Abstract
The authors describe the preparation of carbon nanofibers (CNFs) with a three-dimensional network structure by one-step carbonization of bacterial cellulose at 800 °C. The 3D CNFs wrapped with Nafion polymer were cast on a glassy carbon electrode (GCE) which then enables sensitive detection of Cd(II) and Pb(II). Under optimized conditions and at typical stripping peaks of around −0.80 and −0.55 V (vs Ag/AgCl), the electrode exhibits high sensitivity and a wide analytical range of 2–100 μg·L−1 for both Cd(II) and Pb(II). The detection limits are 0.38 μg·L−1 for Cd(II) and 0.33 μg·L−1 for Pb(II), respectively. The modified GCE was successfully employed to the determination of trace amounts of Cd(II) and Pb(II) in both tap water and waste water.

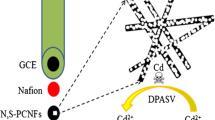
A three-dimensional carbon nanofiber network wrapped with Nafion polymer was employed to prepare a chemically modified electrode, which is shown to enable simultaneous detection of trace amounts of Cd(II) and Pb(II) in both tap water and waste water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) and lead (Pb) are listed as toxic substances by Environmental Protection Agency (EPA), and can induce serious adverse health effects in various organs and tissues, lead to acute or chronic poisoning and even cause cancer [1, 2]. Thus, monitoring trace Pb(II) and Cd(II) is necessary and urgent for the health and safety of humans. Anodic stripping voltammetry (ASV), a kind of sensitive, rapid, and low-cost electrochemical determination method, has been considered as one of the most effective tools for monitoring trace amount of heavy metals in environment [3, 4]. The remarkable sensitivity of ASV depends on an effective accumulation step at the surface of the chemically modified electrode [5]. Therefore, it is a great importance to design electrode materials with good conductivity and ability to achieve this accumulation. Hence, the electrode-modified material is a key factor that strongly affects the electroanalysis performance towards heavy metals [6].
A variety of carbon-based nanomaterials such as graphene [7, 8], carbon nanotube [9, 10] and mesoporous carbon [6, 11], have been used to fabricate the chemically modified electrode due to their high surface area, low charge-transfer resistance and chemical stability [12–14]. Among them, CNF has attracted particular attention owing to its robust mechanical properties, high-volume and corrosion resistance [5, 15]. Some reports on nanomaterial electrodes modified with CNF, prepared by electrospinning technology have also been reported. Zhao et al. reported that electrospun CNF, papered by 11 wt% of polyacrylonitrile (PAN) in DMF at high voltage 16 ~ 18 kV, can be applied for simultaneous detection of Cd(II) and Pb(II) by ASV [5]. Guo et al. demonstrated Pd-Ni alloy nanoparticle/CNF, electrospinning of PAN/Pd(acac)2/Ni(acac)2, for non-enzymatican alysis glucose [16]. Although the electrospinning technology can be used for the production of CNF, it still needs toxic and expensive chemical reagents, such as PAN. In this regard, a non-toxic and economic method is desirable for CNF synthesis.
Biomass materials such as bacterial cellulose (BC, (C6H10O5)n), a kind of natural cellulose produced on industrial scales via the microbial fermentation process, have received increasing interest in the fields of both electrocatalysis and electroanalysis [17, 18]. Due to its low cost, renewability and porous nature, BC has been widely considered as a promising precursor for CNF network [19]. To our knowledge, there are only few reports of CNF network derived from BC for electrochemical assay of trace toxic heavy metal ions. Here, we report for the first time that a green CNF network derived from freeze-dried BC is successfully applied for determination of Cd(II) and Pb(II) content in aqueous solutions by using ASV with high sensitivity and selectivity. The resulting data, and a comparison to other relevant systems, shows that CNF is a highly potential candidate for preparing the chemically modified electrode for fast quantification of Pb(II) and Cd(II) in aqueous environmental samples.
