method
To obtain the equilibration time, a series of solution containing 10 mg zirconium ions were transferred in 10 mL beakers and the pH value was adjusted to the desired value with 0.1 mol L−1 HCl and 0.1 mol L−1 NaOH. Then the volume was adjusted to 10 mL with double distilled water, 1 g of PAN modified zeolite was added to each sample, and the mixture was shaken vigorously for different period of time (2–30 min). After centrifugation, the concentrations of the zirconium ions in the solution were directly measured by ICP-AES. The equilibration time of 10 min obtained from the experiment, showed that the adsorption process is kinetically fast and application of column method is possible.
Column experiment
An aliquot of zirconium solution containing 0.25 to 45 μg zirconium was taken in a 50 mL beaker. The total volume of the metal ion solution was approximately 30 mL. The pH of solution was adjusted. This solution was then passed through the column at a flow rate of 2.0 mL min−1. Finally, the column was washed with 10 mL of deionized water. The adsorbed metal ions on the column were eluted with 5.0 mL of 2 mol L−1 HCl solution at a flow rate of 1.0 mL min−1. The eluent was collected in a 5.0 mL volumetric flask. The final solution was aspirated directly into the ICP-AES against a blank prepared in the same manner, but without the addition of zirconium ions.
Results and discussion
Effect of reaction parameters
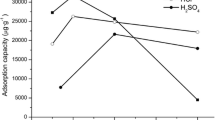
The reaction parameters were investigated with initial solutions containing 1.0 μg mL−1 of zirconium. Adsorptions were carried out at different values of pH, while keeping the other variables constant. It was found that zirconium was quantitatively adsorbed on modified zeolite in the pH range of 3.0–4.0 (Fig. 1). In pH < 3.0 the electrons of oxygen and nitrogen of PAN is attracted by H3O+ and in pH > 4.0 zirconium ions form hydroxide species. In subsequent studies, the pH was maintained at approximately 4.0.
To study the effect of the zirconium concentration on the column efficiency, the volume of the aqueous phase containing 1 μg mL−1 of zirconium was varied in the range of 20–800 mL under the optimum conditions. It was observed that the recovery was almost 100% up to 650 mL of the aqueous phase. A number of eluents were examined for desorption of metal ions from the column. Organic solvents cannot be used as eluent, because of removing the PAN reagent from the column. Preliminary observations indicated that HCl is the best eluent. The concentration of hydrochloric acid was varied from 0.1 to 3.5 mol L−1 and it was observed that the optimum recoveries of zirconium ions were obtained in the 2.0–3.5 mol L−1 concentration range (Fig. 2). In another set of experiments, the volume of eluent was varied in the range of 1–15 mL under the optimum conditions. Lowest volume with the best recovery (>98%) was found to be 5.0 mL. Therefore, 5.0 mL of 2 mol L−1 HCl solution was used for elution. The enrichment factor (130) is calculated as the ratio of the maximal sample volume (650 mL) to the minimal eluent volume (5.0 mL).
The flow rate of the sample was varied from 0.1 to 5.0 mL min−1. The highest value of recovery was obtained up to 2.5 mL min−1. Therefore a flow rate of 2.0 mL min−1 was recommended in all experiments. The flow rate of the eluent was varied form 0.1–1.4 mL min−1. It was found that desorption was approximately complete in the interval of 0.1–1.0 mL min−1. Therefore, a flow rate of 1.0 mL min−1 was recommended in all experiments.
Calibration and sensitivity
Calibration curve under the optimized conditions was linear between 0.05–9.0 μg mL−1 of zirconium in the final solution with correlation factor of 0.9993. Five replicate measurements on the solutions containing of 0.9 and 5.4 μg mL−1 zirconium in the final solution gave relative standard deviations of ±1.5% and ±1.8%, respectively. The detection limit was 0.1 ng mL−1. Sample volume was 30 mL.
Detection limit was calculated from the equation:
where Sb is the standard deviation of blank and m is the slope of calibration curve.
Sorption capacity of modified zeolite for zirconium
The sorption capacity of the PAN-coated clinoptilolite was evaluated. In this case, a column containing 1.0 g of modified zeolite was used and different volumes of zirconium solution (50 μg mL−1) were passed through it until zirconium was detected in the solution. The breakthrough curve has been presented in Fig. 3. The modified zeolite had a sorption capacity of 1.15 mg of zirconium per gram which was calculated according to the following equation:
V1% is volume related to C/C0 = 0.01 where C and C0 are zirconium concentration in effluent and influent solutions.
Interferences, recovery and accuracy tests
Various salts and metal ions were added individually to a solution containing 5.0 μg of zirconium ions and the general procedure was applied. The tolerance limit was set as the concentration ratio of foreign substances to zirconium required to cause a ±4% error. The obtained results are given in Table 1. There are no observed any interfering effect on the determination of zirconium. Some of these ions (Fe3+, Cu2+, Zn2+, Cr3+, Pb2+, Al3+) have lower tolerance limit. This is attributed to complexation of these ions with the reagent.
