Abstract
The authors describe a colorimetric assay for the determination of As(III) in aqueous solution using citrate capped gold nanoparticles (AuNPs) which, in the presence of As(III), undergo aggregation due to the interaction of citrate ion with As(III). This results in an easily detectable color change from wine-red to blue. The ratio of the absorbances at 661 and 519 nm is linearly related to the As(III) concentration in the 4 to 100 ppb range, with a detection limit as low as 1.8 ppb (at 3σ) which is below the guideline value of 10 ppb. The method is rather simple in that it does not require the surface of the AuNPs to be modified. It was successfully applied to the determination of As(III) in spiked drinking water where it gave adequate recoveries.

The ratio of the absorbances at 661 and 519 nm is linearly related to the As(III) concentration in the 4 to 100 ppb range, with a detection limit as low as 1.8 ppb (at 3σ) which is below the guideline value of 10 ppb.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic is a highly poisonous carcinogen with wide distribution that more than twenty countries have been reported to suffer from arsenic contamination, provoking acute and chronic health issues such as skin lesions, problems with the circulatory system and high risk of cancer in the skin, lungs, bladder and kidneys [1–3]. More serious is that arsenic cannot be removed from the body and accumulates in human tissues [4, 5]. Arsenic predominantly presents as inorganic arsenic (As(III)) and arsenate(As(V)) salts, of which As(III) was considered as the most toxic substance [6, 7]. Drinking water is the main route of arsenic exposure which often exceed World Health Organization’s guideline value of 10 ppb, putting about 140 million people’s lives at risk [8].
Attributed to its high toxicity, the detection of arsenic has been carried out by a variety of techniques including atomic absorption spectrometry (AAS) [9, 10], inductively coupled plasma spectrometry (ICP) [11–13] and high-performance liquid chromatography (HPLC) [14] which always provide satisfying results for their good accuracy. However, there is still necessary to develop sensitive, time-saving, capable of on-site and cost-effective methods [15–21]. Among reliable methods for As(III) detection, several colorimetric sensors based on gold nanoparticles (AuNPs) have been blossoming for their high sensitivity and simplistic analyte recognition via bare eyes or cost-effective instrument requirement [22–27].
Ray and co-workers reported a glutathione, dithiothreitol, and cysteine modified gold-nanoparticle based on photometric detection and dynamic light scattering assay with a detection limit of 1 ppb and 10 ppt separately [28]. A sensor was also fabricated to detect arsenic down to 0.76 ppb by modifying silver nanoparticles with glutathione using surface-enhanced Raman scattering platform [29]. Pei Zhou et al. used arsenic-binding DNA aptamer first discovered by Y. H. Kim and J. Min for the colorimetric detection of As(III) [8, 30–33]. Most of the methods can meet arsenic test standard, but the complicated thiolated or other costly labeled probe preparation processes may lead to a poor repeatability and limit their applications to some extent for the increased steps of modification [34]. In this work, we find out that just after dialyzed by pure water to remove remnant sodium citrate in solution, citrate-capped AuNPs can be used to directly detect As(III) by a UV-vis spectrophotometer or even bare eyes. And for all we know, the single-shot assay based on citrate capped AuNPs reduced by sodium citrate for As(III) detection has not been reported yet.
