Abstract
The authors describe a method for the trace determination of Hg(II) in human fluids using magnetic dispersive solid phase extraction. A silica powder magnetized with Fe3O4 nanoparticles was functionalized with 3-mercaptopropyl groups, and the resulting sorbent was characterized by scanning electron microscopy, energy dispersive X-ray, X-ray diffraction, vibrating sample magnetometry and FTIR. Following microwave-assisted digestion of the sorbent, mercury was quantified by continuous-flow CV-AAS. Factors affecting the sorption and desorption of Hg(II) were optimized. The calibration plot is linear in the 0.2–50.0 μg L−1 Hg(II)) concentration range. The accuracy in real sample analysis is in the range from 87 to 114%, and precision varies bteween 2.4 and 8.9%. The method was successfully applied to the determination of low levels of Hg(II) in plasma, urine and saliva.

Herein, a simple method for the trace monitoring of Hg(II) in some human fluids using nanomagnetic dispersive solid phase extraction was developed. The nano magnetic silica-based powder was functionalized with 3-mercaptopropyl. The total amounts of mercury were determined by continuous-flow cold vapor atomic absorption spectrometry following a microwave-assisted digestion treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury is widely distributed in nature, circulating among several media, and is found in different states and chemical forms, exhibiting various degrees of toxicity. Mercury has also been considered as a human health hazard because it may cause kidney toxicity, neurological damage, paralysis, chromosome breakage, and birth defects [1, 2]. Therefore, efficient assessment of suspected mercury intoxication through determination of mercury levels in various human fluid matrices, particularly blood, saliva and urine, is vitally important [3,4,5,6].
The most frequently used procedures for the extraction of mercury from biological samples are based on ultrasound or microwave assisted alkaline or acidic leaching. Ultrasound- and microwave assisted extractions have gained strength, since they offer enhanced sample throughput and require lower amounts of the reagents [6,7,8,9,10,11,12,13,14].
To date, several methods such as liquid extraction [15], column packed solid-phase extraction (SPE) [16] and magnetic solid-phase extraction (MSPE) [17] have been extensively applied for the separation of mercury from wide variety of media. Among these methods, MSPE appears to be one of the most effective selections owing to its ease of automation, high extraction efficiency and rapid phase separation [18,19,20,21,22,23].
Several analytical instruments have been used to detect concentration of mercury. These include: chromatographic methods such as gas chromatography (GC) [24] and liquid chromatography (LC) [15], and predominately non-chromatographic methods such as graphite furnace atomic absorption spectrometer (GF-AAS) [8], inductively coupled plasma-mass spectrometry (ICP-MS) [3] and cold vapor atomic absorption spectrometry (CV-AAS) [24, 25]. CV-AAS is widely applied and preferred over the formers due to its high sensitivity, simplicity and relatively low cost determination [26]. It should be noted that electrochemical method can be used for detection of mercury ions [27].
Hg(II) has been determined in various human fluid samples [3, 16, 24]. We have applied a nanomagnetic silica-based thiol-functionalized sorbent for solid phase extraction for CV-AAS measurement of Hg(II) concentration in human fluid samples. The nano magnetic silica-based powder was functionalized with 3-mercaptopropyl and the resulting sorbent was characterized by scanning electron microscopy, energy dispersive X-ray, X-ray diffraction, vibrating sample magnetometer and Fourier transform Infra-red techniques. Finally, the introduced method was successfully applied for determination of low levels of Hg(II) in various human fluids such as plasma, urine and saliva samples.
Exprimental
Chemicals
3-Mercaptopropyltrimethoxysilane (3-MPTS) was purchased from Aldrich Company. Hg (NO3)2.H2O, FeCl2.4H2O, FeCl3.6H2O, Sodium silicate (Na2SiO3), Sodium hydroxide (NaOH), Phosphoric acid, Nitric acid (HNO3), Hydrogen peroxide (H2O2), Sodium borohydride (NaBH4) and Hydrochloric acid (HCl) were all supplied from Merck (Darmstadt, Germany, www.merck.de). Double distilled water was used throughout for the preparation of all aqueous solutions. Other reagents used in this study were of analytical grade. Argon (99.99%) was used as the carrier gas. Working aqueous standards were prepared from a stock solution of 10 mg L−1 Hg(II) prepared in 0.1 mol L−1 HNO3. Calibrators were prepared at Hg(II) concentrations of 0.2, 0.5, 1.0, 2.0, 5.0, 10.0, 20.0 and 50.0 μg L−1 by dilution with water (aqueous calibrators) or spiking into the pooled lot of blank plasma, saliva or urine (matrix matched calibrators) from the non-exposed volunteers. Of the calibrators, three concentrations (0.5, 2.0 and 5.0 μg L−1) were used as quality controls (QC).
