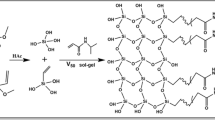
Abstract
An inorganic-organic hybrid monolith incorporated with stellated mesoporous silica nanoparticles (SMSNs) was prepared. Using binary solvents, deep eutectic solvents and room temperature ionic liquids, an SMSN-incorporated poly(butyl methacrylate-co-ethylene glycol dimethacrylate) monolith demonstrated uniform structure with good column permeability. A systematic investigation of preparation parameter was performed, including SMSN content, crosslinking monomer content, and the component of binary solvent. The optimized monoliths were characterized by field emission scanning electron microscopy, transmission electron microscopy, area scanning energy dispersive spectrometry, and nitrogen adsorption. Column performance was tested by separating four groups of analytes (alkylbenzenes, anilines, naphthalenes and phenols) by capillary electrochromatography (CEC). Baseline separation of all analytes was obtained with column efficiencies of up to 266,000 plates m−1. The performance of the resulting monolith was further investigated in detail by separating mixtures of polycyclic aromatic hydrocarbons (PAHs), nonsteroidal antiinflammatory drugs (NSAIDs), and hydroxybenzoic acid isomers. Compared with the corresponding SMSN-free monolith, the CEC performance was improved by about six times. Successful extraction of PAHs and quinolones (QNs) were also performed using this capillary. Improved extraction efficiency (20.2%) for complex samples, lake water, was also found when the material was applied to solid phase microextraction of fluoranthene.

A poly(butyl methacrylate-co-ethylene glycol dimethacrylate) monolith incorporated with stellated mesoporous silica nanoparticles was prepared. It demonstrated column efficiency up to 266,000 plates m−1 in capillary electrochromatography and ability as solid phase microextraction for organic small molecules with good column permeability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymer-based monolithic columns are versatile materials extensively applied in analytical science. The polymer monoliths reported have demonstrated several attractive merits, such as fast mass transfer, ease of preparation, controllable porosity and excellent permeability [1, 2]. They have been applied for pharmaceuticals, biomedicine, food control, environmental analysis and agricultural research [3,4,5]. Nevertheless, preparing polymer monoliths with column efficiency comparable with particulate and monolithic silica-based stationary phases has been proven to be difficult since the original monolithic polymer has smaller surface areas due to absence of nanopores and mesopores in polymer matrix [2, 3].
For overcoming the disadvantages above, plentiful strategies were studied, including innovative polymerization conditions, nanomaterial incorporation and hypercrosslinking [6,7,8]. Among the above strategies, multifunctional polymer nanocomposites are popular and extensive fields. The merit of such nano-entities separation media is that the stationary phases can be formed with more surfaces area [9]. To date, plenty of nano materials, including carbon nanotubes (CNT) [10], graphene oxide (GO) [11], metal-organic frameworks [12], mesoporous molecular sieve [13] and hydroxyapatite [14] have been incorporated into neat monolithic polymer. Surface chemistry with tailored selectivity can be observed on the hybrid monoliths due to the unique surface properties of nanoparticles.
Silica nanoparticles have attracted wide attention for their remarkable properties, including great surface area, high organic solvent resistance, good biocompatibility and easily postmodification with distinct functional groups [15]. Typically, silica nanoparticles can be functionalized at the first step with the following step of dynamical coating on the inner of the capillary to alter EOF or adding to background electrolyte solution [16]. For example, fumed silica nanoparticles (FSNPs) having specific surface of ~ 200 m2/g and an average primary particle size of ~ 12 nm were used to prepare polymethacrylate-based monolithic column [17, 18]. With successful separation of small solutes and proteins, incorporating distinct properties of silica nanoparticles into monolithic column is considered as another valid method to improve chromatographic performance successfully for hydrophilic interaction high performance liquid chromatography and reversed-phase chromatography.