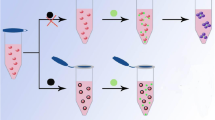
A citrate-mediated reduction of HAuCl4 is considered one of the most classic methods for the preparation of AuNPs which can be used to give sensitive and selective analysis for colorimetric detection. It is crucial to know the surface species of the citrate-reduced AuNPs and solution species present during reduction by citrate. In 1995, Smith and co-workers proved the presence of citrate and its decomposition products, acetoacetic acid and formate in solution throughout colloid formation and the relevant experimental results show that citrate but neither acetoacetic acid nor formate was detected as being adsorbed on the surface. A model of interaction and orientation of citrate at the colloid surface was further presented that is one terminal carboxylate and the tertiary carboxylate to the colloid while the second terminal carboxylate remained unbound to form negatively charged colloid surface [35]. Afterwards, glutathione and cysteine and other small molecules containing carboxyl group have been fabricated as colorimetric probes for arsenic detection [28, 29, 36]. Therefore, it implies that the citrate-capped AuNPs that contains abundant carboxyl groups is sensitive to As(III). Based on the above consideration, we use citrate capped AuNPs prepared by the citrate reduction method as sensitive colorimetric probe for the detection of As(III) (Fig. 1). As(III) can bind with citrate-capped AuNPs and directly induce the aggregation of AuNPs, causing a remarkable change in color. As we all know, the peak position of SPR band is closely related to the distance between AuNPs. When exposed to As(III), the state of AuNPs changes from dispersive to aggregated and an obvious red-shift of the SPR peak can be observed. Compared to existing colorimetric sensors for As(III), the method shows multifaceted advantages. First, the single-shot assay greatly simplify the complicated thiolated or other costly labeled probe preparation processes which may lead to a poor repeatability and limit their applications to some extent for the increased steps of modification. Second, citrate capped AuNPs shows excellent anti-disturbed ability to the common interfering analytes in the detection of As(III). Third, the method is rather sensitive to detect As(III) just by using water dialyzed AuNPs. Particularly, the method can be successfully applied to the detection of As(III) in drinking water.
Experimental
Reagents
HAuCl4·4H2O was purchased from Sinopharm Chemical Reagent Co., Ltd. (www.sinoreagent.com). Trisodium citrate, sodium hydroxide, hydrochloric acid and all of the metal salts used in this study were obtained from the Beijing Chemical Reagent Company (www.beijingchemworks.com). Sodium arsenite was bought from Xiya Chemical Industry Co Ltd. (www.xiyashiji.com) and sodium arsenate-dibasic heptahydrate was bought from Chem Service, Inc. (www.chemnet.com). Dimethylarsinic acid (DMA) was bought from www.aladdin-e.com. Sodium methylarsonate (MMA) was obtained from www.accustandard.com. All the chemicals were of analytical reagent grade and Milli-Q-purified distilled water was used throughout the experiments.
Apparatus
The UV-vis spectra were recorded with a UV-2550 spectrophotometer (www.shimadzu.com). Transmission electron microscopy (TEM) images were acquired on a FEI Tecnai G2 F20 (www.fei.com). Inductively coupled plasma optical emission spectrometer (ICP-OES) experiments were carried out by using a PQ 9000 spectrometer (www.analytik-jena.com). All the photographs were taken by a DSC-W150 camera (www.sony.com).
AuNPs synthesis and dialysis
As reported previously, citrate capped AuNPs were synthesized via trisodium citrate reduction method as shown in Fig. 1 [37]. Briefly, the AuNPs were synthesized by adding 15 mL of trisodium citrate solution (38.8 mM) rapidly to a boiling solution of HAuCl4 (1.0 mM, 150 mL) and stirred for 30 min to form a wine-red solution. The resulting solution was cooled to room temperature while being stirred continuously, then stored at 4 °C for further use.
The above AuNPs were diluted with the ratio of 1:3 by Milli-Q-purified distilled water before dialysis. 5 mL of diluted AuNPs was placed in a membrane bag with molecular weight cut off 1000 under continuous stirring for 6 h, then water dialyzed AuNPs was obtained.
Colorimetric detection of As(III)
In a 1.5 mL centrifuge tube, 20 μL of As(III) solution with varying concentrations were pipetted into water dialyzed AuNPs respectively. After incubating for 6 min at room temperature, the above solutions were measured with UV-Vis spectrophotometry and recorded by photographs. The quantitative analysis of As(III) was achieved by the absorption ratio (A661/A519). Limit of detection (LOD) values were calculated using the following formula:
Limit of detectable response = average response of the blank + (3 × standard deviation of the blank) [38].
