Abstract
The authors describe the synthesis, characterization and electrochemical sensing performance of a PrFeO3-MoS2 nanocomposite. Graphene-like MoS2 sheets and a perovskite-type PrFeO3 were synthesized via a hydrothermal and a sol-gel method, respectively. Finally, PrFeO3-MoS2 nansheets were synthesized by using sodium molybdate as a source for molybdenum and thiourea as the source for sulfur. The nansheets were characterized by transmission electron microscopy and X-ray diffraction. The electrochemical behavior of the nanosheets deposited on a glassy carbon electrode was studied via electrochemical impedance spectroscopy and cyclic voltammetry. The modified electrodes display strong response to nitrite. At a scan rate of 100 mV·s−1, the current at the oxidation peak at 0.85 V (vs. SCE) increases linearly in the 0.005 to 3 mM nitrite concentration range. The detection limit is 1.67 μmol·L−1 (S/N = 3). The sensor is selective, stable and reproducible. It was successfully applied to the determination of nitrite in (spiked) real samples, and appropriate recoveries were obtained.

PrFeO3-MoS2 nanocomposites were employed to fabricate an electrochemical sensor for nitrite. The sensor exhibits excellent electrochemical catalysis towards the oxidation of nitrite with good stability and reproducibility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
MoS2 is a typical layered transition metal sulfide with the graphene-like structure. The layered structure is formed by the accumulation of three atomic layers (S-Mo-S) via van Edward force [1]. This endows MoS2 with excellent electrical properties and large surface area. Nowadays, MoS2 is widely used in catalysis, energy storage, electronics, optoelectronics, lubricant, etc. [2]. For example, the composite of de-layered MoS2 and N-doped graphene exhibits high electrochemical catalytic performance for hydrogen evolution reaction (HER) [3]. Besides these, MoS2 is proved of owning the excellent biocompatibility which makes it attractive in the field of biosensor [4]. It is employed as the ideal nanomaterial to construct the biosensor for detection of glucose [5], H2O2 [6, 7], and micro-RAN [8], etc. For the purpose of quantifying H2O2 in living cells, MoS2-based PtW/MoS2 hybrid nanocomposite was synthesized and used to construct an enzyme-free H2O2 sensor with high sensitivity, low detection limit and good specific response. This nanocomposite was featured with good selectivity and highly specific response for the detection of H2O2 released from breast cancer 4 T1 cell [9].
Perovskite type composite oxide (ABO3) is a new type of material with unique physical and chemical properties [10]. This kind of material has the characteristics of stable crystal structure, unique electromagnetic property, excellent isomerization and electrocatalytic property [11]. PrFeO3 is such a classical perovskite type of rare earth-based ABO3 compound. It can be synthesized via the hydrothermal method [12], self-combustion method [13] and sol-gel reaction [14], etc. It is widely used in the fields of photocatalysis [15], magnetic study [16], and electrical conduction study [17]. However, till now, there are few papers issued about the utilization of PrFeO3 as the electrode modified nanomaterial in the field of electrochemical sensor.
As an important precursor of carcinogen, nitrite is widely existed in our daily foods [18,19,20], such as sausage, bacon and other pickled foods. Nitrite will be oxidized in the body so as to lose the ability of transporting oxygen [21, 22]. Therefore, it is very important to effectively detect the content of nitrite. At present, the methods for detection of nitrite include chromatography [23, 24], fluorescence [25], chemiluminescence [26], spectrophotometry [27] etc. For example, an ion-exchange chromatographic method with postcolumn derivatisation was developed by Indyk’s group for the determination of nitrite and nitrate in dairy products [24]. Attribute to the dissolution, centrifugation and other techniques, it realized the the low analyte levels generally encountered in infant formulas, or in protein hydrolysates. In acidic media, nitrite can react with aminopyrene to form nonfluorescent nitroso derivative. Based on the fluorescence quenching caused by this specific reaction, fluorescent sensing of nitrite was achieved by Xiao et al. [25] with good sensitivity and selectivity. However, these methods are often time-consuming. They also require expensive instruments and highly trained technicians.
Electroanalytical methods are particularly effective in sensing nitrite because of their fast response, good sensitivity and low detection limits. The key step remains on seeking appropriate nanomaterial as the substrate. Herein, combining the excellent electrochemical catalysis of MoS2 and the unique electronic property of PrFeO3, PrFeO3-MoS2 nanocomposite was prepared via hydrothermal method and employed to construct the electrochemical sensor for the detection of nitrite.