Stellated mesoporous silica nanoparticles (SMSN) are novel nanoparticles with a special structure of stellated embossment around silica nanoparticles, thus higher specific surface area (> 500 m2/g) and average diameter (80 nm) than FSNPs. Traditionally, the smaller the size of the nanoparticles, the greater the specific surface area of the resultant materials. Thus, the nature of SMSN opposed to conventional nanoparticles. It is expected that incorporation of SMSN into monolithic matrix will improve the specific surface area and avoid low column permeability. In view of the facts above, it is intriguing for us to investigate whether extraordinary nature of SMSN can be utilized to increase column efficiency of monolith. However, the challenge of preparing SMSN-based hybrid monolithic matrix with homogeneous structure is the difficulty in suspending SMSN in conventional porogen due to the larger size [15].
A binary porogen system, room temperature ionic liquids (RTILs) and deep eutectic solvents (DESs), has been found to afford hybrid monolithic matrix with homogeneous structure and good permeability [10, 13]. When RTILs were used alone as porogenic solvents in preparing multifunctional nanocomposites, nanoparticles suspension can be sustained for fairly long time in prepolymerization mixture [19]. It has also been found that DESs can provide good solubility for polymerization composition [20,21,22,23,24,25]. In this study, SMSN-incorporated poly(butyl methacrylate-co-ethylene glycol dimethacrylate) monolith was prepared with the mixture of RTILs and DESs. To obtain SMSN-incorporated with robust column structure, SMSN were modified with silanization reagent 3-(trimethoxysilyl) propyl methacrylate (γ-MPS) to yield the “hybrid” methacryloyl silica nanoparticle monomer. The optimized SMSN incorporated monolithic column was characterized with area scanning energy dispersive spectrometry, field emission scanning electron microscopy, N2 adsorption experiment and transmission electron microscopy. Evaluation of chromatographic performance, including separation ability and column efficiency, was conducted in capillary electrochromatography (CEC) mode. In addition, the SMSN incorporated monolith was also used as solid phase microextraction (SPME) for extraction of complex samples.
Experimental materials and methods
Chemical reagents and materials
Chemicals and reagents employed were at least analytical grade in this work. Anthracene, acenaphthylene, fluorine, fluoranthene, pyrene, naphthalene, benzo (b) fluoranthene, benzo (a) anthracene, 1-methylnaphthalene, 1-choronaphthalene, 1-bromonaphthalene, 1-naphthol, resorcinol, m-cresol, 2,6-dichlorphenol, toluene, ethylbenzene, propylbenzene, butylbenzene, choline chloride (ChCl) were all obtained from Aladdin (Shanghai, China, www.aladdin-e.com). 2,5-Dihydroxyacetophenone (98%), butyl methacrylate (BMA) and azobisisobutyronitrile (AIBN) were purchased from J&K Scientific Ltd. (Beijing, China, www.jkchemical.com). All the RTILs (98%) were purchased form Chengjie Chemical Co. Ltd. (Shanghai, China, www.ionicliquid.com.cn). Acetonitrile (ACN, HPLC grade), 3-(trimethoxysilyl) propyl methacrylate (γ-MPS, 98%), 2-acrylamido-2-methyl-propanesulfonic acid (AMPS, 98%), butyrophenone (99%) and ethylene glycol dimethacrylate (EDMA, 98%) were purchased form Sigma-Aldrich (St. Louis, MO, USA, www.sigmaaldrich.com). Other reagents were supplied by Tianjin Chemical Reagent Co. Ltd. (Tianjin, China, www.630451.atobo.com.cn). Real water sample was collected by Jingyi Lake in Tianjin Medical University. Stellate mesoporous silica nanosphere (SMSN) was purchased from XFNANO Materials Tech. Co., Ltd. (Nanjing, China, www.xfnano.com). Fused-silica capillary (375 μm OD, 100 μm ID; 375 μm OD, 250 μm ID) was purchased from Xinnuo Optic Fiber Plant (Hebei, China, www.11467.com/handan/co/68426.htm).
Silylation modification of pristine SMSN
Surface modification of pristine SMSN with silanization reagent γ-MPS was carried out according to our previous report [13]. Synthesis method can be seen in the Electronic Supporting Material (ESM).