Results and discussion
The responses of dialyzed and undialyzed AuNPs for As(III)
Dialyzed and undialyzed AuNPs were performed in the absence and presence of As(III) separately. Figure 2a shows the color change and absorbance spectra. A decrease of maximal absorption at 519 nm and an enhancement of a new peak at 661 nm indicating that the state of the AuNPs changed from dispersion to aggregation. In the presence of As(III), the color of dialyzed AuNPs changed from red to blue (d of Fig. 2(A)) indicating the aggregation of the AuNPs, which is in accordance with the result of UV-vis spectroscopy. While the presence of 909 ppb As(III) did not induce any change in the undialyzed AuNPs (c of Fig. 2(B)). Therefore, the dialyzed AuNPs show a higher sensitivity to As(III). The As(III) significantly stimulates aggregation of AuNPs which is also evidenced by TEM images (Fig. 2b). In the presence of As(III), the citrate-capped AuNPs undergo aggregation due to the interaction of citrate ion with As(III).
Optimization of method
The following parameters were optimized: (a) The size of the AuNPs (Fig. S1); (b) Concentration of remnant sodium citrate in solution (Fig. S2); (c) Impact of dialytic time (Fig. S3); (d) Impact of pH (Fig. S4); (e) Impact of incubation time (Fig. S5). Respective data and Figures are given in the Electronic Supporting Material. We found the following experimental conditions to give best results: (a) AuNPs size of 13 nm; (b) Without addition of sodium citrate in solution; (c) Dialytic time of 6 h; (d) pH 11; (e) Incubation time of 6 min; (f) Stability for two days.
Colorimetric detection of As(III)
To examine the assay for the direct colorimetric detection of As(III), 20 μL of different concentration of As(III) solution were added into 1 mL of water-dialyzed AuNPs under optimal experimental conditions and tested after mixed for about 6 min. The increase of As(III) concentration can induce a decrease of maximal absorption at 519 nm and an enhancement of a new peak at 661 nm (Fig. 3a). In addition, a gradual color change from wine-red to blue can also be observed that means we can discriminate the concentration of As(III) with bare eyes (the inset of Fig. 3A). The relationship between the ratio of A661/A519 and As(III) concentration in the range from 4 to 100 ppb shows excellent linearity with a correlation coefficient of 0.9914 and a detection limit of 1.8 ppb (3σ) which is below guideline value of 10 ppb (Fig. 3B), indicating that the method based on citrate capped AuNPs definitely meets the requirements of routine detection.
A UV–Vis spectra of citrate capped AuNPs in the presence of different concentrations of As(III). Insert portion was the corresponding photographic images. B Absorption ratio A661/A519 of citrate capped AuNPs versus the concentration of As(III). Insert portion shows the linear dependence of A661/A519 at low As(III) concentration. (error bars represent the standard deviation of three measurements)
We have listed typical methods for As(III) detection to make intuitive comparison. The method described in reference [28] is highly innovative. It can detect arsenic by photometric detection and dynamic light scattering assay with a detection limit of 1 ppb and 10 ppt separately. The detection limit achieved with this DLS probe represents the lowest among all the reported methods. Although gold nanoparticle sensors for As(III) are described in the literature [28, 29, 36], they usually require some kinds of receptor molecules attached to the particles which result in the specific binding of As(III), like glutathione or aptamer. Here we present a new method for arsenic detection with no need for additional surface modification. We just use the citrate-stabilized gold nanoparticles, utilizing the citrate-induced specific binding of As(III) which greatly simplify the complicated thiolated or other costly labeled probe preparation processes [34]. In order to improve the sensitivity, we utilize dialysis to remove remnant sodium citrate in solution, for that excess sodium citrate can play a counteractive role in the response to As(III) due to the unavoidable binding to As(III) (Fig. S2). The dialysis method can be applied to improve the sensitivity of all the reported colorimetric detection based on gold nanoparticles. We also demonstrate the quantification and the selectivity (by checking with other ions), and finally demonstrate a detection of As(III) down to 1.8 ppb in drinking water (see Table 1). These results indicate that the present sensing system is a promising method for the detection of As(III).
Selectivity of the colorimetric method
The selectivity of the method for As(III) was examined by evaluating the absorbance ratio A661/A519 of citrate capped AuNPs in the presence of competing metal ions, including K(I), Cu(II), Mn(II), Zn(II), Mg(II), Na(I), Hg(II), Fe(II), Fe(III), Ca(II), Ni(II), Pb(II), Cd(II), Cr(III), Al(III), As(V), MMA and DMA.