Experimental
Chemical reagent and instruments
The pure BC was purchased from Hainan Yeguo Foods Co., Ltd. (Hainan, China, https://hnyeguo.1688.com/). 5% Nafion stock solution and 1000 mg·L−1 standard Cd(II) solution were purchased from Sigma-Aldrich (Shanghai, China, http://www.sigmaaldrich.com/china-mainland/contact-us.html). Standard Pb(II) solution (1000 mg·L−1) was purchased from NCS Testing Technology Co., Ltd. (Beijing, China, http://www.ncscrm.com/Member/login.aspx?back=car.aspx). A 0.1 M acetate buffer (HAc-NaAc) was prepared by mixing the stock solutions of 0.1 M NaAc and HAc. All chemicals are analytical grade and used as received. Ultrapure water (≥18.2 MΩ·cm) was prepared for all electrochemical test solutions.
The morphology of CNF was characterized by a field-emission scanning electron microscope (FE-SEM, Carl Zeiss, Germany). The structure analysis of CNF was made on a transmission electron microscope (TEM, JEOL, Japan) at 200 kV. Surface element and relative chemical valence analysis of CNFs was carried out on an X-ray photoelectron spectroscopy (XPS, Kratos Analytical Ltd., UK). The content of Cd(II) and Pb(II) was measured by the inductively coupled plasma-optical emission spectrometry (ICP-OES, VISTA-PRO CCD, Palo Alto, California, USA).
Preparation of CNF and fabrication of CNF-Nafion/GCE
30 g BC was quenched in liquid nitrogen and then freeze-dried for 24 h in order to maintain its vulnerable network according to former report [19]. After freeze-drying, BC was heated up to 800 °C with a rate of 5 °C∙min−1 and then kept for 5 h at nitrogen atmosphere. After cooling down to room temperature, a fluffy black substrate was collected and labeled as CNF. Prior to electrode modification, a glassy carbon electrode (GCE, 3 mm in diameter) was carefully polished with 0.3 and 0.05 μm alumina slurry on a polishing cloth, respectively. After that, the electrode was sonicated in ultrapure water and ethanol successively for 5 min, and then dried by flowing N2. 5 wt% Nafion was diluted to 0.3 wt% with isopropyl alcohol-water (1:4, v/v) solution. Then 3 mg CNF was added in 3 mL of the above solution and further sonicated for 30 min to get homogeneous suspension. Finally, 5 μL suspensions were pipetted and casted on the pretreated GCE surface. After drying for 12 h at room temperature, the CNF-Nafion/GCE was prepared. The preparation procedure of CNF and CNF-Nafion/GCE was illustrated as Scheme.1.
DPASV analysis
The electrochemical measurements were performed on CHI-1040C workstation with a conventional three-electrode cell (bare or modified GCE as working electrode; a Ag/AgCl or saturated calomel electrode (SCE) as reference electrode; a Pt wire as auxiliary electrode) containing 10 mL HAc-NaAc. At first, Cd(II) and Pb(II) in the electrolyte solution were electrochemically reduced and then deposited on the surface of CNF-Nafion/GCE at −1.2 V for 240 s under stirring. Then the solution was kept staticfor 10 s, and finally, differential pulse voltammetry (DPV) curve was recorded in the potential range from −1.0 V to −0.4 V using amplitude 50 mV, pulse width 50 ms and potential step of a 4 mV.
Results and discussion
Characterization of CNF
The morphology and structure of the CNF network were characterized by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The SEM image in Fig. 1a undoubtedly shows the well-connected network of CNF nanowire, which is also confirmed by Fig. 1b inset. The interconnected 3D network structure can expand the specific surface area, shorten the transport pathway of heavy metal ion and accelerate diffusion, which improves electrical analysis heavy metal process [20, 21]. As shown in Fig. 1a inset, the average diameter of CNF was determined to 35 ± 33 nm with a wide size range from 10 nm to 200 nm. A high magnification TEM image (Fig. 1b) reveals that CNF networks are mostly constructed by amorphous carbon.