Preparation of SMSN incorporated monolithic column
Prior to the preparation of monolithic capillary, the inner wall of fused silica capillary was dealt with NaOH solution (1 mol L−1) for 30 min and washed with deionized water to neutral, respectively. The capillary was then treated by pumping γ-MPS/acetic acid aqueous solution (4/96, v/v) (6 mmol L−1) for 90 min. The derivatized capillary was then washed with deionized water to neutral and dried with N2 before use.
The DESs were prepared by mixing choline chloride (ChCl) with different type of alcohols. After a 24 h pretreatment of vacuum drying at 60 °C, the mixture of ChCl and alcohols was heated and stirred in oil bath of 100 °C for 3 h. The DESs obtained were homogeneous and viscous colourless liquid. The DESs were placed in drier before use.
For the preparation of SMSN incorporated polymer monolith (Table S1), various contents of modified SMSN were dispersed in a mixture containing BMA (0.175 mmol, functional monomers), EDMA (0.075 mmol, cross-linking monomers) and binary porogen, RTIL (65%, v/v) and DES (35%, v/v). AMPS (1%, wt/wt%) and AIBN (1%, wt/wt%) were also contained in the pre-polymerization mixture as electroosmotic flow provider and initiator, respectively. After vortexed and sonicated, the homogenous pre-polymerization mixture was then filled into the derived capillary. Both outlet and inlet was sealed using rubber stopper. In situ polymerization was carried out in water bath of 65 °C for 30 min. After reaction, the resulting capillary column was washed with acetonitrile (ACN) to remove unreacted reagents. A detection window of 2–3 mm length was burned at distance of 10 cm from the outlet. The corresponding monolith without SMSN was made with same process in absence of modified SMSN.
Electrochromatography
CEC was conducted using Kaiao K1050 high performance capillary electrophoresis instrument (Beijing, China) equipped with ultraviolet detector. Detection wavelength of 254 nm was used and separation was performed at an operating voltage of 15 kV. Chromatographic workstation, CXTH-3000, was applied for instrumental controlling and data analysis. Before CEC analysis, all capillaries were rinsed with mobile phase, which was filtered through 0.2 μm membrane before experiment. The mobile phase was a various ratio mixture of acetonitrile and acetate buffer, consisted of the solution of acetic acid and sodium acetate with different proportion and concentration.
Electroosmotic flow (EOF) was evaluated by eq. (1) [26]:
where μ refers to EOF, t is dead time, V is operating voltage. L0 (32 cm) and L (42 cm) are stationary-based length and total length of capillary monolithic column, respectively.
Column permeability was evaluated by eq. 2 [26].
where BEK refers to electrokinetic permeability.
Solid phase microextraction (SPME) and chromatographic analysis
The SPME procedures included pre-conditioning, sample loading, washing as well as desorption. A syringe pump (RSP04-B, RISTRON, Zhejiang, China, www.chinamot.com) was employed for the SPME procedure. The procedure in detail for SPME of PAHs and lake water was as follows. For pre-conditioning, 0.2 mL methanol was in-drafted into the syringe and injected through the SMSN incorporated monolith at 0.05 mL min−1, and then 0.5 mL phosphate solution (100 mM, pH 4.5) was injected at a flow rate of 0.10 mL min−1. Likewise, 1.0 mL sample solution was introduced into the syringe and passed though the monolith at 0.10 mL min−1. After that, 0.2 mL phosphate solution (100 mM, pH 4.5) was introduced into the monolith at a flow rate of 0.10 mL min−1. Subsequently, 0.1 mL mixture of methanol and H2O (80/20, v/v) was injected into the monolith at 0.05 mL min−1 and the eluate was collected into a vial for the subsequent HPLC determinations. The SPME procedure of quinolones (QNs) was performed according to previous report [27].
