Abstract
A polydopamine-based molecularly imprinted polymer was deposited on the surface of magnetite (ferroferric oxide) nanoparticles (Fe3O4@PDA MIPs) and is shown to be an efficient and fairly specific sorbent for the extraction of various ochratoxins. The MIPs were characterized by IR spectroscopy and transmission electron microscopy. The adsorption capacities, evaluated through the langmuir adsorption isotherm model, are 1.8, 0.23 and 0.17 mg·g−1 for ochratoxin A, ochratoxin B and ochratoxin C, respectively. Parameters such as the amount of magnetic MIPs, pH value, time for ultrasonication, elution solvent and volume were optimized. Following desorption from the MIP with acetonitrile, the ochratoxins were quantified by HPLC with fluorometric detection. Under optimal experimental conditions, the calibration plots are linear in the range of 0.01–1.0 ng·mL−1 of OTA, 0.02–2.0 ng·mL−1 of OTB, and 0.002–0.2 ng·mL−1 of OTC. The LODs are between 1.8 and 18 pg·mL−1, and the recoveries from spiked samples are 71.0% - 88.5%, with RSDs of 2.3–3.8% in case of rice and wine samples. The MIPs can be re-used for at least 7 times.

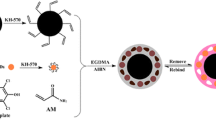
Schematic of the preparation of a magnetic molecularly imprinted polymer based on self-polymerization of dopamine in weakly alkaline solution. Ochratoxins are recognized owing to homologous cavities in the MIPs, and quantified by HPLC after desorption with acetonitrile.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ochratoxins (OTs) are a group of chemically related mycotoxins that can be present in food [1]. Ochratoxin A (OTA), ochratoxin B (OTB) and ochratoxin C (OTC) are the main ochratoxins among the seven analogues [2] (Scheme S1). Considering their nephrotoxic, teratogenic and immunotoxic effects to human health [3], OTA was classified as a possible carcinogenic compound (Group 2B) for human by the International Agency of Research on Cancer (IARC) [4]. Some countries and organizations have set limitations of OTA for foods, the European Union stipulated the maximum limits as 5 and 3 μg·kg−1 for raw cereal and processed cereal products, respectively [5], China and Australia marked 5 μg·kg−1 as the maximum limit for cereal [2, 6]. OTB and OTC were also detected in foods [7], and they additionally become attention focus due to the potential toxicity [2], especially for OTC, which can convert to OTA through oral and intravenous administration [8]. However, the maximum amounts of OTB and OTC have not been limited by any country or organization.
High performance liquid chromatography (HPLC) with fluorometric detection is the main approach to detect OTs in foods [9], often along with a clean-up step, such as solid phase extraction (SPE) [10] or immunoaffinity column (IAC) [7]. Although these pre-concentrations technologies offered advantages like high sensitivity and selectivity, the practical limitations were remained owing to high operation cost, or complicated operating process. Therefore, proposing a robust and rapid pre-treatment way for complex food samples was necessary.
Molecularly imprinted polymers (MIPs) possess predetermined recognition ability, high stability, and a wide range application for analytes [11]. MIPs have been successfully applied in the determination of OTA in wine [12, 13]. However, these MIPs have several limits such as poor accessibility, low affinity binding, high diffusion barrier and the uncompleted removal of template [14]. To overcome the problems above, nanomaterials with small size and high surface-to-volume ratio had been attempted to support the surface imprinting procedure, such as Fe3O4 [15], TiO2 [16], and quantum dots [17]. Fe3O4 nanoparticles (Fe3O4 NPs) coated MIPs had gained considerable attention owing to the unique size and satisfied physical properties [18]. However, The common synthesis procedure of magnetic MIPs was complicated, which involved functional monomers, cross linkers, initiators and relative harsh conditions, such as oxygen free [19]. Therefore, enhancing the general synthesis procedure of magnetic MIPs was another requirement.