Instruments
A Perkin Elmer AAnalyst 700 (Norwalk, CT, USA) atomic absorption spectrometer equipped with MHS-15 mercury/hydride System was used in this study. A hallow cathode lamp operating at 6 mA was used and a spectral bandwidth of 0.7 nm was selected to isolate the 253.7 nm mercury line. A domestic microwave oven was also employed for total digestion of the fluid samples.
For characterization of the functional groups on the surface of the samples, fourier transform infrared spectroscopy (FT-IR) spectra were recorded using a Perkin-Elmer (Germany) spectrometer under dry air at room temperature by the KBr pellets method. The spectra were collected over the range from 400 to 4000 cm−1. The X-ray diffraction (XRD) studies were performed with a Philips XRD instrument (Siemens D-5000, Germany) using Cu Kα radiation (λ = 1.5406 Å) at wide-angle range (2Ө value 4–80°) at 40 kV of accelerating voltage and at 30 mA of emission current. The surface morphology of Fe3O4 and TF-SCMNPs nanoparticles was obtained studied by a field emission scanning electron microscopy (Mira3, Tescan, Czech Republic). The SEM images were further supported by energy dispersive X-ray (EDX) to provide direct evidence for the purity, existence and distribution of specific elements in a solid sample. The magnetic property of nanoparticles was characterized by vibrating Sample Magnetometer (VSM, MDKFD, Iran).
Sample preparation and analytical procedure
Plasma samples were taken from non-exposed volunteers and placed in 10 mL tubes containing citrate and immediately centrifuged (1850×g, 4 °C, 10 min) upon their arrivals. The resulting supernatants were transferred into polypropylene tubes and kept at −20 °C until the time of analysis. Saliva and urine samples were also collected from non-exposed volunteers and kept at −20 °C and 4 °C, respectively. All mentioned-above fluid samples were rapidly thawed in a water bath and immediately kept on ice and used as blank (later reconfirmed to be mercury free) or spiked with Hg(II) solution, unless mentioned otherwise. A microwave assisted acid digestion procedure was carried out to obtain Hg(II) which as follows:
An aliquot of 1.0 mL of each sample was transferred to a Teflon cup of acid digestion bomb. Nitric acid (65% w/v, 4.0 mL) and hydrogen peroxide (30% v/v, 1.0 mL) were added. The digestion bomb flask was closed and placed at room temperature for 10 min. The flasks were closed and submitted to the microwave for 3 min at 650 W. The digested samples were then transferred to a 50 ml of volumetric flask and volume was made up with 0.1 mol L−1 HCl. Firstly, 80 mg of the nanomagnetic sorbent was dispersed into 50 mL of the fluid samples for 10 min at room temperature followed by the addition of the phosphate buffer (pH 6.0, 0.2 mol L−1) to achieve pH 6.0. Secondly, the resulting mixture was vortexed vigorously for 5 min. Then, the nanomagnetic sorbent was separated from the solution by using a magnet and washed three times with double-distilled water. Finally, 3 mL of the eluent containing HCl 1 M and 5% (w/v) thiourea was added to the solution to remove Hg(II) from the nanomagnetic sorbent followed by its measurement by CVAAS. Sodium borohydride was used as the reducing agent in the determination of mercury. The solution was freshly prepared containing 0.3% NaBH4 in 0.5% NaOH. The hydrochloric acid solution (3 mol L−1) was prepared by adequate dilution of the concentrated HCl. The analytical measurement was based on peak height. Reading time and argon flow rate were 10 s and 50 mL min−1, respectively.