To investigate the surface contents and chemical valence of CNF, X-ray photoelectron spectroscopy (XPS) was measured in the energy region from 0 to 900 eV. As shown in Fig. 1c, the XPS survey of CNF exhibits a weak N1 s peak around 399.4 eV and two strong peaks representing C1s (284.8 eV) and O1s (532.0 eV) [22]. According to elemental analysis, the content of C, O and N on the CNF surface was 86.3 at%, 9.3 at% and 2.0 at%, respectively. In order to elucidate the detailed configuration, a high resolution N1 s spectrum was employed and analyzed in Fig. 1d, revealing that the N in CNF are in the form of pyridinic N (398.3 eV), pyrrolic N (400.2 eV) and graphitic N (401.2 eV) [19, 22]. In the C1s spectrum in Fig. 1e, four peaks are observed and assigned to C-C (284.5 eV), C-O (285.8 eV), C = O (286.9 eV) and O-C = O (289.1 eV) [23, 24]. The core-level XPS spectra of O1s (Fig. 1f) reveals two characteristic oxygen states of C = O (531.5 eV) and C-O (533.0 eV), which are consistent with previous report [25].
Electrochemical characterization
In order to investigate the electrochemical sensitivity of CNF towards Cd(II) and Pb(II), differential pulse anodic stripping voltammetry (DPASV) measurements were carried out on GCE, Nafion/GCE, CNF/GCE and CNF-Nafion/GCE. It can be seen from Fig. 2a that anodic stripping peaks of Cd(II) and Pb(II) are observed for all these four electrodes. The bare GCE presents weak peaks towards both Cd(II) and Pb(II). After casting by Nafion polymer, the electrochemical response signals of both Cd(II) and Pb(II) are improved for the Nafion/GCE, due to the negative anion groups of the polyelectrolyte polymer that favor the statistic accumulation of positive heavy metal ions [26]. For CNF/GCE, two sharper and more pronounced stripping peaks for Cd(II) and Pb(II) can be observed, attributed to the good conductivity and the efficient ions diffusion in the 3D network structure. Due to the synergistic effect of CNF and Nafion at the GCE, the stripping peaks of Cd(II) and Pb(II) recorded at CNF-Nafion/GCE are much larger than for the other electrodes.
a DPASV records of bare GCE (black line), Nafion/GCE (purple line), CNF/GCE (red line) and CNF-Nafion/GCE (blue line) in 0.1 M HAc-NaAc buffer (pH 4.5) containing 50 μg·L−1 Cd(II) and 50 μg·L−1 Pb(II). b A Nyquist diagram of EIS recorded at CNF/GCE (green square), Nafion/GCE (red square) and CNF-Nafion/GCE (blue square) in the presence of 0.1 M KCl solution containing 5 mM Fe(CN)6 3−/4-. c Cyclic voltammograms of bare GCE and d CNF-Nafion/GCE at various scan rates: 25, 50, 75, 100, 125, 150, 175, 200 mV·s−1 in 0.1 M KCl containing 0.1 mM K3[Fe(CN)6] (inset is relationship between the square of the scan rates and reduction peak currents)
Electrochemical impedance spectroscopy (EIS) is an effective and convenient tool to reveal the interface properties of the electrode materials. It can give information about the impedance changes before and after the electrode modification process. To calculate the electron transfer resistance (Rct), the Randle’s equivalent circuit (inset of Fig. 2b) was chosen to fit the impedance data (Rs: ohmic resistance of the electrolyte solution; W: Warburg impedance; C: interfacial capacitance). As seen in Fig. 2b, the CNF modified GCE shows a lower electron transfer resistance of 67 Ω, due to the 3D electric conductivity network of CNF. After decorating GCE with Nafion, a clear interfacial Rct is observed (108 Ω), indicating the formation of an insulating Nafion layer between GCE surface and the redox probe. When using CNF-Nafion nanocomposite to modify GCE, a moderate value of 76 Ω is obtained for CNF-Nafion/GCE, resulting from the combined action of CNF and Nafion.