Dopamine was often used to synthesize the magnetic MIPs owing to the ability of self-polymerization in a weak alkaline solution [20, 21]. Herein, dopamine was covered on the surface of the Fe3O4 NPs as a adsorption temperate by self-polymerization to synthesize the magnetic MIPs in the Tris-HCl buffer (pH 8.5) (Scheme 1), the product was called Fe3O4@polydopamine molecularly imprinted polymers (Fe3O4@PDA MIPs). Fe3O4@PDA MIPs was able to recognize and enrich the presented OTs in the rice and wine samples through the specific position. Besides, the features and functions of Fe3O4@PDA MIPs, which contained MIPs amount, desorption condition and extraction efficiency, were investigated respectively.
Experimental
Reagents and materials
The standard compounds contained OTA and OTB with the purities 98.14%, and 99.14%, respectively, purchased from Stanford Chemicals Company (Oregon, America, www.stanfordchem.com), OTC with the purity 98%, bought from Toronto Research Chemicals (Toronto, Canada, www.trc-canada.com), aflatoxin B1 with the purity >99%, purchased from Pribolab Company (Singapore, www.pribolab.com), fumonisin B1 and zearalenone with the concentration 50.5 μg·mL−1 and 102.2 μg·mL−1, purchased from Romer Company (Germany, www.romerlabs.com) and Sigma-Aldrich Company (Australia, www.sigmaaldrich.com), respectively. All chemical reagents for the synthesis of Fe3O4 NPs and Fe3O4@PDA MIPs were of analytical grade and used without any further purification. Ferric chloride (FeCl3·6H2O), ethylene glycol, anhydrous sodium acetate, hydrochloric acid, sodium hydroxide, dopamine hydrochloride bought from Sinopharm Chemical Reagent (Shanghai, China, www.sinoreagent.com). Methanol and acetonitrile were HPLC grades and purchased from Merck (Darmstadt, Germany, www.merck-chemicals.com).
Standard solutions were prepared as follows: 2.0 mg of OTA, 2.5 mg of OTB and 0.5 mg of OTC were dissolved in acetonitrile and stored in a 1 mL flask, respectively, and then 2.0 mg·mL−1 OTA, 2.5 mg·mL−1 OTB and 0.5 mg·mL−1 OTC was gained. An intermediate standard solutions of OTs at the concentrations of 0.5 μg·mL−1 OTA, 1.0 μg·mL−1 OTB and 0.1 μg·mL−1 OTC were prepared in acetonitrile. And the working standard solutions of their desired concentrations were obtained in acetonitrile. All the standard solutions were stored at 4 °C in the refrigerator. Ultrapure water used from a Milli-Q water purifier (18.2 MΩ·cm) (Millipore, Molsheim, France, www.millipore.com) was used throughout the work.
Preparation of magnetic molecularly imprinted polymers
Fe3O4 NPs were synthesized according to our previous work [22]. The magnetic MIPs were prepared according to the document [23] with some modification. Briefly, Fe3O4 NPs (0.2 mg) were dissolved in Tris-HCl buffer (80 mL), and the solution was mechanically stirred for 1 h at room temperature until well-suspended. Afterwards, OTA (5 mL 1.6 μg·mL−1) was added to the solution, stirring for 2 h continually. Then dopamine hydrochloride (100 mg) was added and the reaction was continued for another 4 h at ambient temperature. The Fe3O4@PDA MIPs acquired were washed with ultrapure water and the solution of 3% (v/v) acetic acid and 20% (v/v) acetonitrile to extract the template molecules until OTA was not detected by HPLC. Finally, the Fe3O4@PDA MIPs were dried. For comparison, Fe3O4@polydopamine molecularly non-imprinted polymers (Fe3O4@PDA MNIPs) were prepared and washed using the same protocol but without OTA as the template molecule in the self-polymerization stage.
Preparation of real samples
In this study, rice and wine were purchased at local supermarkets (Nanchang, China) randomly. And three samples of each kind were taken. Each sample was replicated for five times. All the samples were extracted according to the procedure in GB 5009.96-2016 [24] (See the Electronic Supporting Material).