The SPME device was fabricated according to Li’s group [28]. HPLC analysis was performed on an Agilent 1260 liquid chromatography system, equipped with a multiple wavelength detector, and a quaternary pump and degasser. ChemStation software was applied for instrumental controlling and data analysis. A reverse phase 100–5-C18 column (4.6 × 250 mm, Kromasil, Sweden) was employed for the chromatographic analysis. Before analysis, a series of PAHs and QNs standard solutions and the lake water were filtered with a membrane filter of 0.22 μm.
Results and discussion
Characterization of the SMSN-loaded monolith
Particle size distribution of the SMSN is showed in Fig. 1. Over 90% of SMSN had a particle size of about 90 nm. Furthermore, the morphology of the SMSN incorporated monoliths was tested by field emission scanning electron microscopy (FESEM). Compared with the SMSN-free monolith, the microstructure in the SMSN incorporated capillary monolith is denser with lower interstitial porosity (Fig. 2). As shown in Fig. 3, the successful incorporation of SMSN into polymer matrix is further demonstrated by transmission electron microscopy (TEM). Moreover, area scanning of energy dispersive spectrometer (EDS) is employed to confirm a homogeneous distribution of SMSN in the resulting monolith (Fig. S1).
Pore property of both the incorporated monolith and the free monolith were studied by N2 adsorption experiments. The isotherms of nitrogen adsorption-desorption for the SMSN incorporated and SMSN-free monolith are exhibited in Fig. S2. The Brunauer-Emmett-Teller specific surface area of the SMSN incorporated monolith is increased about 28.18% by comparing the surface area of the incorporated (41.892 m2 g−1) and free monolith (32.683 m2 g−1). Both the incorporated monolith and free monolithic column demonstrated incomplete isotherms of type II and hysteresis loops of type H3 [29]. Distinction of pore structure between the monolithic columns was observed. As a whole, the incorporation of SMSN with polymer matrix affects the resulting hybrid monolithic column system positively and significantly.
Optimization of polymerization variables
In order to obtain the best monolith, the influence of preparation variables was systematically studied. The following parameters were optimized: (a) SMSN content; (b) crosslinking monomer content; (c) type of RTILs cation and anion; (d) type of DESs; (e) percentage of DESs in binary porogens. Respective data and figures are given in the ESM. The following experimental conditions were found to give the best results: (a) SMSN content: 7.5% (wt/wt%); (b) crosslinking monomer content: 30%; (c) type of RTILs cation and anion: [HMIM]BF4; (d) type of DESs: ChCl-PG (1, 2-propylene glycol); (e) percentage of DESs in binary porogens: 35% (v/v). The resulting capillary monolith demonstrated uniform structure with good column permeability. Baseline separation of all analytes measured was obtained.
Electrochromatographic evaluation
To investigate the performance of the formed SMSN incorporated monolith and corresponding neat SMSN-free monolith, the separation for small molecules was performed by CEC. Four groups of small molecules were chosen as model compounds, including alkylbenzenes, anilines, naphthalenes and phenols. The SMSN incorporated monolith exhibited improved performance in terms of resolution and column efficiency for the model compounds above compared to the SMSN-free monolith (Fig. 4).
Comparison of incorporated monolith and free monolith on separation. Conditions: capillary, 100 μm inner diameter, 42 cm total length and 32 cm effective length; separation voltage, 15 kV; temperature, 25 °C; UV-vis detector, 254 nm. Mobile phase: acetonitrile/acetate buffer (pH 6.0, a mixture of acetic acid and sodium acetate solution) (70/30, v/v). Analytes: a alkylbenzenes; b anilines; c naphthalenes; d phenols
For alkylbenzene samples containing eight compounds, baseline separation and high column efficiency (266,000 plates m−1) are obtained in less than 15 min on the SMSN incorporated monolith (Fig. 4a). Elution order was as follow: acetone, 2, 5-dihydroxyacetophenone, acetophenone, butyrophenone, toluene, ethylbenzene, propylbenzene, butylbenzene. On the contrary, the SMSN-free monolith exhibited unsatisfied separation performance with unsymmetrical peak shape and baseline separation was not realized in the same experimental conditions. The stronger retention on the SMSN incorporated monolith is due to the introduction of silanized SMSN onto the polymer matrix.