Figure 4 and Fig. S6 show that only As(III) can induce a remarkable aggregation of AuNPs. The results demonstrate that all of the studied ions displayed slight and negligible interferences for As(III) detection. The possible reason is as follows: when pH at 11, only As(III) predominantly presents as H3AsO3 and H2AsO3 − in the form of stable covalent compound [41, 42], which can form hydrogen bond with citrate ions and induce the obvious aggregation of citrate capped AuNPs [43].
Application in water samples
To validate the applicability of the method, the analysis of As(III) in drinking water was performed. A series of As(III) standard solution was added into the drinking water, and then detected utilizing citrate capped AuNPs based colorimetric method and Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES) (see Table 2). The results show that the recoveries of 16 ppb, 32 ppb and 64 ppb As(III) are 95.0%, 98.1% and 102.8%, which is in good agreement with the results obtained by ICP-OES (96.9%, 100.3%, 104.1% respectively), demonstrating the potential application of the colorimetric assay for As(III) detection in water samples.
Conclusion
Citrate capped gold nanoparticles were synthesized via citrate reduction method and reported as sensitive, selective, and colorimetric single-shot assay for As(III). As(III) can bind with citrate-capped AuNPs and directly induce the aggregation of citrate capped AuNPs, resulting in an obvious color change from wine-red to blue. It was successfully applied to the determination of As(III) in spiked drinking water where it gave adequate recoveries. However, our method cannot online monitor over time like a pH electrode or an oxygen electrode for that completely aggregated AuNPs no longer show a linearity between the ratio of A661/A519 and As(III) concentration.
References
Haque R, Mazumder DN, Samanta S, Ghosh N, Kalman D, Smith MM, Mitra S, Santra A, Lahiri S, Das S, De BK, Smith AH (2003) Arsenic in drinking water and skin lesions: dose-response data from West Bengal, India. Epidemiology 14:174–182
Hung DQ, Nekrassova O, Compton RG (2004) Analytical methods for inorganic arsenic in water: a review. Talanta 64:269–277
Smith AH, Hopenhayn-Rich C, Bates MN, Goeden HM, Hertz-Picciotto I, Duggan HM, Wood R, Kosnett MJ, Smith MT (1992) Cancer risks from arsenic in drinking water. Environ Health Perspect 97:259–267
Cullen WR, Reimer KJ (1989) Arsenic speciation in the environment. Chem Rev 89:713–764
Mandal BK, Ogra Y, Suzuki KT (2003) Speciation of arsenic in human nail and hair from arsenic-affected area by HPLC-inductively coupled argon plasma mass spectrometry. Toxicol Appl Pharmacol 189:73–83
Ensafi AA, Ring AC, Fritsch I (2010) Highly sensitive Voltammetric speciation and determination of inorganic arsenic in water and alloy samples using ammonium 2-amino-1-Cyclopentene-1-Dithiocarboxylate. Electroanal 22:1175–1185
Moriarty MM, Koch I, Gordon RA, Reimer KJ (2009) Arsenic speciation of terrestrial invertebrates. Environ Sci Technol 43:4818–4823
Wu Y, Zhan S, Wang F, He L, Zhi W, Zhou P (2012) Cationic polymers and aptamers mediated aggregation of gold nanoparticles for the colorimetric detection of arsenic(III) in aqueous solution. Chem Commun 48:4459–4461
Pantuzzo FL, Silva JC, Ciminelli VS (2009) A fast and accurate microwave-assisted digestion method for arsenic determination in complex mining residues by flame atomic absorption spectrometry. J Hazard Mater 168:1636–1638
Sounderajan S, Udas AC, Venkataramani B (2007) Characterization of arsenic (V) and arsenic (III) in water samples using ammonium molybdate and estimation by graphite furnace atomic absorption spectroscopy. J Hazard Mater 149:238–242