Synthesis of the nanomagnetic silica-based thiol-functionalized sorbent
The Fe3O4 nanoparticles were prepared using the co-precipitation method described in pervious works. The appropriate amount of FeCl3.6H2O and FeCl2.4H2O were dissolved in 200 mL deionized water. NH4OH 25% (25 mL) was added drop-wise to the precursor solution to obtain an alkaline medium (pH = 8) producing a black and gelatinous precipitate of Fe3O4 nanoparticles under nitrogen gas. It was heated at 80 °C for 2 h with continuous stirring. The desired Fe3O4 nanoparticles was collected by a permanent magnet and then washed with deionized water and ethanol for five times and dried at 80 °C in vacuum for 5 h. Then, appropriate amount of the TEOS (Tetraethyl orthosilicate), ethanol and NH3 were added to the previous stage to obtain Fe3O4@SiO2 under nitrogen gas while agitating for 2 h. Meanwhile, According to the Pearson’s hard soft acid–base theory (HSAB) mercury is classified as a soft acid, which tends to form very strong bonds with soft Lewis base groups, such as –CN, −RS, and –SH [14]. Therefore, 3-MPTS as a common silanizing reagent was chosen to modify Fe3O4@SiO2 to obtain a mercaptopropyl functionalized sorbents (Fe3O4@SiO2–SH) for the extraction of mercury from human fluid solutions. Thiol-function nanoparticles were prepared by appropriate addition of the MPTMS, TEOS, ethanol and NH3 to the latter stage under nitrogen gas and agitation for 24 h. The obtained thiol-functionalized nanoparticles was collected by a permanent magnet and then washed with deionized water and ethanol for five times. Then it was dried at 80 °C in vacuum for 5 h. The procedure of synthesis of functionalized nanoparticles (TF-SCMNPs) is summarized in Fig. 1.
Results and discussion
Characterization of the sorbent
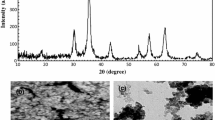
The information regarding the FT-IR and XRD of the synthesized sorbent was presented in the electronic supplementary materials.
SEM images of TF-SCMNPs nanoparticles are shown in Fig. 2. As can be seen, the average size of TF-SCMNPs from SEM image was around 10 nm. EDX microanalysis was used to characterize the elemental composition of the Fe3O4 and TF-SCMNPs nanoparticles. EDX pattern of the Fe3O4 and TF-SCMNPs nanoparticles are depicted in Fig. 3. According to the EDX analysis, the major elements were Fe (57.63%), O (29.18%), Si (5.88%), and S (3.15%) indicating good hybridization thiol-functionalized silica-coated magnetite nanoparticles. VSM was used to measure magnetite property of Fe3O4 and TF-SCMNPs. The VSM magnetization curve of the Fe3O4 and TF-SCMNPs nanoparticles at room temperature is depicted in Fig. 4. The saturated magnetization value of Fe3O4 and TF-SCMNPs nanoparticles were determined 58.97 and 32.43 emu.g−1, respectively. These results also indicates that the TF-SCMNPs nanoparticles showed an excellent magnetic response to a magnetic field. Therefore, it can be separated rapidly due to this high magnetic sensitivity.
Optimization of extraction parameters
It is evident that the extraction of Hg(II) ions using the nanomagnetic sorbent is strongly affected by some conditions such as pH of sample solution, the type of eluent and its concentration, amounts of sorbent, and the selectivity of method. Therefore, these parameters should be optimized before using the sorbent for measurement of Hg(II) in real fluid samples. It is noted that the aqueous calibrator of 1.0 μg L−1 was used for all experiments regarding the optimization process. The interaction can be described by the following chemical reactions long known from alkyl thiols:
-
Two SiO2-propyl-SH + Hg(II) == > SiO2-propyl-S-Hg-S-propyl-SiO2
The following parameters were optimized: (a) Sample pH value; (b) Eluent; and (c) Sorbent amount. Respective data and Figures are given in the Electronic Supporting Material [section 3.2.1; 3.2.2 and 3.2.3]. It was found that the following experimental conditions yielded the best results: (a) A sample pH value of 6.0; (b) 5 mL of 1.0 M HCl containing 5 w/w % thiourea as the elution solvent; and (c) 80 mg of the sorbent.