Naphthalenes were used as analytes to study the potential of SMSN incorporated monolith in separation enhancement of π-electron rich compounds. With increasing elution time of 1-bromonaphthalene >1-chloronaphthalene >1-methylnaphthalene >1-naphthol, baseline separation is achieved (Fig. 4b). Compared with the SMSN-free monolith, the increasing resolution on the SMSN incorporated monolith can be due to successful combination of marcopores from polymer matrix with mesopores from SMSN.
To further display the versatility of the SMSN incorporated monolith, alkaline analytes, and anilines were also separated in CEC mode. The retention time increased with an order of 1-naphthylamine >2-nitroaniline >4-fluoroaniline > acetanilide (Fig. 4c). All the analytes are baseline separated in ten minutes (Rs > 1.5). In contrast, the SMSN-free monolith failed to separate 4-fluoroaniline and 2-nitroaniline. Practically, the hybrid capillary fabricated with SMSN enhances separation for aniline compounds.
In contrast to the unsuccessful separation on the SMSN-free monolith, three phenols were separated perfectly on the SMSN incorporated monolith. The elution time of phenols is in an order of resorcinol < m-cresol <2, 6-dichlorophenol, indicating that hydrophobicity acts as a main role during phenols elution (Fig. 4d). Hydrophobic interaction between stationary phase and analytes increases as the hydrophobicity of phenols increasing, which leads to the successful separation of phenols analytes.
For the purpose of investigating applicability, polycyclic aromatic hydrocarbons (PAHs), nonsteroidal antiinflammatory drugs (NSAIDs) and hydroxybenzoic acid (HBA) isomers were separated on the SMSN incorporated monolith. Baseline separation of PAHs, NSAIDs and HBA isomers is observed on SMSN incorporated monolith (C1) in CEC mode (Fig. 5). The result indicates that the SMSN incorporated monolithic column possesses adequate separation ability for PAHs, NSAIDSs and HBA isomers. Quinolones (QNs) were also chosen to investigate the separation selectivity of the SMSN incorporated monolith stationary phase (Fig. S3). However, QNs cannot be separated on the resulting monolith. The result indicates that the resulting monolith has selectivity for the substances containing carboxyl group.
a Application of incorporated monolith and free monolith on PAHs separation. Conditions were same as Fig. 4. b Application of SMSN incorporated monolith and SMSN-free monolith on NSAIDs separation. Conditions were same as Fig. 4. c Application of incorporated monolith and free monolith on HBA isomers separation. Conditions: UV-vis detector, 210 nm. Mobile phase: acetonitrile/0.01 mol L−1 sodium hydrogen phosphate (pH 10.0) (20/80, v/v). Other conditions were same as Fig. 4
A number of monoliths with incorporation of mesoporous silica nanoparticles to monoliths have been reported [15, 30,31,32]. Table 1 compares the analytes for separation, column efficiency and other characteristic features of our work with other reports. Although the column efficiency of our columns was lower than the result of Lei and Wan’s group [30, 31], the SMSN incorporated monolith showed a remarkable enhanced retention accompanied with a better batch-to-batch repeatability. Because repeatability of the hybrid monolithic column is mainly determined by the stability of the column with nanoparticles embedded, it seemed that the silylation modification of pristine SMSN played an important role in the SMSN incorporated monolith. Moreover, much more types of analytes can be separated on the SMSN incorporated monolith than other mesoporous silica nanoparticles incorporated monoliths.
The major limitation of this method is a need of the accurate control of polymerization temperature and time during the preparation. No change of the temperature (65 °C) and time (30 min) had to be used. Otherwise, other polymerization temperature or time would result in block monolith. Thus, the variation in the polymerization temperature and time can not be utilized as polymerization variables for the optimization of monolith preparation.