Extraction procedures
In this work, Fe3O4@PDA MIPs (15 mg) was added in 100 mL beaker with ultrapure water (50 mL) at OTs concentrations (0.01–1.0 ng·mL−1 OTA, 0.02–2.0 ng·mL−1 OTB and 0.002–0.2 ng·mL−1 OTC). The solution was adjusted at pH 3 through HCl (0.1 M). After performing the extraction by an ultrasonic water bath for 5 min at room temperature, the magnetic MIPs were separated through a commercial NdFeB magnet (10 × 5 × 4 cm) and the supernatant was decanted. Acetonitrile (1.0 mL) was used to desorb the target compounds from the magnetic MIPs. Acetonitrile solution above (0.5 mL) was mixed with 1% acetic acid solution (0.5 mL), which was the initial composition of the mobile phase during the chromatiographic separation, and then 10 μL of the solution was injected into HPLC for further analysis.
HPLC analysis
All of measurements of OTs were detected by UltiMate 3000 HPLC with Fluorescence Detector (FLD) (ThermoFisher, USA, www.thermofisher.com). The column of separation used was Eclipse Plus C18(150 mm × 4.6 mm, 3 μm) (Agilent; www.agilent.com) at 35 °C as the column temperature. The conditions of the gradient elution were carried out according to GB 5009.96-2016 [24] with a minor modification (See the Electronic Supporting Material).
Results and discussion
Preparation and characterization of magnetic molecularly imprinted polymers
The synthesis of magnetic MIPs which utilized OTA as the template molecule was depicted in Scheme 1. The magnetic MIPs films were formed on the surface of Fe3O4 NPs through the unique adhesive effect based on self-polymerization of dopamine. The template OTA was imprinted in the polymer layer through the non-covalent interactions of –NH2 and –OH groups on PDA as well as the –COOH and –NH groups on OTA. Subsequently, the modified OTA templates were extracted in acidic solution, resulting homologous cavities for the recognition of target OTA, OTB and OTC.
The infrared spectra characterization was performed by the Fourier Transform Infrared Spectrometer (FT-IR) (Bruker, Germany, www.bruker.com). Compared with the absorption bands in Fig. S1A, the Fig. S1B show that two new absorption bands at 1609 cm−1 and 1467 cm−1 emerged, which corresponded to the C=C stretching vibration in the aromatic rings [25], and the C–C stretching vibration from PDA [20], respectively. Fe3O4 NPs and Fe3O4@PDA MIPs were also characterized by a JEM-2100 Transmission Electron Microscope (TEM) (JEOL, Japan, www.jeol.cn). Their average diameters (inset of Fig. S1A and B) were 252 nm and 301 nm, respectively. This shows that Fe3O4 NPs were fully covered by PDA. Therefore, these results indicate that the PDA film was modified on the surface of Fe3O4 NPs. As shown in Fig. S2, Fe3O4@PDA MIPs have excellent dispersibility after ultrasonication and can rapidly be separated through an extra magnet, which suggested a satisfied magnet-controlled property.
Extraction condition optimization
It is significant to choose optimal extraction conditions that improve the absorption efficiency of OTs. The recovery was used as an absorption efficiency index in the whole procedure. Therefore, the following parameters were optimized: (a) sample pH value; (b) amount of sorbent; (c) ultrasonic time; (d) desorption conditions; (e) solution volume. Respective data and figures are given in Fig. S3, (See the Electronic Supporting Material). The following experimental conditions were found to give best results: (a) best sample pH value: 3.0; (b) optimal amount of sorbent: 15 mg; (c) ultrasonic time: 5 min; (d) desorption conditions: 1.0 mL acetonitrile; (e) solution volume: 50 mL.
Moreover, Fe3O4@PDA MNIPs were used to adsorb three OTs under the above optimized conditions. The recoveries were only 23.2%, 15.8%, and 37.6% for OTA, OTB, and OTC, respectively, which was possibly attributed to the non-covalent combination between target compounds and polydopamine through π-π and hydrogen bonds [21]. The standard chromatogram of OTs under the different materials was showed in Fig. 1. Comparing to the standard chromatogram produced by the pretreatment utilized direct injection (d), the retention time and the peak area of OTs pretreated by Fe3O4@PDA MIPs (c) are almost the same, however, three OTs pretreated by Fe3O4@PDA NMIPs (b) have much lower peak areas. This indicates the imprinted cavities of Fe3O4@PDA MI Ps were formed and exhibited the high affinity toward target compounds.