Experimental
Reagents and apparatus
NaMoO4·2H2O was purchased from Guanghua Sci. Tech. Co., Ltd. (Guangdong, China, http://www.jinhuada.com/en). Pr6O11 was purchased from Shanghai Environmental Protection Tech. Co., Ltd. (Shanghai, China, http://www.tongna.com). Chitosan (low molecular weight) was purchased from Sigma-Aldrich (www.sigma-aldrich.com) and used without further purification. Thiourea and sodium nitrite were purchased from Xilong Chemical Co., Ltd. (Beijing, China, http://xilongchemical.en.made-in-china.com). All other reagents were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China, http://en.reagent.com.cn). Millipore ultrapure water (R ≥ 18.2 MΩ·cm) was used in all experiments.
X-ray diffraction (XRD) patterns were recorded on an X-ray diffractometer (PANalytical X’Pert Pro) with Cu Kα radiation (λ = 0.15418 nm) for crystal phase identification. The XRD was operated at 40 KV accelerating voltage and 40 mA tube current, with the degree range of 5° ~ 80°. Transmission electron microscopy (TEM) was recorded on a Tecnai G-20 Transmission Electron Microscope. Electrochemical impedance spectroscopy (EIS) analyses were performed on an Autolab PGSTAT12 (Ecochemie, BV, The Netherlands) with the frequency range of 10−2 ~ 104 Hz. CHI660D Electrochemical Workstation (Shanghai CH Instruments Co., China) was used for the cyclic voltammograms (CVs). All the electrochemical measurements were operated with the conventional three-electrode system, which consisted of a modified glassy carbon electrode (GCE) as working electrode, a platinum wire as auxiliary electrode and a saturated calomel electrode (SCE) as reference electrode.
Preparation of MoS2
MoS2 nanomaterials were prepared according to the reported method with some modifications [28]. In brief, 61 mg of NaMoO4·2H2O and 121.2 mg of cysteine were added to 10 mL of N-methyl pyrrolidone. After being stirred for 30 min, the homogeneous solution was transferred into a Teflon-lined autoclave (30 mL) and held at 220 °C in an oven for 16 h. After cooling to room temperature naturally, the black product was collected by centrifugation at 7000 rpm (5369 g) for 6 min and washed three times with an ethanol/water mixture.
Preparation of PrFeO3
Typical preparation of PrFeO3 was described as follows: Firstly, 0.1 mmol Pr6O11 was dissolved in 10 mL nitric acid (1 mol⋅L−1), followed by mixing with 10 mL Fe(NO3)3·9H2O solution (0.01 mmol⋅mL−1) in 20 °C bath conditions. After stirring for 10 min, 10 mL of the freshly-prepared citric acid solution (0.2 mol⋅L−1) was added into the mixed solution. The mixture was maintained stirring for 1 h until it was changed into the sol colloid. The temperature was then heated and maintained at 80 °C for 30 min until the sol colloid changed into gel colloid. Afterwards, the gel colloid was further dried at 90 °C. Finally, the product was put in a muffle furnace and calcined at 900 °C for 4 h. After cooling to room temperature and grinding, the final powder PrFeO3 products were obtained for further use.
Synthesis of the PrFeO3-MoS2 nanocomposite
1 mmol NaMoO4·2H2O and 5 mmol thiourea were firstly dissolved in 60 mL water. Then 0.05 g of the pre-synthesized PrFeO3 was added into the solution. After stirred homogeneously, the solution was transferred into the 100 mL Teflon-lined autoclave, and heating at 200 °C for 16 h. After cooled naturally, the product was centrifuged at 7000 rpm (5369 g) for 6 min and washed for 3 times with water and ethanol, respectively. The final product was dried at 80 °C for 12 h.