To compare with the active surface area of bare GCE and the CNF-Nafion/GCE, cyclic voltammetry with different scan rates was carried out in the presence of 0.1 mM K3[Fe(CN)6] containing 0.1 M KCl. The results are presented in Fig. 2c and Fig. 2d. According to the Randles–Ševčik equation, the active surface area of electrode can be evaluated by [27]:
(Ipc: the reduction peak current, A; n: the electron transfer number, n = 1; A: the effective surface area of the working electrode, cm2; D: the diffusion coefficient, D = 7.6×10−6 cm2∙s−1; ν: scanning speed, V∙s−1; c: the concentration of electrolyte, c = 1×10−7 mol∙cm−3).
The linear dependences of the reduction peak current of bare GCE and CNF-Nafion/GCE versus the square of the scan rates are shown in inset of Fig. 2c and Fig. 2d. The electroactive surface area was calculated to 0.0198 cm2 for bare GCE and for 0.0302 cm2 CNF-Nafion/GCE, respectively. The result suggests that CNF-Nafion/GCE exhibits a relatively high electrochemical activity for heavy metals detection, which is ascribed to the great conductivity and pre-accumulation interaction of the CNF-Nafion membrane on GCE.
Electrochemical determination ofCd(II) and Pb(II)
It is well known that Nafion concentration, accumulation time, deposition potential and solution acidity, seriously affect the sensitivity and detection limitation of the CNF-Nafion/GCE towards heavy metal ion detection. Therefore the following parameters were carefully optimized: (a) Nafion concentration; (b) accumulation potential; (c) deposition time; (d) pH. Corresponding data and figures were presented in the Electronic Supporting Material. From Fig. S1, it can be concluded that the best results was observed for a Nafion concentration of 0.3 wt%, an accumulation potential of −1.2 V, a deposition time of 240 s and a pH of 4.5, respectively.
Under the optimum experimental conditions, the CNF-Nafion/GCE was applied for simultaneous determination of Cd(II) and Pb(II) in 0.1 M HAc-NaAc by DPASV. As presented in Fig. 3a, when the concentrations of Cd(II) and Pb(II) increase synchronously, their peak currents at around −0.80 and −0.55 V increase accordingly. A linear regression equation was calculated in the concentration ranges of 2–100 μg·L−1 for both Cd(II) and Pb(II). The average data, calculated from the measurements with three different electrodes, allows drawing two linear regressions for Cd(II) and Pb(II) (Fig. 3b). They are described with the following equations:
a DPASV curves of CNF-Nafion/GCE in 0.1 M HAc-NaAc with different concentration of Cd(II) and Pb(II) (from bottom to top, 0, 2, 4, 6, 8, 10, 30, 50, 70, 100 μg·L−1, respectively) along with the response to the blank solution (dash line). b Calibration plot of anodic peak current of CNF-Nafion/GCE as a function of Cd(II) (black square) and Pb(II) (red dot) concentrations
The limit of detection (LOD) was calculated at a signal-to-noise ratio of three. The estimated values are 0.38 and 0.33 μg·L−1 for Cd(II) and Pb(II), respectively.
In Table 1, the performance of the CNF-Nafion/GCE is compared with other materials for the simultaneous determination of Pb(II) and Cd(II). In terms of linear range and a detection limit, the CNF-Nafion/GCE remarkably improves analytical performance towards both Cd(II) and Pb(II).
Selectivity, repeatability and reproducibility of CNF-Nafion/GCE
In respect of selectivity, the interference of other metal ions was investigated in 0.1 M HAc-NaAc containing 50 μg·L−1 Cd(II) and 50 μg·L−1 Pb(II) in the presence of 20-fold excess of Ca2+, Mg2+, Al3+, Cr3+, Fe3+, Mn2+and Zn2+, respectively. The results are summarized in Fig. 4. It indicates that, except for Fe3+, other metal ions do not lead to serious interference for target heavy metal ion detection, meaning that the CNF-Nafion/GCE exhibits relatively high selectivity for Cd(II) and Pb(II).