Reusability of Fe3O4@PDA MIPs
To test the reusability of Fe3O4@PDA MIPs, 10 mL of acetonitrile as the optimum desorption solvent were used to rinse the MIPs to recover the template for twice before applying in the next. Fig. S5 indicates that the adsorption efficiencies for the three OTs had no significant decrease after at least seven consecutive adsorption-desorption cycles, and the recoveries were more than 86.8%, 82.6% and 80.2% for OTA, OTB and OTC, respectively. This manifested that Fe3O4@PDA MIPs have satisfied stability.
Adsorption isotherms
The binding properties of the Fe3O4@PDA MIPs for the three OTs were studied. In general, two models which the Langmuir for describing monolayer adsorption, and the Freundlich for assuming heterogeneous surface energy were applied to evaluate the adsorption property [26]. The isotherms data was then fitted to the Langmuir or Freundlich models to assess the adsorption capacity and the adsorption mechanisms of the adsorbent at a certain temperature. Equilibrium isotherm was determined by using batch studies with various initial concentrations of the three OTs (0.016–4 mg·L−1 for OTA, 0.032–0.16 mg·L−1 for OTB and 0.0032–0.4 mg·L−1 for OTC) at 25 °C under the optimal conditions.
The parameters of the Langmuir equation and Freundlich equation were calculated according to our previous report [3]. Table S1 shows that the correlation coefficients of OTA, OTB and OTC were 0.994, 0.995 and 0.997 from the Langmuir equation, respectively, which given better fitting than those provided by the Freundlich model (R2 = 0.986, 0.929 and 0.938, respectively). This illustrates that the Langmuir isotherm model provided with more satisfied the experimental data, and the results suggest the mechanism that three OTs were adsorbed on the surface of Fe3O4 @PDA MIPs through the monolayer molecular adsorption. The maximum adsorption capacities were calculated by the Langmuir equation as 1.8 mg·g−1, 0.23 mg·g−1 and 0.17 mg·g−1, respectively, which were far outweighed to the limitation level of OTA (5 μg·kg−1) in China.
Coexisting compounds studies
Aflatoxin B1, fumonisin B1 and zearalenone are common several mycotoxins presented in food samples. Thus, to evaluate the selectivity of the method, the interference of the three mycotoxins to the recoganition of Fe3O4@PDA MIPs was investigated. The presence of following amounts of foreign substances compared with the concentration of 1.0 ng·mL−1 OTA resulted in less than ±5%: 50 fold (50 ng·mL−1) aflatoxin B1 and fumonisin B1, and 20 fold (20 ng·mL−1) zearalenone. Additionally, three batches of each coexisting compound were performed under the same batch of magnetic MIPs. Fig. S6 show that the recoveries of OTs under each coexisting compound were more than 80% with RSDs which below 3.8%. Since the measured amounts of coexisting substances in real sample were lower than the tolerable concentrations, the extraction capacity of Fe3O4@PDA MIPs to the target was proved.
Analytical performance
Under the optimized conditions, the calibration curves were obtained in the range of 0.01–1.0 ng·mL−1 OTA, 0.02–2.0 ng·mL−1 OTB and 0.002–0.2 ng·mL−1 OTC for a sample volume of 50.0 mL with the calibration curves eq. Y = 37,812.9X + 5532.0, Y = 12,856.0X + 105.3 and Y = 56,021.5X + 91.2 (Y, the peak area and X, ng·mL−1 of the target), the correlation coefficient (R) were 0.9995, 0.9995 and 0.9996, respectively. The limits of detection (LODs) with three times of the S/N ratio were 1.8 pg·mL−1 for OTA, 18 pg·mL−1 for OTB and 3.2 pg·mL−1 for OTC. To verify the reproducibility of the method, three different batches magnetic MIPs were prepared according to the synthesized protocol strictly. The recoveries were measured under the optimized conditions. The results show that the recovery deviations of the three materials were less 5.0%. This illustrated an excellent reproducibility of the magnetic MIPs for extraction of OTs.