Fabrication of the electrochemical sensor
The pre-synthesized PrFeO3-MoS2 nanomaterial was dispersed in water/ethanol (V H2O : V ethanol = 4 : 1), followed by ultrasonicating 30 min to form a stable suspension with the concentration of 4 mg·mL−1. The glassy carbon electrode (GCE, 4 mm in diameter) was polished with 1.0, 0.3 and 0.05 μm alumina powder on polishing cloth, and dried by flowing N2 before it was used. For fabrication of the electrochemical sensor, 10 μL of the above suspension was dropped onto the surface of the pre-treated GCE and dried at room temperature. Finally, 3 μL of 5 mg·mL−1 chitosan solution was dripped onto the PrFeO3-MoS2/GCE for sealing. The MoS2/GCE, PrFeO3/GCE were fabricated through a similar procedure for comparison.
Results and discussion
Choice of materials
Nowadays, the rare earth based-nanomaterials have gained considerable attention and are widely used in many fields like luminescent detection [29], catalyst [30] and solid oxide fuel cell [31], etc. Several works proved that rare earth nanomaterials owned the excellent catalysis efficiency in the field of electrochemical sensor [32, 33]. As a classical perovskite type of rare earth-based ABO3 compound, PrFeO3 already exhibited its excellent performances in the fields of photocatalysis, magnetic study, and electrical conduction study. In order to extend the great potential utilization of rare earth-based ABO3 compound, also to search the suit materials for the fabrication of electrochemical sensor, PrFeO3 was synthesized and utilized to form the PrFeO3-MoS2 nanocomposite for electrochemical sensor.
Characterizations of the nanomaterials
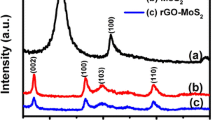
The MoS2 was synthesized via hydrothermal method with NaMoO4 as the Mo resource. The morphology was confirmed by a TEM image which is shown in Fig. 1a. It can be seen from Fig. 1a that MoS2 is layered structure. Fig. 1b is the morphology of PrFeO3-MoS2. The nanocomposite is composed of PrFeO3 nanoparticles and the layered MoS2 nanosheets. The PrFeO3 nanoparticles are inserted in the MoS2 sheets. Fig. 1c is the XRD patterns of the MoS2, PrFeO3 and their reference patterns. As shown in Fig. 1c, MoS2 displays three characteristic broad diffraction peaks at about 15.6°, 33.1° and 56.4° (JCPDS No. 37–1492) [8], which illustrates the successful synthesis of MoS2 nanomaterials. Four well-defined peaks at about 28.5°, 33°, 47° and 56° appear on the XRD curve of PrFeO3 in Fig. 1c. These peaks are well indexed to PrFeO3 diffraction peaks (JCPDS file No. 78–2424) [10]. Compare with the single component, the XRD curve of PrFeO3-MoS2 nanocomposite (Fig. 1d) displays all the characteristic peaks of PrFeO3 and MoS2.
Electrochemical behaviors of the modified electrodes
EIS is a powerful technique to inspect the surface feature of the electrode [34]. The traditional impedance spectrum contains a semicircle portion at higher frequencies and a linear portion at lower frequencies. The diameter of the semicircle equals the electron-transfer resistance (Ret). In this system, electrodes with different modification stages were characterized by EIS. Shown in Fig. 2a are the EIS spectra of electrodes with different modifications. The MoS2 modified electrode (curve a in Fig. 2a) shows relative small electron-transfer resistance (Ret) as compared to the PrFeO3 modified electrode (curve b in Fig. 2a). This can be attributed to the better electronic conductivity of the layered MoS2 than PrFeO3. Correspondingly, the dual nanocomposites PrFeO3-MoS2 modified electrode exhibits a moderate Ret (curve c in Fig. 2a), which is smaller than the PrFeO3 and the larger than the MoS2 modified electrode.
In the field of electrochemistry, the cyclic voltammogram (CV) of ferricyanide is considered to be a valuable tool for investigating the electrochemical behavior of the modified electrode. Therefore, for the purpose of further understanding the electrochemical behavior of the modified electrode, the CVs of the PrFeO3-MoS2/GCE at various scan rates in 0.1 mol⋅L−1 KCl + 2 mmol⋅L−1 [Fe(CN)6]3−/4- were carried out, which were shown in the Fig. 2b. As the scan rate (v) increased from 10 to 500 mV⋅s−1, the redox peak currents increased with the slightly enlargement of potential peak-to-peak separation.