Dufailly V, Noel L, Guerin T (2008) Optimisation and critical evaluation of a collision cell technology ICP-MS system for the determination of arsenic in foodstuffs of animal origin. Anal Chim Acta 611:134–142
Gil RA, Ferrua N, Salonia JA, Olsina RA, Martinez LD (2007) On-line arsenic co-precipitation on ethyl vinyl acetate turning-packed mini-column followed by hydride generation-ICP OES determination. J Hazard Mater 143:431–436
Gomez-Ariza JL, Sanchez-Rodas D, Giraldez I, Morales E (2000) A comparison between ICP-MS and AFS detection for arsenic speciation in environmental samples. Talanta 51:257–268
Al-Assaf KH, Tyson JF, Uden PC (2009) Determination of four arsenic species in soil by sequential extraction and high performance liquid chromatography with post-column hydride generation and inductively coupled plasma optical emission spectrometry detection. J Anal Atom Spectrom 24:376–384
Alizadeh A, Abdi G, Khodaei MM (2016) Colorimetric and visual detection of silver(I) using gold nanoparticles modified with furfuryl alcohol. Microchim Acta 183:1995–2003
Chen Y, Yao L, Deng Y, Pan D, Ogabiela E, Cao J, Adeloju SB, Chen W (2015) Rapid and ultrasensitive colorimetric detection of mercury(II) by chemically initiated aggregation of gold nanoparticles. Microchim Acta 182:2147–2154
Chen Z, Zhang C, Tan Y, Zhou T, Ma H, Wan C, Lin Y, Li K (2014) Chitosan-functionalized gold nanoparticles for colorimetric detection of mercury ions based on chelation-induced aggregation. Microchim Acta 182:611–616
Huang P, Li J, Liu X, Wu F (2015) Colorimetric determination of aluminum(III) based on the aggregation of Schiff base-functionalized gold nanoparticles. Microchim Acta 183:863–869
Liang Y, He Y (2015) Arsenazo III-functionalized gold nanoparticles for photometric determination of uranyl ion. Microchim Acta 183:407–413
Liu L, Leng Y, Lin H (2016) Photometric and visual detection of Cr(VI) using gold nanoparticles modified with 1,5-diphenylcarbazide. Microchim Acta 183:1367–1373
Zarlaida F, Adlim M (2016) Gold and silver nanoparticles and indicator dyes as active agents in colorimetric spot and strip tests for mercury(II) ions: a review. Microchim Acta 184:45–58
Chen Z, Tan L, Wang S, Zhang Y, Li Y (2016) Sensitive colorimetric detection of K(I) using catalytically active gold nanoparticles triggered signal amplification. Biosens Bioelectron 79:749–757
Elghanian R, Storhoff JJ, Mucic RC, Letsinger RL, Mirkin CA (1997) Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 277:1078–1081
Liu JW, Lu Y (2003) A colorimetric lead biosensor using DNAzyme-directed assembly of gold nanoparticles. J Am Chem Soc 125:6642–6643
Liu X, Wu Z, Zhang Q, Zhao W, Zong C, Gai H (2016) Single gold nanoparticle-based colorimetric detection of Picomolar mercury ion with dark-field microscopy. Anal Chem 88:2119–2124
Storhoff JJ, Elghanian R, Mucic RC, Mirkin CA, Letsinger RL (1998) One-pot colorimetric differentiation of polynucleotides with single base imperfections using gold nanoparticle probes. J Am Chem Soc 120:1959–1964
Zhao W, Brook MA, Li Y (2008) Design of gold nanoparticle-based colorimetric biosensing assays. Chembiochem 9:2363–2371
Kalluri JR, Arbneshi T, Khan SA, Neely A, Candice P, Varisli B, Washington M, McAfee S, Robinson B, Banerjee S, Singh AK, Senapati D, Ray PC (2009) Use of gold nanoparticles in a simple colorimetric and ultrasensitive dynamic light scattering assay: selective detection of arsenic in groundwater. Angew Chem 48:9668–9671
Li J, Chen L, Lou T, Wang Y (2011) Highly sensitive SERS detection of As3+ ions in aqueous media using glutathione functionalized silver nanoparticles. ACS Appl Mater Interfaces 3:3936–3941