Analysis of real samples
In order to evaluate the accuracy and practicability of the method, three different levels of OTs were added in real samples of rice and wine with five parallels tests for the recoveries. Table S2 presents the recoveries, the OTA, OTB and OTC in the rice and wine were 70.0–90.0%, in addition, the RSDs were 2.3–3.8% for real samples. However, for coffee sample, the recoveries of OTs (no more than 40%) were low due to matrix effects. This illustrated that the method exhibited a favourable accuracy and excellent precision for the determinations of OTs in rice and wine samples.
Many pre-treatment and analytical methods have been used to enrich and detect OTs in food samples. Compared with these reported methods, the magnetic MIPs as a sample pre-treatment technology owned many cavities to recognize OTs effectively. Simultaneously, the magnetic MIPs were reused for many times (more than 7 cycles). Although the recoveries were lower than other methods, the magnetic MIPs can recognize and enrich three OTs with the LODs of 1.8–18 pg·mL−1. The comparison results were given in Table 1. This indicated that Fe3O4@PDA MIPs was an ideal extraction technique in the pre-treatment of OTs in rice and wine.
Conclusions
A pre-concentration technology based on Fe3O4@PDA MIPs for the extraction of OTs was established for food samples. The magnetic MIPs possessed both of magnetic nanoparticles and MIPs properties. In comparison with the traditional pre-concentration ways, this strategy possessed small amounts of Fe3O4@PDA MIPs, high separation rate and good adsorption capacity for the targets. In addition, Fe3O4@PDA MIPs were easy to regenerate at least 7 cycles without the obvious decrease of recovery after washing procedures. Hence, the Fe3O4@PDA MIPs were applied with HPLC as a sensor to separate and detect the OTs in rice and wine samples. Future work will be done to purify the matrix from coffee sample and further improve the specificity for the magnetic MIPs.
References
Kumar R, Ansari KM, Saxena N, Dwivedi PD, Jain SK, Das M (2012) Detection of ochratoxin A in wheat samples in different regions of India. Food Control 26:63–67. https://doi.org/10.1016/j.foodcont.2012.01.004
Zhang YQ, Wang LT, Shen X, Wei XQ, Huang XN, Liu YJ et al (2017) Broad-specificity immunoassay for simultaneous detection of ochratoxins A, B, and C in millet and maize. J Agric Food Chem 65:4830–4838. https://doi.org/10.1021/acs.jafc.7b00770
Wu XM, Hu J, Zhu BH, Lu L, Huang XD, Pang DW (2011) Aptamer-targeted magnetic nanospheres as a solid-phase extraction sorbent for determination of ochratoxin A in food samples. J Chromatogr A 1218:7341–7346. https://doi.org/10.1016/j.chroma.2011.08.045
Xie L, Sheng P, Kong W, Zhao X, Ou-Yang Z, Yang M (2015) Solid-phase extraction using molecularly imprinted polymer for determination of ochratoxin A in human urine. World Mycotoxin J 8:37–44. https://doi.org/10.3920/WMJ2013.1633
Mashhadizadeh MH, Amoli-Diva M, Pourghazi K (2013) Magnetic nanoparticles solid phase extraction for determination of ochratoxin a in cereals using high-performance liquid chromatography with fluorescence detection. J Chromatogr A 1320:17–26. https://doi.org/10.1016/j.chroma.2013.10.062
National Standard of People’s Republic of China (GB 2761-2017), Mycotoxins limited in food, National Health and Family Planning Commission of the People’s Repubic of China, Beijing, 2017. http://mall.foodmate.net/goods-85839.html
Remiro R, González-Peñas E, Lizarraga E, de Cerain AL (2012) Quantification of ochratoxin A and five analogs in Navarra red wines. Food Control 27:139–145. https://doi.org/10.1016/j.foodcont.2012.03.006