As can be seen from Fig. 2c, when v ≤ 100 mV·s−1, the redox peak currents increase linearly with v 1/2, the corresponding linear equation for the anodic peak current is I pa (μA) = 6.478v 1/2(mV⋅s−1) + 3.425 with the square of correlation coefficient R2 = 0.999. The corresponding linear equation for the cathodic peak current is I pc (μA) = −8.67v 1/2(mV⋅s−1) - 1.547 with the square of correlation coefficient R2 = 0.999. This result illustrates that the redox reaction is a diffusion-controlled electrochemical process under lower scan rates. However, when v > 100 mV·s−1, I pa increases linearly directly with the increase of v (Fig. 2d). The anodic peak current of I pa (μA) = 0.167v (mV⋅s−1) + 59.93 with the square of correlation coefficient R2 = 0.9943. The cathodic peak current of I pc (μA) = −0.196v - 8.17 (mV⋅s−1) with the square of correlation coefficient R2 = 0.9934. The redox peak currents increase linearly with the increase of the scan rate. This confirms that under higher scan rate, this redox reaction is a surface-controlled electrochemical process [35].
Electrochemical detection of nitrite
By utilization of the excellent electrochemical property, the synthesized dual nanocomposite was employed to construct the electrochemical sensor for detection of nitrite. In order to confirm the electrochemical catalysis effect of the nanocomposite, the electrochemical responses of nitrite on different electrodes with different modified material were examined. Figure 3a was the CV of different modified electrodes in acetate buffer (0.1 mol·L−1 pH 4.5) containing 1.0 mmol⋅L−1 NaNO2 at the scan rate of 100 mV·s−1. The results show that the oxidation potential of nitrite at a bare GCE (curve a in Fig. 3a) is as high as 0.98 V. This is relative high for nitrite detection. As MoS2 was modified onto the electrode (curve b in Fig. 3a), the oxidation peak potential was obviously reduced to 0.87 V compared to that of the bare GCE, with the oxidation peak current enhanced from 1.95 μA to 2.98 μA. And the oxidation peak current further increased to 4.70 μA on the PrFeO3/GCE (curve c in Fig. 3a). Nevertheless, this value dramatically enlarged to 7.05 μA on PrFeO3-MoS2/GCE (curve d in Fig. 3a), and the oxidation peak potential was obviously reduced to 0.85 V. These results proved that the PrFeO3-MoS2 nanocomposite owned the excellent electrochemical catalysis toward the oxidation of nitrite. As a typical layered transition metal sulfide, MoS2 owns excellent electrical property and large surface area. It can be served as the matrix with good electrical conductivity. This MoS2 matrix can provide adsorption sites for the attachment of PrFeO3. It also can be treated as the electron mediator to accelerate electrons transfer between the target analyte and the electrode surface. The nanoparticle-structured PrFeO3, acting like nanoscale electrodes, provided the necessary pathways between the analyte and the electrode. Herein, the synergistic effect between PrFeO3 and MoS2 endows the prepared electrochemical sensor with excellent electrocatalytic activity toward the oxidation of NO2 −.
a CVs of bare GCE (a), MoS2/GCE (b), PrFeO3/GCE (c) and PrFeO3-MoS2/ GCE (d) in acetate buffer (pH 4.5, 0.1 mol⋅L−1) containing 1.0 mmol⋅L−1 NaNO2 (b) CVs of PrFeO3-MoS2/GCE in acetate buffer (0.1 mol⋅L−1) containing 1.0 mmol⋅L−1 NaNO2 at different pH value of 4.5 (a), 6.0 (b), 7.0 (c), 7.5 (d), 8.0 (e), the inset is the peak current vs. pH value
To further investigate the electrocatalytic performance of this sensor, the effect of pH value on the electrochemical response was studied by CV. With pH value increased from 4.5 to 8.0 (Fig. 3b), the peak current decreased accordingly. Insert in Fig. 3b was the calibration of the peak current vs. pH value. As the pKa of HNO2 is 3.3, most nitrite ions are protonated in the acidic solutions [36]. As the value of pH becomes higher, the electrocatalytic oxidation of nitrite might become more difficult due to the shortage of protons [37].