The effect of potentially interfering ions
To evaluate the selectivity of the method, the influence of several ions - predominantly present in human fluids - such as K+, Na+, Mg2+, Ca2+, Cl−, F− and CO3 2− on the extraction and measurement of Hg(II) was studied. The aqueous solutions containing Hg(II) at 1.0 μg L−1 and the above-mentioned ions at various concentrations were monitored. By definition, the effect of an ion is considered significant when its presence causes a variation greater than 5% in the value of the analytical response. Accordingly, no significant interference was observed due to the presence of the above-mentioned ions (Table 1).
Method validation
For validation of the method, the following parameters were determined: linearity range, intra- and inter assay precision, accuracy, recovery and limit of quantification (LOQ). It is noted that in all validation tests to come the calibrators (as described in section 2.3.1.) were used.
Linear range and determination of LOQ
The calibration curves (eight calibrators in duplicate) were fairly linear over the concentration ranges listed in Table 1S (ESM) for each fluid sample. The correlation coefficients were found to be greater than 0.98 within the mentioned concentration ranges.
The LOQ was measured based on the following definition: the lowest concentration at which the accuracy fell between 85% and 115% and the precision had a value of ≤10% under five measurements. Accordingly, the LOQs were determined to be 0.2, 0.3, 0.4 μg L−1 for urine, saliva and plasma samples, respectively.
The analytical figure of merits for the method and previously published articles is presented in the Table 2 [23, 28,29,30,31].
Precision and accuracy
Accuracy (measured value/nominal value × 100) and precision (coefficient of variation, C.V.: standard deviation of measured values/mean measured values × 100) were determined for the QC samples (Table 3). Intra-day precision (%) and accuracy (%) were evaluated by extracting and analyzing the fluid samples at three QC levels (0.5, 2.0 and 5.0 μg L−1) five times each. Inter-day precision was evaluated by analyzing the QC levels 3 times each over three consecutive days. As can be seen in Table 3, the precision and accuracy of the method is relatively good for all fluid samples except for the plasma due to its highly complicated matrix.
Recovery
Mean recoveries were evaluated by spiking Hg(II) into the blank fluid samples to the QC levels five times each and by measuring the Hg(II) concentrations before and after the spiking. The results shown in Table 3 indicate that the more the fluid’s medium is complicated, the less the extraction recovery will be.
Application to real sample analysis
In order to investigate the accuracy and applicability of the optimized method in real analysis, the concentration of Hg(II) was measured in human fluid samples such as plasma, urine and saliva. The plasma and urine samples were taken from our lab’s colleagues. Various saliva samples were taken from both individual volunteers who have/not had mercury amalgam dental fillings. All above-mentioned samples underwent the same optimized sample preparation as discussed earlier in section “Sample preparation and analytical procedure”. As can be deducted from the analytical results shown in Table 4., the method fulfills the necessary criteria for the measurement of Hg(II) in selected human fluids.
Conclusion
A simple dispersive solid-phase extraction method was successfully employed for validation and measurement of Hg(II) in human fluids by using a nanomagnetic silica-based thiol-functionalized sorbent. The sorbent was characterized and proved to be highly efficient for adsorption of mercury. Having been undergone the microwave digestion treatment, the fluid samples were introduced to a continuous-flow cold vapor atomic absorption spectrometer for final measurement of Hg(II). Once the method of analysis was optimized, it was validated by investigating various analytical parameters such as accuracy, precision, extraction recovery and limits of quantification. Finally, the method was successfully applied for determination of low levels of Hg(II) in various human fluids such as plasma, urine and saliva. Other heavy metals such as silver, lead, Bi and Cd also to be bound by the sorbent. It should be noted that, the mentioned metal ions will not interfere here because CVAAS and borohydride reduction are applied.