Extraction of PAHs and quinolones (QNs)
To evaluate further the performance of the incorporated monolith, capillary C1 was selected for SPME to PAHs and QNs. Increasing the quantity of polymer in volume may improve the SPME extraction efficiency, and therefore the preparation conditions and extraction device were modified accordingly: (1) the ID of capillary to prepare SMSN incorporated monolith was enlarged to 250 μm; (2) the length of the incorporated monolith was shortened to 20 cm to speed up the procedure; (3) the time of polymerization was prolonged to 1 h. The washing liquid and the eluate after the SPME were collected for HPLC analysis. Four PAHs (anthracene, fluoranthene, phenanthrene and fluorene) and four QNs (ciprofloxacin, norfloxacin, enrofloxacin and gatifloxacin) were selected as model analytes to study the potential in extraction and enrichment. The concentration of each component in the mixed standard sample was 0.1 μg mL−1. HPLC chromatograms are shown in Fig. S4. Peak area of each analyte before and after SPME was used to determine the extraction efficiency of the resulting capillary monolith (Table 2). The recovery of the analytes was all above 80%, showing that the SMSN incorporated monolith is a reliable SPME material for extraction of PAHs and QNs. Moreover, compared with QNs, the resulting capillary monolith is more suitable for extraction of PAHs.
Extraction of fluoranthene in lake water by SPME
The incorporated monolith was used to determine the trace fluoranthene, a common environmental pollution, in lake water. HPLC chromatograms of fluoranthene in the lake water after SPME are shown in Fig. 6a. The result indicates that the lake water contains only tiny amounts of fluoranthene. The recovery of fluoranthene of the lake water after SPME was 93.43%. As shown in Fig. 6b, the SMSN incorporation into monolithic column can significantly improve extraction efficiency and the hybrid monolith exhibits greater separation ability for complex samples.
In our study, the concentration ranging from 0.2 to 1.8 μg mL−1 of standard fluoranthene solutions was chosen to validate the method for the quantification and the linearity. There was a good linear relationship with R2 of 0.9994, the limit of detection (LOD, 3 times the standard deviation of the baseline noise) was 0.01 μg mL−1 and limit of quantitation (LOQ, 10 times the standard deviation of the baseline noise) was 0.04 μg mL−1. The relative standard deviations (RSDs), the intra-capillary and inter-capillary reproducibility of the method were all less than 3.0% (three replicate experiments). These results suggested that the application of the resulting SMSN incorporated monolithic column for SPME is reliable.
Reproducibility of SMSN incorporated monolithic column
The resulting incorporated monolith can afford baseline separation to the analytes after more than one hundred times of injections in CEC mode, and no obvious damage of the structure of the monolith was found. The reproducibility of the monolithic column fabricated with C1 was evaluated by investigating the migration times, selectivity factor and retention factor of alkylbenzenes in a series of injections (Table S2). The RSDs of retention times and selectivity factor for batch-to-batch and run-to-run of the incorporated monolith were all less than 0.6%. The RSDs of retention parameters for batch-to-batch and run-to-run of the SMSN incorporated monolithic column were less than 2.0% (n = 3) and 1.0% (n = 5), respectively.
Conclusion
In this work, a novel SMSN incorporated monolith was successfully prepared and used as stationary phase for CEC and solid phase microextraction. The binary porogens, [HMIM]BF4 and ChCl-PG, exhibited excellent dispersible property for SMSN and can lead to hybrid monolithic column with good column permeability. The formed hybrid monolith incorporated SMSN demonstrated improved chromatographic performance for small organic molecules, including alkylbenzenes, anilines, naphthalenes and phenols. The highest column efficiency achieved was 266,000 plates m−1 for alkylbenzenes analysis. Further investigation for the separation of PAHs, NSAIDs, HBA isomers and extraction of PAHs, quinolone drugs and trace fluoranthene in the lake water demonstrated the applicability of the monolith. As a conclusion, using “hybrid” methacryloyl silica nanoparticle monomer to prepare SMSN incorporated monolith is a promising approach to inorganic-organic hybrid column for separation.