Under the optimal condition, the electrochemical response of PrFeO3-MoS2/GCE to different concentrations of nitrite was measured. Shown in Fig. 4a is the oxidation peak current intensity against the concentrations of nitrite from 0.005 to 3 mmol·L−1. With more nitrite added to the acetate buffer, the oxidation current was increased, in other words, the higher nitrite concentration caused the increase of the oxidation current. Fig. 4b is the CV response calibration curve of the modified electrode vs. the concentration of nitrite. The calibration curve corresponding to the peak current is linear with the concentration of nitrite ranging from 5 ~ 3000 μmol·L−1. The linear equation is I p (μA) = 52.12C NaNO2 (mmol⋅L−1) + 3.05 with the square of the correlation coefficient R2 = 0.9961. According to the linear equation of I p vs. v 1/2 in Fig. 2c and the Randles–Sevcik equation:
It can be calculated that the electrochemical effective electrode area is 15.94 mm2, which is larger than its physical area. The sensitivity of this electrochemical sensor is 0.327 μA·μM−1·cm−2.
For the purpose of evaluating the performance of this electrochemical sensor, the critical comparison of this sensor to other reported electrochemical sensors for detection of nitrite was listed in Table 1. As shown in Table 1, these results are comparable or better than nitrite detection with other electrochemical sensors. This indicated that the PrFeO3-MoS2 based-composite might be a hopeful material for nitrite detection.
Stability and selectivity
To investigate the selectivity of the electrochemical sensor, a certain concentration of NaNO2, Na2SO4, KCl, NaNO3, K2CO3, NaF and Na2HPO4 were added in the buffer respectively and the corresponding i-t electrochemical responses were measured. Fig. 5 is the amperometric response of PrFeO3-MoS2/GCE upon the successive injection of 5.0 μL 1 mmol⋅L−1 different ions in stirred acetate buffer (0.1 mol⋅L−1, pH 4.5). It can be drawn from the result that the amperometric current increased immediately after the addition of NaNO2, but the current remained almost unchanged after the addition of the interfering substance, which included Na2SO4, KCl, NaNO3, K2CO3, NaF and Na2HPO4. The current was amplified again after the second addition of NaNO2. The result strongly explained that the existence of interfering substances didn’t affect the detection of nitrite, proving the good selectivity of PrFeO3-MoS2/GCE towards the detection of nitrite.
The stability of the prepared electrochemical sensor was test by placing the modified electrode in the refrigerator for a week at 4 °C. The current response retained 95.1% of its initial value in acetate buffer (pH 4.5) containing 1 mmol⋅L−1 nitrite, indicating the good stability of the sensor. In order to further learn the reproducibility of the sensor, four branches of the same PrFeO3-MoS2 modified electrodes were prepared and the CV were measured in the same experimental conditions, the relative standard deviation (RSD) was 4.6%, proved that the electrochemical sensor owned good reproducibility.
Analytical application
To validate the applicability of the fabricated electrochemical sensor for real sample analysis, the PrFeO3-MoS2/GCE was applied for determination of nitrite in tap water, local river water and wastewater using a standard addition method. It can be seen from Tables 2, 3 and 4 that the fabricated electrochemical sensor owned appropriate recovery of nitrite analysis in water samples. The result clearly indicated the potential application of the fabricated sensor for the detection of nitrite in water samples.
Conclusion
Nanocomposite of PrFeO3-MoS2 was prepared by hydrothermal method and characterized. By utilization of the excellent electrochemical property, the synthesized dual nanocomposite was employed to construct the electrochemical sensor for detection of nitrite. The experimental results showed that the PrFeO3-MoS2 modified glassy carbon electrode owned excellent electrocatalytic activity for the NO2 − oxidation. In addition, the prepared electrochemical sensor exhibited good selectivity, stability and reproducibility. This experiment provided a reliable method for the development of NO2 − electrochemical sensor, which is expected to be applied to the test of some real samples. Furthermore, it can be expanded to other rare earth-based nanomaterials, so as to enlarge the electrochemical research of rare earth elements.