References
Manahan SE (1994) Environmental chemistry, 6th ed. Lewis Publishers, Ann Arbor, p 185
Baldwin DDR, Marshall WJ (1999) Heavy metal poisoning and its laboratory investigation. Ann Clin Biochem 36:267–300
Fong BMW, Siu TS, Lee JSK, Tam S (2007) Determination of mercury in whole blood and urine by inductively coupled plasma mass spectrometry. J Anal Toxicol 31:281–287
Kalate Bojdi M, Behbahani M, Omidi F, Hesam G (2016) Application of a novel electrochemical sensor based on modified siliceous mesocellular foam for electrochemical detection of ultra-trace amounts of mercury ions. New J Chem 40:4519–4527
Sedghi R, Heidari B, Behbahani M (2016) Synthesis, characterization and application of poly (acrylamide-co-methylenbisacrylamide) nanocomposite as a colorimetric chemosensor for visual detection of trace levels of hg and Pb ions. J Hazard Mater 285:109–116
Hossien-poor-Zaryabi M, Chamsaz M, Heidari T, Arbab Zavar MH, Behbahani M, Salarian M (2014) Application of dispersive liquid–liquid micro-extraction using mean centering of ratio spectra method for trace determination of mercury in food and environmental samples. Food Anal Methods 7:352–359
Krishna MVB, Ranjit M, Karunasagar D, Arunachalam J (2005) A rapid ultrasound-assisted thiourea extraction method for the determination of inorganic and methyl mercury in biological and environmental samples by CVAAS. Talanta 67:70–80
Moraes PM, Santos FA, Cavecci B, Padilha CCF, Vieira JCS, Roldan PS, Padilha PM (2013) GFAAS determination of mercury in muscle samples of fish from Amazon, Brazil. Food Chem 141:2614–2617
Babaee S, Daneshfar A, Khezeli T (2017) Determination of carboxylic acids in non-alcoholic beer samples by an ultrasonic-assisted dispersive micro-solid phase extraction based on Ni/cu-al layered double hydroxide nanocomposites followed by gas chromatography. Ultrason Sonochem 34:847–855
Arabi M, Ghaedi M, Ostovan A, Tashkhourian J, Asadallahzadeh H (2016) Synthesis and application of molecularly imprinted nanoparticles combined ultrasonic assisted for highly selective solid phase extraction trace amount of celecoxib from human plasma samples using design expert (DXB) software. Ultrason Sonochem 33:67–76
Al-Dhabi NA, Ponmurugan K, Jeganathan PM (2017) Development and validation of ultrasound-assisted solid-liquid extraction of phenolic compounds from waste spent coffee grounds. Ultrason Sonochem 34:206–213
Asfaram A, Ghaedi M, Goudarzi A (2016) Optimization of ultrasound-assisted dispersive solid-phase microextraction based on nanoparticles followed by spectrophotometry for the simultaneous determination of dyes using experimental design. Ultrason Sonochem 32:407–417
Kolaei M, Dashtian K, Rafiee Z, Ghaedi M (2016) Ultrasonic-assisted magnetic solid phase extraction of morphine in urine samples by new imprinted polymer-supported on MWCNT-Fe3O4-NPs: central composite design optimization. Ultrason Sonochem 33:240–248
Dastkhoon M, Ghaedi M, Asfaram A, Arabi M, Ostovan A, Goudarzi A (2017) Cu@SnS/SnO2 nanoparticles as novel sorbent for dispersive micro solid phase extraction of atorvastatin in human plasma and urine samples by high-performance liquid chromatography with UV detection: application of central composite design (CCD). Ultrason Sonochem 36:42–49
Batista BL, Rodrigues JL, Souza SS, Souza VCO, Barbosa F (2011) Mercury speciation in seafood samples by LC–ICP-MS with a rapid ultrasound-assisted extraction procedure: application to the determination of mercury in Brazilian seafood samples. Food Chem 126:2000–2004
Lemos VA, dos Santos LO (2014) A new method for preconcentration and determination of mercury in fish, shellfish and saliva by cold vapour atomic absorption spectrometry. Food Chem 149:203–207