Remiro R, Ibáñez-Vea M, González-Peñas E, Lizarraga E (2010) Validation of a liquid chromatography method for the simultaneous quantification of ochratoxin A and its analogues in red wines. J Chromatogr A 1217:8249–8256. https://doi.org/10.1016/j.chroma.2010.11.004
Savastano ML, Losito I, Pati S (2016) Rapid and automatable determination of ochratoxin A in wine based on microextraction by packed sorbent followed by HPLC-FLD. Food Control 68:391–398. https://doi.org/10.1016/j.foodcont.2016.04.016
Rodríguez-Cabo T, Rodríguez I, Ramil M, Cela R (2016) Liquid chromatography quadrupole time-of-flight mass spectrometry selective determination of ochratoxin A in wine. Food Chem 199:401–408. https://doi.org/10.1016/j.foodchem.2015.12.036
Cao JL, Kong WJ, Zhou SJ, Yin LH, Wan L, Yang MH (2013) Molecularly imprinted polymer-based solid phase clean-up for analysis of ochratoxin A in beer, red wine, and grape juice. J Sep Sci 36:1291–1297. https://doi.org/10.1002/jssc.201201055
Maier NM, Buttinger G, Welhartizki S, Gavioli E, Lindner W (2004) Molecularly imprinted polymer-assisted sample clean-up of ochratoxin A from red wine: merits and limitations. J Chromatogr B 804:103–111. https://doi.org/10.1016/j.jchromb.2004.01.014
Giovannoli C, Passini C, Nardo FD, Anfosso L, Baggiani C (2014) Determination of ochratoxin A in Italian red wines by molecularly imprinted solid phase extraction and HPLC analysis. J Agric Food Chem 62:5220–5225. https://doi.org/10.1021/jf5010995
Niu MH, Pham-Huy C, He H (2016) Core-shell nanoparticles coated with molecularly imprinted polymers: a review. Microchim Acta 183:2677–2695. https://doi.org/10.1007/s00604-016-1930-4
Li HF, Xie T, Ye LL, Wang YW, Xie CG (2017) Core-shell magnetic molecularly imprinted polymer nanoparticles for the extraction of triazophos residues from vegetables. Microchim Acta 184:1011–1019. https://doi.org/10.1007/s00604-017-2096-4
Zhang WL, Li Y, Wang Q, Wang C, Wang PF, Mao K (2013) Performance evaluation and application of surface-molecular-imprinted polymer-modified TiO2 nanotubes for the removal of estrogenic chemicals from secondary effluents. Environ Sci Pollut R 20:1431–1440. https://doi.org/10.1007/s11356-012-0983-0
Shi XX, Xu L, Duan HQ, Huang YP, Liu ZS (2011) CEC separation of ofloxacin enantiomers using imprinted microparticles prepared in molecular crowding conditions. Electrophoresis 32:1348–1356. https://doi.org/10.1002/elps.201000515
Turan E, Sahin F (2016) Molecularly imprinted biocompatible magnetic nanoparticles for specific recognition of Ochratoxin A. Sensors Actuators B 227:668–676. https://doi.org/10.1016/j.snb.2015.12.087
Zare F, Ghaedi M, Daneshfar A, Ostovan A (2015) Magnetic molecularly imprinted polymer for the efficient and selective preconcentration of diazinon before its determination by high-performance liquid chromatography. J Sep Sci 38:2797–2803. https://doi.org/10.1002/jssc.201500383
Huang ZZ, Lee HK (2015) Study and comparison of polydopamine and its derived carbondecorated nanoparticles in the magnetic solid-phaseextraction of estrogens. J Chromatogr A 1414:41–50. https://doi.org/10.1016/j.chroma.2015.08.039
Yang B, Lv SF, Chen F, Liu C, Cai CQ, Chen CY et al (2016) A resonance light scattering sensor based on bioinspired molecularlyimprinted polymers for selective detection of papain at trace levels. Anal Chim Acta 912:125–132. https://doi.org/10.1016/j.aca.2016.01.030