References
Zhang Y, Chen P, Wen F, Yuan B, Wang H (2016) Fe3O4 nanospheres on MoS2 nanoflake: Electrocatalysis and detection of Cr (VI) and nitrite. J Electroanal Chem 761:14–20. doi:10.1016/jelechem.2015.12.004
Liu YM, Shi GF, Zhang JJ, Zhou M, Cao JT, Huang KJ, Ren SW (2014) A novel label-free electrochemiluminescence aptasensor based on layered flowerlike molybdenum sulfide–grapheme nanocomposites as matrix. Colloids Surf, B 122:287–293. doi:10.1016/colsurfb.2014.07.011
Ye JB, Yu ZT, Chen WX, Chen QN, Xu SR, Liu R (2016) Facile synthesis of molybdenum disulfide/nitrogen-doped graphene composites for enhanced electrocatalytic hydrogen evolution and electrochemical lithium storage. Carbon 107:711–722. doi:10.1016/carbon.2016.06.074
Gan XR, Zhao HM, Quan X (2017) Two-dimensional MoS2: a promising building block for biosensors. Biosens Bioelectron 89:56–71. doi:10.1016/bios.2016.03.042
Parlak O, İncel A, Uzun L, Turner AP, Tiwari A (2017) Structuring au nanoparticles on two-dimensional MoS2 nanosheets for electrochemical glucose biosensors. Biosens Bioelectron 89:545–550. doi:10.1016/bios.2016.03.024
Li XY, Du XZ (2017) Molybdenum disulfide nanosheets supported au-Pd bimetallicnanoparticles for non-enzymatic electrochemical sensing of hydrogenperoxide and glucose. Sensors Actuators B 239:536–543. doi:10.1016/snb.2016.08.048
Zheng W, Li GJ, Liu LH, Chen W, Weng WJ, Sun W (2016) Electrochemical behaviors of horseradish peroxidase on MoS2 nanosheets modified electrode. Int J Electrochem Sci 11:7584–7593. doi:10.20964/2016.09.15
Shuai HL, Huang KJ, Chen YX, Fang LX, Jia MP (2016) Au nanoparticles/hollow molybdenum disulfide microcubes based biosensor for microRNA-21 detection coupled with duplex-specific nuclease and enzyme signal amplification. Biosens Bioelectron 89:989–997. doi:10.1016/bios.2016.10.051
Zhu LL, Zhang Y, Xu PC, Wen WJ, Li XX, Xu JQ (2016) PtW/MoS2 hybrid nanocomposite for electrochemical sensing of H2O2 released from living cells. Biosens Bioelectron 80:601–606. doi:10.1016/bios.2016.02.019
Xu H, Hu XL, Zhang LZ (2008) Generalized low-temperature synthesis of nanocrystalline rare-earth orthoferrites LnFeO3 ( Ln = La, Pr, Nd, Sm, Eu, Gd ). Cryst Growth Des 8:2061–2065. doi:10.1021/cg800014b
Zhang L, Hu JF, Song P, Qin HW, Liu XD, Jiang MH (2005) Formaldehyde-sensing characteristics of perovskite La0.68Pb0.32FeO3 nano-material. Physica B 370:259–263. doi:10.1016/physb.2005.09.020
Yuan L, Huang KK, Wang S, Hou CM, Wu XF, Zou B, Feng SH (2016) Crystal shape tailoring in perovskite structure rare-earth ferrites REFeO3 (RE = La, Pr, Sm, Dy, Er, and Y) and shape-dependent magnetic properties of YFeO3. Cryst Growth Des 16:6522–6530. doi:10.1021/acs.cgd.6b01219
Morales LA, Sierra-Gallego G, Barrero CA, Arnache O (2016) Relative recoilless F-factors in REFeO3 (RE = rare-earth La, Pr, Nd and Sm) orthoferrites synthesized by self-combustion method. Mater Sci Eng B 211:94–100. doi:10.1016/mseb.2016.06.005
Saha S, Chanda S, Dutta A, Sinha TP (2016) Dielectric relaxation of PrFeO3 nanoparticles. Solid State Sci 58:55–63. doi:10.1016/solidstatesciences.2016.05.013
Thirumalairajan S, Girija K, Ganesh I, Mangalaraj D, Viswanathan C, Balamurugan A, Ponpandian N (2012) Controlled synthesis of perovskite LaFeO3 microsphere composed of nanoparticles via self-assembly process and their associated photocatalytic activity. Chem Eng J 209:420–428. doi:10.1016/cej.2012.08.012
Mihalik M, Jaglicic Z, Fitta M, Kavecansky V, Csach K, Budziak A, Briancin J, Zentkova M, Mihalik M (2016) Structural and magnetic study of PrMn1-xFexO3 compounds. J Alloys Compd 687:652–661. doi:10.1016/jallcom.2016.06.177