The repeatability and reproducibility of the CNF-Nafion/GCE were also evaluated by the electrochemical detection of 50 μg·L−1 Cd(II) and 50 μg·L−1 Pb(II). The repeatability of the modified electrode was investigated by using one electrode to detect heavy metal ion concentration for 5 times. Here, the relative standard deviation (RSD) is 5.0% (N = 5) for Cd(II) and 3.9% (N = 5) for Pb(II), respectively. A similar experiment was performed with five electrodes and the RSD is 5.5% for Cd(II) and 6.2% for Pb(II), implying high reproducibility of the CNF-Nafion modified GCE.
Application to real analysis
For practical applicability, CNF-Nafion/GCE was used to detect Cd(II) and Pb(II) in both tap water and urban waste water. Two kind of water samples were spiked with 10 and 50 μg·L−1 for Cd(II) and Pb(II), respectively. Each sample was analyzed three times and the analysis results were listed in Table 2. CNF-Nafion/GCE, with recoveries of 93.0–110.4% for Cd(II) and 90.1–104.6% for Pb(II), which are in good agreement with the values determined by ICP-OES method. It reveals that the CNF-Nafion modified electrode can be successfully employed to detect Cd(II) and Pb(II) in environmental samples.
Conclusion
In conclusion, a three-dimensional cross-linked carbon nanofiber network derived from bacterial cellulose was prepared by a one-step annealing method. The interconnected network was then wrapped with electronegative Nafion polymer, making the CNF-Nafion modified GCE electrode can expand specific surface area, good ion-diffusion and pre-accumulation ability towards toxic heavy metal ions lead and cadmium. Combining the great conductivity of CNFs and excellent statistic accumulation interaction between Nafion and heavy metal ions, the analytical performance (high sensitivity and low detection limitation) of the CNF-Nafion modified electrode shows that the CNF-Nafion modified electrode is the most potential candidate for the heavy metal analysis in aqueous solution like tap water, industrial waste water, and soil solution after some needful improvements for the aim to completely meet with the strict environmental quality standard.
References
Aragay G, Pons J, Merkoci A (2011) Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem Rev 111(5):3433–3458
Bagheri H, Afkhami A, Khoshsafar H, Rezaei M, Sabounchei SJ, Sarlakifar M (2015) Simultaneous electrochemical sensing of thallium, lead and mercury using a novel ionic liquid/graphene modified electrode. Anal Chim Acta 870:56–66
Çelik GK, Üzdürmez AF, Erkal A, Kılıç E, Solak AO, Üstündağ Z (2016) 3,8-DiaminobenzoCinnoline Derivatived graphene oxide modified graphene oxide sensor for the Voltammetric determination of Cd2+ and Pb2+. Electrocatalysis 7(3):207–214
Cerovac S, Guzsvany V, Konya Z, Ashrafi AM, Svancara I, Roncevic S, KukoveczA DB, Vytras K (2015) Trace level voltammetric determination of lead and cadmium in sediment pore water by a bismuth-oxychloride particle-multiwalled carbon nanotube composite modified glassy carbon electrode. Talanta 134:640–649
Chamjangali MA, Kouhestani H, Masdarolomoor F, Daneshinejad H (2015) A voltammetric sensor based on the glassy carbon electrode modified with multi-walled carbon nanotube/poly(pyrocatechol violet)/bismuth film for determination of cadmium and lead as environmental pollutants. Sensors Actuators B Chem 216:384–393