Kim M, Um HJ, Bang S, Lee SH, Oh SJ, Han JH, Kim KW, Min J, Kim YH (2009) Arsenic removal from Vietnamese groundwater using the arsenic-binding DNA aptamer. Environ Sci Technol 43:9335–9340
Wu Y, Liu L, Zhan S, Wang F, Zhou P (2012) Ultrasensitive aptamer biosensor for arsenic(III) detection in aqueous solution based on surfactant-induced aggregation of gold nanoparticles. Analyst 137:4171–4178
Wu Y, Zhan S, Xing H, He L, Xu L, Zhou P (2012) Nanoparticles assembled by aptamers and crystal violet for arsenic(III) detection in aqueous solution based on a resonance Rayleigh scattering spectral assay. Nanoscale 4:6841–6849
Wu YG, Wang FZ, Zhan SS, Liu L, Luo YF, Zhou P (2013) Regulation of hemin peroxidase catalytic activity by arsenic-binding aptamers for the colorimetric detection of arsenic(III). RSC Adv 3:25614–25619
Shellaiah M, Simon T, Sun KW, Ko F-H (2016) Simple bare gold nanoparticles for rapid colorimetric detection of Cr3+ ions in aqueous medium with real sample applications. Sensors Actuators B Chem 226:44–51
Munro CH, Smith WE, Garner M, Clarkson J, White PC (1995) Characterization of the surface of a citrate-reduced colloid optimized for use as a substrate for surface-enhanced resonance Raman-scattering. Langmuir 11:3712–3720
Dominguez-Gonzalez R, Gonzalez Varela L, Bermejo-Barrera P (2014) Functionalized gold nanoparticles for the detection of arsenic in water. Talanta 118:262–269
Turkevich JS, Stevenson PC, Hillier J (1951) A study of the nucleation and growthprocesses in the synthesis of colloidal gold. Discuss Faraday Soc 11:55–75
Sener G, Uzun L, Denizli A (2014) Lysine-promoted colorimetric response of gold nanoparticles: a simple assay for ultrasensitive mercury(II) detection. Anal Chem 86:514–520
Forzani ES, Foley K, Westerhoff P, Tao N (2007) Detection of arsenic in groundwater using a surface plasmon resonance sensor. Sensors Actuators B Chem 123:82–88
Wang X, Lv Y, Hou X (2011) A potential visual fluorescence probe for ultratrace arsenic (III) detection by using glutathione-capped CdTe quantum dots. Talanta 84:382–38639
Loehk TM, Plane KA (1968) Raman spectra and structures of Arsenious acid and Arsenites in aqueous solution. Inorg Chem 7:1708–1714
Sellers P, Sunner S, Wadso I (1964) Heats of ionization of Arsenious and arsenic acids. Acta Chem Scand 18:202–206
Buschmann J, Kappeler A, Lindauer U, Berg M, Sigg L (2006) Arsenite and arsenate binding to dissolved humic acids: influence of pH, type of humic acid, and aluminum. Environ Sci Technol 40:6015–6020
Acknowledgements
This work was sponsored by the National Natural Science Foundation of China (Grant Nos. 21272263, 21205132 and 21302008), the State Key Laboratory of Natural and Biomimetic Drugs (No. K20160203), the national key research and development plan(2016YFF0203700), a grant from the Major National Scientific Research Plan of China (973 Program) (Grant No. 2011CB933202), College Student Innovation Training Program of Chinese Academy of Sciences (No. 118900EA12), Science and Education Integration Innovation of Molecule Science of Institute of Chemistry Chinese Academy of Sciences (No. Y52902HED2), the Special Fund of UCAS for Scientific Research Cooperation between Faculty and Institutes (Grant No. Y552016Y00), and the University of Chinese Academy of Sciences Grant (No. O8JT011J01).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 11930 kb)
Rights and permissions
About this article
Cite this article
Gong, L., Du, B., Pan, L. et al. Colorimetric aggregation assay for arsenic(III) using gold nanoparticles. Microchim Acta 184, 1185–1190 (2017). https://doi.org/10.1007/s00604-017-2122-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2122-6