Finally, the applicability and reliability of this preconcentration method for the analysis of Zr was investigated in tap water and Zayanderood river water sample. First, 30 mL water sample was passed through the column at optimal conditions by proposed procedure and it was shown that the concentration of Zr was lower than the limit of detection of our method. Then, for studying the matrix effect on the method efficiency, various amount of zirconium was spiked to water sample and the proposed method was applied for these new samples. The summarized results are presented in Table 2. The results show that the recoveries of the spiked sample are good and at >95% confidence level.
The column was used for 15 adsorption-desorption cycles and the data showed that the column can be used only 8 times for quantitative preconcentration and determination of zirconium (recovery >97%). Although we can use 15 times if recovery >92% can be acceptable.
To study the accuracy of the method, zirconium ICP standard (NIST ZrOCl2 in HCl 7% 1,000 mg L−1) was used. The result showed that there was no significant difference between certified and obtained concentration of Zr (999.45 ± 1.02).
Conclusions
A solid-phase extraction method using clinoptilolite for the preconcentration of trace amounts of zirconium in aqueous sample solutions was investigated. The trace element is adsorbed onto a column of PAN-coated clinoptilolite. The metal ions are quantitatively desorbed with 2 mol L−1 hydrochloric acid. The main advantages of this procedure are: (1) Natural clinoptilolite is very cheap; (2) The preparation of the extraction system is simple and fast; (3) The column can be used several times because of remaining the PAN reagent in the surfactant modified zeolite during the desorption of zirconium; (4) A good enrichment factor (130) can be achieved. The achieved recovery (measured by standard addition technique) shows that the proposed procedure is fairly accurate. The proposed procedure was applied for zirconium determination in water sample. Comparative data from some literature on preconcentration studies for determination of zirconium are summarized in Table 3. The detection limit of the proposed method is better than the methods in Table 3. According to literature, selectivity of proposed method in the case of some ions such as K+, Ca2+, Mg2+, NH +4 , Na+, Cl−, and CH3COO− is very good or comparable with some works in which Zr have been preconcentrated on the other sorbents.
References
Hedrick JB (2000) Geological survey minerals yearbook.
Ayata S, Kaynak İ, Merdivan M (2009) Solid phase extractive preconcentration of silver from aqueous samples. Environ Monit Assess 153:333–338
Amin AS, Saber AL, Mohammed TY (2009) Study on solid phase extraction and spectrophotometric determination of vanadium with 2, 3-dichloro-6-(2, 7-dihydroxy-1-naphthylazo) quinoxaline. Spectrochim Acta Part A 73:195–200
Al-Degs YS, El-Sheikh AH, Al-Ghouti MA, Hemmateenejad B, Walker GM (2008) Solid-phase extraction and simultaneous determination of trace amounts of sulphonated and azo sulphonated dyes using microemulsion-modified-zeolite and multivariate calibration. Talanta 75:904–915
Moghimi A (2008) Solid phase extraction of thallium (III) on micro crystalline naphthalene modified with N, N′-Bis(3-methylsalicylidene)- ortho-phenylenediamine and determination by spectrophotometry. Chin J Chem 26:1831–1836
Bingol A, Aslan A, Cakici A (2009) Biosorption of chromate anions from aqueous solution by a cationic surfactant-modified lichen (Cladonia rangiformis (L.)). J Hazard Mater 161:747–752
Burham N (2008) Uses of 5-methylresorcin-bonded polyurethan foam as a new solid phase extractor for the selective separation of mercury ions from natural water samples. Cent Eur J Chem 6:641–650
Yu MQ, Liu GQ, Jin Q (1983) Determination of trace arsenic, antimony, selenium and tellurium in various oxidation states in water by hydride generation and atomic-absorption spectrophotometry after enrichment and separation with thiol cotton. Talanta 30:265–270
Vanderborght BM, Van Grieken RE (1977) Enrichment of trace metals in water by adsorption on activated carbon. Anal Chem 49:311–316
Burba P, Willmer PG (1983) Cellulose: a biopolymeric sorbent for heavy-metal traces in waters. Talanta 30:381–383
Hosseini MS, Hosseini-Bandegharaei A, Hosseini M (2009) Column-mode separation and pre-concentration of some heavy metal ions by solvent-impregnated resins containing quinizarin before the determination by flame atomic absorption spectrometry. Int J Environ Anal Chem 89:35–48