Chen L, Huang Z, Liang H, Gao H, Yu S (2014) Three-dimensional heteroatom-doped carbon nanofiber networks derived from bacterial cellulose for supercapacitors. Adv Funct Mater 24(32):5104–5111
Zhu W, Yue X, Dua J, Zhang Y, Zhang W, Yu S, Wang Y, Zhang D, Wang J (2016) Electrochemically coreduced 3D GO C60 nanoassembly as an efficient nanocatalyst for electrochemical detection of bisphenol S. Electrochim Acta 188:85–90
Yue X, Song W, Zhu W, Wang J, Wang Y (2015) In situ surface electrochemical co-reduction route towards controllable construction of AuNPs/ERGO electrochemical sensing platform for simultaneous determination of BHA and TBHQ. Electrochim Acta 182:847–855
Joshi A, Nagaiah TC (2015) Nitrogen-doped carbon nanotubes for sensitive and selective determination of heavy metals. RSC Adv 5(127):105119–105127
Keawkim K, Chuanuwatanakul S, Chailapakul O, Motomizu S (2013) Determination of lead and cadmium in rice samples by sequential injection/anodic stripping voltammetry using a bismuth film/crown ether/Nafion modified screen-printed carbon electrode. Food Control 31(1):14–21
Li L, Zhou Y, Li Z, Ma Y, Pei C (2014) One step fabrication of Mn3O4/carbonated bacterial cellulose with excellent catalytic performance upon ammonium perchlorate decomposition. Mater Res Bull 60:802–807
Li W, Zhang Z, Kong B, Feng S, Wang J, Wang L, Yang J, Zhang F, Wu P, Zhao D (2013) Simple and green synthesis of nitrogen-doped photoluminescent carbonaceous nanospheres for bioimaging. Angew Chem 52(31):8151–8155
Liang H, Wu Z, Chen L, Li C, Yu S (2015) Bacterial cellulose derived nitrogen-doped carbon nanofiber aerogel: an efficient metal-free oxygen reduction electrocatalyst for zinc-air battery. Nano Energy 11:366–376
Zhu W, Liu W, Li T, Yue X, Liu T, Zhan W, Yu S, Zhang D, Wang J (2014) Facile green synthesis of graphene-au nanorod nanoassembly for on-line extraction and sensitive stripping analysis of methyl parathion. Electrochim Acta 146:419–429
Liu Y, Lu T, Sun Z, Chua D, Pan L (2015) Ultra-thin carbon nanofiber networks derived from bacterial cellulose for capacitive deionization. J Mater Chem A 3(16):8693–8700
Madhu R, Sankar KV, Chen S, Selvan RK (2014) Eco-friendly synthesis of activated carbon from dead mango leaves for the ultrahigh sensitive detection of toxic heavy metal ions and energy storage applications. RSC Adv 4(3):1225–1233
Matovic V, Buha A, Ethukic CD, Bulat Z (2015) Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association 78:130–140
Meng F, Li L, Wu Z, Zhong H, Li J, Yan J (2014) Facile preparation of N-doped carbon nanofiber aerogels from bacterial cellulose as an efficient oxygen reduction reaction electrocatalyst. Chin J Catal 35(6):877–883
Pérez-López B, Merkoçi A (2012) Carbon nanotubes and graphene in analytical sciences. Microchim Acta 179(1–2):1–16
Perez RC, Serrano N, Diaz JM, Arino C, Esteban M (2015) Penicillamine-modified sensor for the voltammetric determination of cd(II) and Pb(II) ions in natural samples. Talanta 144:569–573
Promphet N, Rattanarat P, Rangkupan R, Chailapakul O, Rodthongkum N (2015) An electrochemical sensor based on graphene/polyaniline/polystyrene nanoporous fibers modified electrode for simultaneous determination of lead and cadmium. Sensors Actuators B Chem 207:526–534
Shen H, Qin D, Li Y, Li S, Yang C, Yuan Q, Wagberg T, Hu G (2016) In situ Magnesiothermal synthesis of mesoporous MgO/OMC composite for sensitive detection of lead ions. Electroanalysis 28:1–9