Mashhadizadeh MH, Amoli-Diva M, Shapouri MR, Afruzi H (2014) Solid phase extraction of trace amounts of silver, cadmium, copper, mercury, and lead in various food samples based on ethylene glycol bis-mercaptoacetate modified 3-(trimethoxysilyl)-1-propanethiol coated Fe3O4 nanoparticles. Food Chem 151:300–305
Ziaei E, Mehdinia A, Jabbari A (2014) A novel hierarchical nanobiocomposite of graphene oxide–magnetic chitosan grafted with mercapto as a solid phase extraction sorbent for the determination of mercury ions in environmental water samples. Anal Chim Acta 850:49–56
Behbahani M, Bide Y, Bagheri S, Salarian M, Omidi F, Nabid MR (2016) A pH responsive nanogel composed of magnetite, silica and poly (4-vinylpyridine) for extraction of cd (II), cu (II), Ni (II) and Pb (II). Microchim Acta 183:111–121
Bagheri A, Behbahani M, Amini MM, Sadeghi O, Tootoonchi A, Dahaghin Z (2012) Preconcentration and separation of ultra-trace palladium ion using pyridine-functionalized magnetic nanoparticles. Microchim Acta 178:261–268
Nazari Serenjeh F, Hashemi P, Naeimi H, Zakerzadeh E, Ghiasvand AR (2016) Spherical agarose-coated magnetic nanoparticles functionalized with a new salen for magnetic solid-phase extraction of uranyl ion. Microchim Acta 183:2449–2455
Yang Y, Ma X, Feng F, Dang X, Huang J, Chen H (2016) Magnetic solid-phase extraction of triclosan using core-shell Fe3O4@MIL-100 magnetic nanoparticles, and its determination by HPLC with UV detection. Microchim Acta 183:2467–2472
Cui Y, Liu S, Wei K, Liu Y, Hu Z (2015) Magnetic solid-phase extraction of trace-level mercury(II) ions using magnetic core-shell nanoparticles modified with thiourea-derived chelating agents. Microchim Acta 182:1337–1344
Soleimani M, Mahmodi MS, Morsali A, Khani A, Afshar MG (2011) Using a new ligand for solid phase extraction of mercury. J Hazard Mater 189:371–376
Shah AQ, Kazi TG, Baig JA, Afridi HI, Arain MB (2012) Simultaneously determination of methyl and inorganic mercury in fish species by cold vapour generation atomic absorption spectrometry. Food Chem 134:2345–2349
Tuzen M, Soylak M (2005) Mercury cContamination in mMushroom sSamples from Tokat, Turkey. Bull Environ Contam Toxicol 74:968–972
Lesaint C, Frébault F, Delacôte C, Lebeau B, Marichal C, Walcarius A (2005) Synthesis and characterization of mesoporous silicas functionalized by thiol groups, and application as sorbents for mercury (II). J. Patarin. Stud Surf Sci Catal 156:925–932
Mehdinia A, Jebeliyan M, Kayyal TB, Jabbari A (2017) Rattle-type Fe3O4@SnO2 core-shell nanoparticles for dispersive solid-phase extraction of mercury ions. Microchim Acta 184:707–713
Razmi H, Musevi SJ, Mohammad-Rezaei R (2016) Solid phase extraction of mercury(II) using soluble eggshell membrane protein doped with reduced graphene oxide, and its quantitation by anodic stripping voltammetry. Microchim Acta 183(2):555–562
Zhai Y, He Q, Yang X, Han Q (2010) Solid phase extraction and preconcentration of trace mercury(II) from aqueous solution using magnetic nanoparticles doped with 1,5-diphenylcarbazide. Microchim Acta 169(3–4):353–360
Gao R, Hu Z, Chang X, He Q, Zhang L, Tu Z, Shi J (2009) Chemically modified activated carbon with 1-acylthiosemicarbazide for selective solid-phase extraction and preconcentration of trace cu(II), hg(II) and Pb(II) from water samples. J Hazard Mater 172(1):324–329
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 46 kb)
Rights and permissions
About this article
Cite this article
Sobhi, H.R., Ghambarian, M., Esrafili, A. et al. A nanomagnetic and 3-mercaptopropyl-functionalized silica powder for dispersive solid phase extraction of Hg(II) prior to its determination by continuous-flow cold vapor AAS. Microchim Acta 184, 2317–2323 (2017). https://doi.org/10.1007/s00604-017-2224-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2224-1