References
Eeltink S, Wouters S, Dores-Sousa JL, Svec F (2017) Advances in organic polymer-based monolithic column technology for high-resolution liquid chromatography-mass spectrometry profiling of antibodies, intact proteins, oligonucleotides. and peptides J Chromatogr A 1498:8–21
Hong TT, Yang X, Xu Y, Ji Y (2016) Recent advances in the preparation and application of monolithic capillary columns in separation science. Anal Chim Acta 931:1–24
Ding X, Yang J, Dong Y (2018) Advancements in the preparation of high-performance liquid chromatographic organic polymer monoliths for the separation of small-molecule drugs. J Pharm Anal 8:75–85
Zhang Z, Zhu G, Peuchen EH, Dovichi NJ (2017) Preparation of linear polyacrylamide coating and strong cationic exchange hybrid monolith in a single capillary, and its application as an automated platform for bottom-up proteomics by capillary electrophoresis-mass spectrometry. Microchim Acta 184:921–925
Jandera P (2013) Advances in the development of organic polymer monolithic columns and their applications in food analysis-a review. J Chromatogr A 1313:37–53
Urban J, Svec F, Fréchet JMJ (2010) Efficient separation of small molecules using a large surface area hypercrosslinked monolithic polymer capillary column. Anal Chem 82:1621–1623
Urban J (2016) Current trends in the development of porous polymer monoliths for the separation of small molecules. J Sep Sci 39:51–68
Lv Y, Alejandro FM, Fréchet JMJ, Svec F (2012) Preparation of porous polymer monoliths featuring enhanced surface coverage with gold nanoparticles. J Chromatogr A 1261:121–128
Xu P, Han X, Zhang B, Dua Y, Wang HL (2014) Multifunctional polymer-metal nanocomposites via direct chemical reduction by conjugated polymers. Chem Soc Rev 43:1349–1360
Zhang LS, Gao SP, Huang YP, Liu ZS (2016) Green synthesis of polymer monoliths incorporated with carbon nanotubes in room temperature ionic liquid and deep eutectic solvents. Talanta 154:335–340
Wang MM, Yan XP (2012) Fabrication of graphene oxide nanosheets incorporated monolithic column via one-step room temperature polymerization for capillary electrochromatography. Anal Chem 84:39–44
Fu YY, Yang CX, Yan XP (2013) Incorporation of metal-organic framework UiO-66 into porous polymer monoliths to enhance the liquid chromatographic separation of small molecules. Chem Commun 49:7162–7164
Zhang LS, Zhao QL, Li XX, Li XX, Huang YP, Liu ZS (2016) Green synthesis of mesoporous molecular sieve incorporated monoliths using room temperature ionic liquid and deep eutectic solvents. Talanta 161:660–667
Krenkova J, Lacher NA, Svec F (2010) Control of selectivity via nanochemistry: monolithic capillary column containing hydroxyapatite nanoparticles for separation of proteins and enrichment of phosphopeptides. Anal Chem 82:8335–8341
Xu SJ, Mo RZ, Jin C, Cui XQ, Bai RH, Ji YB (2017) Mesoporous silica nanoparticles incorporated hybrid monolithic stationary phase immobilized with pepsin for enantioseparation by capillary electrochromatography. J Pharmaceut Biomed Anal 140:190–198
Wang B, Chai W, Ding G (2015) The application of functional silica nanoparticles to fulfill the rapid and improved enantioselective capillary electrophoresis separation of amino acid derivatives. J Sep Sci 38:332–338
Aydogan C, Rassi ZE (2016) Monolithic stationary phases with incorporated fumed silica nanoparticles. Part I. Polymethacrylate-based monolithic column with incorporated bare fumed silica nanoparticles for hydrophilic interaction liquid chromatography. J Chromatogr A 1445:55–61
Aydogan C, Rassi ZE (2016) Monolithic stationary phases with incorporated fumed silica nanoparticles. Part II. Polymethacrylate-based monolithic column with “covalently” incorporated modified octadecyl fumed silica nanoparticles for reversed-phase chromatography. J Chromatogr A 1445:62–67