Taher MA (2001) Flame atomic absorption spectrometric determination of trace amounts of manganese in alloys and biological samples after preconcentration with the ion pair of 2-(5-Bromo-2-pyridylazo)-5-diethylaminophenol and ammonium tetraphenylborate on microcrystalline naphthalene or by column method. Anal Sci 17:969–973
Ferreria SLC, de Brito CF, Dantas AF, de Araújo NML, Costa ACS (1999) Nickel determination in saline matrices by ICP-AES after sorption on amberlite XAD-2 loaded with PAN. Talanta 48:1173–1177
Shemirani F, Abkenar SD (2004) Preconcentration and determination of trace nickel using 1-(2-pyridylazo)-2-naphtol (PAN) immobilized on surfactant-coated alumina. J Anal Chem 59:327–330
Thabano JRE, Breadmore MC, Hutchinson JP, Johns C, Haddad PR (2009) Silica nanoparticle-templated methacrylic acid monoliths for in-line solid-phase extraction-capillary electrophoresis of basic analytes. J Chromatogr A 1216:4933–4940
Pourreza N, Ghanemi K (2009) Determination of mercury in water and fish samples by cold vapor atomic absorption spectrometry after solid phase extraction on agar modified with 2-mercaptobenzimidazole. J Hazard Mater 161:982–987
Meney KM, Davidson CM, Littlejohn D (1998) Use of solid-phase extraction in the determination of benzene, toluene, ethylbenzene, xylene and cumene in spiked soil and investigation of soil spiking methods. Analyst 123:195–200
Chen X, Chen S, Liu J, Wang J (2009) Isolation of hemoglobin from human blood using solid phase extraction with lanthanum(III) modified zeolite. Microchim Acta 165:217–222
Faghihian H, Hajishabani A, Dadfarnia S, Zamani H (2009) Use of clinoptilolite loaded with 1-(2-pyridylazo)-2-naphthol as a sorbent for preconcentration of Pb(II), Ni(II), Cd(II) and Cu(II) prior to their determination by flame atomic absorption spectroscopy. Int J Environ Anal Chem 89(4):223–231
Breck DW (1974) Zeolite molecular sieves, structure, chemistry and uses. Wiley, New York
Weikamp J, Puppe L (1999) Catalysis and zeolites fundamental and applications. Springer-Verlag, Berlin, Heidelberg, New York
Sayed SA (1996) Exchange of Zn2+, V2+, Cd+2, and Hg+2 using zeolite A and dinonylnaphthalenesulfonate. Zeolites 17:261–264
Razee S, Masujima T (2002) Uptake monitoring of anilines and phenols using modified zeolites. Anal Chim Acta 464:1–5
Potdar A, Shukla A, Kumar A (2002) Effect of gas phase modification of analcime zeolite composite membrane on separation of surfactant by ultrafiltration. J Membr Sci 210:209–225
Sulikowski B, Find J, Karge HG, Herein D (1997) Solid-state ion exchange in zeolites: Part 8. Interaction of lanthanum(III) chloride with zeolites under anhydrous conditions. Zeolites 19:395–403
Dryfe RAW, Hayes P, Holmes SM (2001) Non-aqueous potentiometry using zeolites. Analyst 126:733–735
Walcarius A, Mariaulle P, Lamberts L (1999) Use of a zeolite-modified electrode for the study of the methylviologen-sodium ion-exchange in zeolite Y. J Electroanal Chem 463:100–108
Shibata S (1972) 2-Pyridylazo compounds in analytical chemistry. In: Flaschka HA, Barnard AJ (eds) Chelates in analytical chemistry, vol. 4. Marcel Dekker Inc, New York
Cheng KL, Bray RH (1955) 1-(2-Pyridylazo)-2-naphthol as possible analytical reagent. Anal Chem 27:782–785
Bahramifar N, Yamini Y (2005) On-line preconcentration of some rare earth elements in water samples using C18-cartridge modified with l-(2-pyridylazo) 2-naphtol (PAN) prior to simultaneous determination by inductively coupled plasma optical emission spectrometry (ICP-OES). Anal Chim Acta 540:325–332
Narin I, Soylak M (2003) The uses of 1-(2-pyridylazo) 2-naphtol (PAN) impregnated Ambersorb 563 resin on the solid phase extraction of traces heavy metal ions and their determinations by atomic absorption spectrometry. Talanta 60:215–221
Maloney MP, Moody GJ, Thomas JDR (1980) Extraction of metals from aqueous solution with polyurethane foam. Analyst 105:1087–1097
Behpour M, Ghoreishi SM, Salehi S (2005) Solid phase extraction of arsenic by sorption on naphthalene-methyltrioctyl ammonium chloride and spectrophotometric determination. Acta Chim Slov 52:323–327
Faghihian H, Amini MK, Nezamzadeh AR (2005) Cerium uptake by zeolite A synthesized from natural clinoptilolite tuffs. J Radioanal Nucl Chem 264:577–582
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 286 kb)
Rights and permissions
About this article
Cite this article
Faghihian, H., Kabiri-Tadi, M. A novel solid-phase extraction method for separation and preconcentration of zirconium. Microchim Acta 168, 147–152 (2010). https://doi.org/10.1007/s00604-009-0273-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-009-0273-9