Hu MH, Huang PC, Suo LL, Wu FY (2017) Cetylpyridinium chloride functionalized silica-coated magnetite microspheres for the solid-phase extraction and pre-concentration of ochratoxin A from environmental water samples with high-performance liquid chromatographic analysis. J Sep Sci 40:2431–2437. https://doi.org/10.1002/jssc.201601464
Yao GH, Liang RP, Huang CF, Wang Y, Qiu JD (2013) Surface plasmon resonance sensor based on magnetic molecularly imprinted polymers amplification for pesticide recognition. Anal Chem 85:11944–11951. https://doi.org/10.1021/ac402848x
National Standard of People’s Republic of China (GB 5009.96-2016), Determination of ochratoxin A in food, National Health and Family Planning Commission of the People’s Repubic of China, Beijing, 2016. http://mall.foodmate.net/goods-88895.html
Chen J, Liang RP, Wang XN, Qiu JD (2015) A norepinephrine coated magnetic molecularly imprinted polymer for simultaneous multiple chiral recognition. J Chromatogr A 1409:268–276. https://doi.org/10.1016/j.chroma.2015.07.052
Yu P, Sun QL, Li JF, Tan ZJ, Yan YS, Li CX (2015) Magnetic imprinted nanomicrosphere attached to the surface of bacillus using miniemulsion polymerization for selective recognition of 2,4,6-trichlorophenol from aqueous solutions. J Ind Eng Chem 29:349–358. https://doi.org/10.1016/j.jiec.2015.04.014
Dai SL, Wu SJ, Duan N, Wang ZP (2016) A luminescence resonance energy transfer based aptasensor for the mycotoxin Ochratoxin A using upconversion nanoparticles and gold nanorods. Microchim Acta 183:1909–1916. https://doi.org/10.1007/s00604-016-1820-9
Qing Y, Li X, Chen S, Zhou XP, Luo M, Xu X, Li CR, Qiu JF (2017) Differential pulse voltammetric ochratoxin A assay based on the use of an aptamer and hybridization chain reaction. Microchim Acta 184:863–870. https://doi.org/10.1007/s00604-017-2080-z
Zhang CC, Tang J, Huang LL, Li YP, Tang DP (2017) In-situ amplified voltammetric immunoassay for ochratoxin A by coupling a platinum nanocatalyst based enhancement to a redox cycling process promoted by an enzyme mimic. Microchim Acta 184:2445–2453. https://doi.org/10.1007/s00604-017-2223-2
Wu H, Liu RJ, Kang XJ, Liang CY, Lv L, Guo ZJ (2018) Fluorometric aptamer assay for ochratoxin A based on the use of single walled carbon nanohorns and exonuclease III-aided amplification. Microchim Acta 185:27. https://doi.org/10.1007/s00604-017-52592-6
Jia W, Chu XG, Ling Y, Huang JR, Chang J (2014) Multi-mycotoxin analysis in dairy products by liquid chromatographycoupled to quadrupole orbitrap mass spectrometry. J Chromatogr A 1345:107–114. https://doi.org/10.1016/j.chroma.2014.04.021
Flores-Flores ME, González-Peñas E (2017) An LC–MS/MS method for multi-mycotoxin quantification in cow milk. Food Chem 218:378–385. https://doi.org/10.1016/j.foodchem.2016.09.101
Limay-Rios V, Miller JD, Schaafsma AW (2017) Occurrence of Penicillium verrucosum, ochratoxin A, ochratoxin B and citrinin in onfarm stored winter wheat from the Canadian Great Lakes region. PLoS One 12:e0181239. https://doi.org/10.1371/journal.pone.0181239
Acknowledgements
We greatly acknowledge the National Natural Science Foundation of China (No. 21765014 and 21505067) for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 763 kb)
Rights and permissions
About this article
Cite this article
Hu, M., Huang, P., Suo, L. et al. Polydopamine-based molecularly imprinting polymers on magnetic nanoparticles for recognition and enrichment of ochratoxins prior to their determination by HPLC. Microchim Acta 185, 300 (2018). https://doi.org/10.1007/s00604-018-2826-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2826-2