Niwa E, Sato T, Watanabe Y, Toyota Y, Hatakeyama Y, Judai K, Shozugawa K, Matsuo M, Hashimoto T (2015) Dependence of crystal symmetry, electrical conduction property and electronic structure of LnFeO3 (Ln: La, Pr, Nd, Sm) on kinds of Ln3+. J Ceram Soc Jpn 123:501–506. doi:10.2109/jcersj2.123.501
Mirvish SS (1995) Role of N-nitrosocompounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Lett 93:17–48. doi:10.1016/0304-3835(95)03786-V
Afkhami A, Soltani-Felehgari F, Madrakian T, Ghaedi H (2014) Surface decoration of multi-walled carbon nanotubes modified carbon paste electrode with gold nanoparticles for electro-oxidation and sensitive determination of nitrite. Biosens Bioelectron 51:379–385. doi:10.1016/bios.2013.07.056
Dai ZH, Bai HY, Hong M, Zhu Y, Bao J, Shen J (2008) A novel nitrite biosensor based on the direct electron transfer of hemoglobin immobilized on CdS hollow nanospheres. Biosens Bioelectron 23:1869–1873. doi:10.1016/bios.2008.03.002
Wang HW, Wang CQ, Yang BB, Zhai CY, Bin D, Zhang K, Yang P, Du YK (2015) A facile fabrication of copper particle-decorated novel graphene flower composites for enhanced detecting of nitrite. Analyst 140:1291–1297. doi:10.1039/C4AN01924E
Huang S-S, Liu L, Mei L-P, Zhou J-Y, Guo F-Y, Wang A-J, Feng J-J (2016) Electrochemical sensor for nitrite using a glassy carbon electrode modified with gold-copper nanochain networks. MicrochimActa 183:791–797. doi:10.1007/s00604-015-1717-z
Khan MR, Wabaidur SM, Alothman ZA, Busquets R, Naushad M (2016) Method for the fast determination of bromate, nitrate and nitrite by ultra performance liquid chromatography–mass spectrometry and their monitoring in Saudi Arabian drinking water with chemometric data treatment. Talanta 152:513–520. doi:10.1016/talanta.2016.02.036
Gapper LW, Fong BY, Otter DE, Indyk HE, Woollard DC (2004) Determination of nitrite and nitrate in dairy products by ion exchange LC with spectrophotometric detection. Int Dairy J 14:881–887. doi:10.1016/idairyj.2004.02.015
Xiao WX, Xiao D, Xia JH, Chen ZC (2011) Fluorescent sensing of nitrite at nanomolar level using functionalized mesoporous silica. Microchim Acta 173:73–78. doi:10.1007/s00604-010-0524-9
Wu J, Wang X, Lin Y, Zheng Y, Lin JM (2016) Peroxynitrous-acid-induced chemiluminescence detection of nitrite based on microfluidic chip. Talanta 154:73–79. doi:10.1016/talanta.2016.03.062
Böhmer A, Pich A, Schmidt M, Haghikia A, Tsikas D (2016) Evidence by chromatography and mass spectrometry that inorganic nitrite induces S-glutathionylation of hemoglobin in human red blood cells. J Chromatogr B 1019:72–82. doi:10.1016/jchromb.2016.01.032
Ding JB, Zhou Y, Li YG, Guo SJ, Huang XQ (2016) MoS2 nanosheet assembling superstructure with a three-dimensional ion accessible site: a new class of bifunctional materials for batteries and electrocatalysis. Chem Mater 28:2074–2080. doi:10.1021/acs.chemmater.5b04815
Sheng Y, Liao LD, Bandla A, Liu YH, Thakor N, Tan MC (2016) Size and Shell effects on the Photoacoustic and luminescence properties of dual modal rare-earth-doped nanoparticles for infrared Photoacoustic imaging. ACS Biomater Sci Eng 2:809–817. doi:10.1021/acsbiomaterials.6b00012
Yan Y, Jia X, Yang Y (2015) Palladium nanoparticles supported on CNT functionalized by rare-earth oxides for solvent-free aerobic oxidation of benzyl alcohol. Catal Today 259:292–302. doi:10.1016/j.cattod.2015.07.021