Sioda RE, Frankowska B (2008) Voltammetric oxidation of naphthalene derivatives. J Electroanal Chem 612(1):147–150
Hu G, Nitze F, Sharifi T, Barzegar HR, Wagberg T (2012) Self-assembled palladium nanocrystals on helical carbon nanofibers as enhanced electrocatalysts for electro-oxidation of small molecules. J Mater Chem 22:85541–88548
Hu G, Nitze F, Gracia-Espino E, Ma J, Barzegar HR, Sharifi T, Jia XE, Shchuckarev A, Lu L, Ma C, Yang G, Wagberg T (2014) Small palladium islands embedded in palladium–tungsten bimetallic nanoparticles form catalytic hotspots for oxygen reduction. Nat Commun 5:5253–5262
Wang S, Wang Y, Zhou L, Li J, Wang S, Liu H (2014) Fabrication of an effective electrochemical platform based on graphene and AuNPs for high sensitive detection of trace Cu2+. Electrochim Acta 132:7–14
Wu Z, Liang H, Li C, Hu B, Xu X, Wang Q, Chen J, Yu S (2014) Dyeing bacterial cellulose pellicles for energetic heteroatom doped carbon nanofiber aerogels. Nano Res 7(12):1861–1872
Wu Z, Liang H, Chen L, Hu B, Yu S (2016) Bacterial cellulose: a robust platform for Design of Three Dimensional Carbon-Based Functional Nanomaterials. Acc Chem Res 49(1):96–105
Xiao L, Xu H, Zhou S, Song T, Wang H, Li S, Gan W, Yuan Q (2014) Simultaneous detection of cd(II) and Pb(II) by differential pulse anodic stripping voltammetry at a nitrogen-doped microporous carbon/Nafion/bismuth-film electrode. Electrochim Acta 143:143–151
Yu W, Lin W, Shao X, Hu Z, Li R, Yuan D (2014) High performance supercapacitor based on Ni3S2/carbon nanofibers and carbon nanofibers electrodes derived from bacterial cellulose. J Power Sources 272:137–143
Zhang C, Zhou Y, Tang L, Zeng G, Zhang J, Peng B, Xie X, Lai C, Long B, Zhu J (2016) Determination of Cd2+ and Pb2+ based on mesoporous carbon nitride/self-doped polyaniline nanofibers and square wave anodic stripping voltammetry. Nano 6(1):1–11
Zhao D, Wang T, Han D, Rusinek C, Steckl AJ, Heineman WR (2015) Electrospun carbon nanofiber modified electrodes for stripping voltammetry. Anal Chem 87(18):9315–9321
Zhou H, Hou H, Dai L, Li Y, Zhu J, Wang L (2016) Preparation of dendritic bismuth film electrodes and their application for detection of trace Pb (II) and cd (II). Chin J Chem Eng 24(3):410–414
Zhou W, Li C, Sun C, Yang X (2016) Simultaneously determination of trace Cd2+and Pb2+ based on L-cysteine/graphene modified glassy carbon electrode. Food Chem 192:351–357
Acknowledgements
This work was supported by National Natural Science Foundation of China (21505154 and 21677171), Xinjiang International Science and Technology Cooperation Project (20166010), 1000-Talent Program (Recruitment Program of Global Expert, In Chinese: Qian-Ren-Ji-Hua), and Project supported by the Special Scientific Research Fund of Agricultural Public Welfare Profession of China (21403014-1). TW acknowledge support from the Artificial Leaf Project Umeå (K&A Wallenberg foundation) and the Swedish Research Council (2013-5252).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 147 kb)
Rights and permissions
About this article
Cite this article
Qin, D., Gao, S., Wang, L. et al. Three-dimensional carbon nanofiber derived from bacterial cellulose for use in a Nafion matrix on a glassy carbon electrode for simultaneous voltammetric determination of trace levels of Cd(II) and Pb(II). Microchim Acta 184, 2759–2766 (2017). https://doi.org/10.1007/s00604-017-2260-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2260-x