Zhou X, Wu T, Ding K, Hu B, Hou M, Han B (2009) The dispersion of carbon nanotubes in water with the aid of very small amounts of ionic liquid. Chem Commun (14):1897–1899
Said B, Grandjean A, Barre Y, Tancret F, Fajula F, Galarneau A (2016) LTA zeolite monoliths with hierarchical trimodal porosity as highly efficient microreactors for strontium capture in continuous flow. Micropor Mesopor Mat 232:39–52
Abbott AP, Ttaib KE, Frisch G, Ryder KS, Weston D (2012) The electrodeposition of silver composites using deep eutectic solvents. Phys Chem Chem Phys 14:2443–2449
Massolo E, Palmieri S, Benaglia M, Capriati V, Perna FM (2016) Stereoselective organocatalysed reactions in deep eutectic solvents: highly tunable and biorenewable reaction media for sustainable organic synthesis. Green Chem 18:792–797
Wang Y, Hou HC, Wu WZ, Liu DD, Ji YA, Ren SH (2016) Roles of a hydrogen bond donor and a hydrogen bond acceptor in the extraction of toluene from n-heptane using deep eutectic solvents. Green Chem 18:3089–3097
Morrison HG, Sun CC, Neervannan S (2009) Characterization of thermal behavior of deep eutectic solvents and their potential as drug solubilization vehicles. Int J Pharm 378:136–139
Mota-Morales JD, Gutierrez MC, Ferrer ML, Jimenez R, Santiago P, Sanchez IC, Terrones M, Monte FD, Luna-Barcenas G (2013) Synthesis of macroporous poly (acrylic acid)-carbon nanotube composites by frontal polymerization in deep eutectic solvents. J Mater Chem A 1:3970–3976
Choudhary G, Horváth C (1997) Dynamics of capillary electrochromatography experimental study on the electrosmotic flow and conductance in open and packed capillaries. J Chromatogr A 781:161–183
Zhang X, Wang C, Yang L, Zhang W, Lin J, Li C (2017) Determination of eight quinolones in milk using immunoaffinity microextraction in a packed syringe and liquid chromatography with fluorescence detection. J Chromatogr B 1064:68–74
Liu W, Qi JR, Yan LN, Jia Q, Yu C (2011) Application of poly (butyl methacrylate-co-ethylene glycol dimethacrylate) monolith microextraction coupled with high performance liquid chromatography to the determination of polycyclic aromatic hydrocarbons in smoked meat products. J Chromatogr B 879:3012–3016
Li YY, Tolley HD, Lee ML (2011) Preparation of monoliths from single crosslinking monomers for reversed-phase capillary chromatography of small molecules. J Chromatogr A 1218:1399–1408
Lei W, Zhang LY, Wan L, Shi BF, Wang YQ, Zhang WB (2012) Hybrid monolithic columns with nanoparticles incorporated for capillary electrochromatography. J Chromatogr A 1239:67–71
Wan L, Zhang L, Lei W, Zhu Y, Zhang W, Wang Y (2012) Novel hybrid organic-inorganic monolithic column containing mesoporous nanoparticles for capillary electrochromatography. Talanta 98:277–281
Weller A, Carrasco-Correa EJ, Belenguer-Sapiña C, Mauri-Aucejo ADLR, Amorós P, Herrero-Martínez JM (2017) Organo-silica hybrid capillary monolithic column with mesoporous silica particles for separation of small aromatic molecules. Microchim Acta 184:1–10
Acknowledgments
This work was supported by National Natural Science Foundation of China (Grant No. 21775109).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 5.82 mb)
Rights and permissions
About this article
Cite this article
Zhou, XJ., Zhang, LS., Song, WF. et al. A polymer monolith incorporating stellate mesoporous silica nanospheres for use in capillary electrochromatography and solid phase microextraction of polycyclic aromatic hydrocarbons and organic small molecules. Microchim Acta 185, 444 (2018). https://doi.org/10.1007/s00604-018-2964-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2964-6