Gao Z, Mogni LV, Miller EC, Railsback JG, Barnett SA (2016) A perspective on low-temperature solid oxide fuel cells. Energy Environ Sci 9:1602–1644. doi:10.1039/c5ee03858h
Huang HP, Xu L, Yue YF, Lv LL (2017) A novel hydrogen peroxide biosensor based on hemoglobin/holmium phosphate nanocomposites. Chin J Anal Chem 45:111–117. doi:10.11895/j.Issn.0253-3820.160678
Huang HP, Yue YF, Li LL, Zhu JJ (2017) Rare earth oxide Dy2O3-au nanocomposite-based electrochemical sensor for sensitive determination of nitrite. J Electrochem Soc 164:H321–H325. doi:10.1149/2.0871706jes
Bard AJ, Faulkner LR (1980) Electrochemical methods: fundamentals and applications. Wiley 275:669–676
Wang JP, Zhao DY, Zhang Y, Li J, Xu C (2014) A highly sensitive sensor for the detection of nitrite based on a nanoporous Fe2O3–CoO composite. Anal Methods 6:3147–3151. doi:10.1039/C4AY00171K
Huang X, Li YX, Chen YL, Wang L (2008) Electrochemical determination of nitrite and iodate by use of gold nanoparticles/poly(3-methylthiophene) composites coated glassy carbon electrode. Sensor Actuat B-Chem 134:780–786. doi:10.1016/snb.2008.06.028
Pham XH, Li CA, Han KN, Huynh-Nguyen BC, Le TH, Ko E, Kim JH, Seong GH (2014) Electrochemical detection of nitrite using urchin-like palladium nanostructures on carbon nanotube thin film electrodes. Sensor Actuat B-Chem 193:815–822. doi:10.1016/snb.2013.12.034
Zhao S, Zhang K, Sun YY, Sun CQ (2006) Hemoglobin/colloidal silver nanoparticles immobilized in titania sol–gel film on glassy carbon electrode: direct electrochemistry and electrocatalysis. Bioelectrochemistry 69:10–15. doi:10.1016/j.bioelechem.2005.09.004
Zhu WL, Zhou Y, Zhang JR (2009) Direct electrochemistry and electrocatalysis of myoglobin based on silica-coated gold nanorods/room temperature ionic liquid/silica sol–gel composite film. Talanta 80:224–230. doi:10.1016/j.talanta.2009.06.056
Yue R, Lu Q, Zhou YK (2011) A novel nitrite biosensor based on single-layer graphene nanoplatelet–protein composite film. Biosens Bioelectron 26:4436–4441. doi:10.1016/j.bios.2011.04.059
Wu WQ, Li YB, Jin JY, Wu HM, Wang SF, Ding Y, Ou JF (2016) Sensing nitrite with a glassy carbon electrode modified with a three-dimensional network consisting of Ni7S6 and multi-walled carbon nanotubes. Microchim Acta 183:3159–3166. doi:10.1007/s00604-016-1961-x
Meng Z, Liu B, Zheng J, Sheng Q, Zhang H (2011) Electrodeposition of cobalt oxide nanoparticles on carbon nanotubes, and their electrocatalytic properties f or nitrite electrooxidation. Microchim Acta 175:251–257. doi:10.1007/s00604-011-0688-y
Chen L, Liu X, Wang C, Lv S, Chen C (2017) Amperometric nitrite sensor based on a glassy carbon electrode modified with electrodeposited poly (3, 4-ethylenedioxythiophene) doped with a polyacenic semiconductor. Microchim Acta 184:2073–2079. doi:10.1007/s00604-017-2189-0
Wang G, Han R, Feng X, Li Y, Lin J, Luo X (2017) A glassy carbon electrode modified with poly (3, 4-ethylenedioxythiophene) doped with nano-sized hydroxyapatite for amperometric determination of nitrite. Microchim Acta 184:1721–1727. doi:10.1007/s00604-017-2180-9
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 21465013, 21005034 and 21501077), China Postdoctoral Science Foundation (Grant Nos. 2014 M551550) and Qingjiang Excellent Young Talents Program of Jiangxi University of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Huang, H., Lv, L., Xu, F. et al. PrFeO3-MoS2 nanosheets for use in enhanced electro-oxidative sensing of nitrite. Microchim Acta 184, 4141–4149 (2017). https://doi.org/10.1007/s00604-017-2446-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2446-2