Abstract
The authors describe a colorimetric method for the determination of Al(III) using surface modified gold nanoparticles (AuNPs). Citrate capped AuNPs were functionalized, by self-assembly, with the Schiff base obtained from 2-hydroxy-1-naphthaldehyde and 2-aminoethanethiol. The modified AuNPs were characterized by transmission electron microscopy and FTIR. Complexation of Al(III) ions by the Schiff base on the AuNPs results in self-aggregation of the AuNPs which is accompanied by a color change from red to blue which can be monitored visually or by UV–vis spectroscopy. Absorbance varies linearly with the Al(III) concentration in the range from 9 to 23 μM, and the lower detection limit is 0.29 μM (at 3 So/k). The method was applied to the determination of Al(III) in (spiked) samples of boiler water and urine.

A colorimetric method for the determination of Al(III) using Schiff base-functionalized gold nanoparticles (AuNPs) is developed based on the complexation of Al(III) leading to the aggregation of the AuNPs and a color change from red to blue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dissolved Al3+ widely exists in the environment due to acidic rain and human activities. Its toxicity not only hampers plant growth but also damages the human nervous system to induce Alzheimer’s disease [1], Parkinson’s disease [2], dialysis encephalopathy [3] etc. To avoid the adverse health problems caused by the uptake of Al3+, an estimated tolerable dietary intake in the human body is 7 mg kg−1 body weight per week [4]. Detection of Al3+ is crucial in controlling its concentration levels in the biosphere and its direct impact on human health.
Al3+ is detected using multiple laboratory analysis platforms, including atomic absorption spectrometry [5], inductively coupled plasma mass spectroscopy (ICP-MS) [6], and voltammetry [7]. Although these methods can enable a high level of qualitative and quantitative analyses, operative inability by a non-specialist and requirement of sophisticated instrumentation severely limit applications which are suitable for point-of-care, field deployment, or other low-resource settings. To date, optical sensors, especially colorimetric ones, have always been the most convenient analytical tool and gained a lot of interest because they provide a simple and economical assay without the aid of instruments. It is reported that colorimetric chemosensors have been widely exploited for Al3+ detection [8–10], which depends on rational design of Al3+-responsive receptors with sufficient or even remarkable sensitivity and selectivity. Although they provide a straightforward and acceptable assay, they always suffer from complicated probe design, multistep synthesis strategy and large reagent consumption. Besides, concerning their practical applications, aqueous media may be the best choice for Al3+ detection, but majority of these sensors are unstable or dysfunctional in aqueous solution.
Compared with chromophoric chemosensors, gold nanoparticles (AuNPs) based colorimetric assays have emerged as a fascinating research field [11–14]. AuNPs can be synthesized in a straightforward manner and remain highly stable after proper surface modification. They possess unique optical properties which depend on interparticle distance, i.e., dispersion/aggregation is directly observed as a solution color switch due to surface plasmon coupling. In addition, they have an high extinction coefficient (from 1 × 108 to 1 × 1010 M−1 cm−1), which is often several orders of magnitude higher than those of organic dyes. More importantly, AuNPs have a high surface area to volume ratio, and their surfaces can be readily chemically tailored by ligands with functional groups. Through these functional groups, specific molecular recognition can be realized, thus allowing for easy colorimetric assays of a wide range of targets, including metal ions, small molecules, proteins, nucleic acids, malignant cells, etc., at fairly low concentrations. Although a large number of sensitive and reliable gold nanoparticle-based colorimetric detections of transition metal ions (Cd2+, Pb2+, Hg2+, Cu2+, etc.) have been introduced [15–21], colorimetric detection of Al3+ using AuNPs probe is not well studied. Li et al. reported the first colorimetric assay based on pentapeptide functionalized AuNPs for detecting Al3+, which achieved great success at monitoring contaminated levels of Al3+ on living cellular surfaces [22]. Moreover, mononucleotide-modified metal nanoparticles were also reported as a colorimetric probe for selective and sensitive detection of Al3+ and used to monitor Al3+ on living cellular surfaces under physiological conditions [23].
To develop a convenient alternative for on-site Al3+ assay that meets the requirements of low cost, high sensitivity and selectivity, water compatibility and environment friendliness, we demonstrated a colorimetric assay for the detection of Al3+ based on the Al3+-induced self-aggregation of AuNPs. The Schiff base of N-(2-hydroxynaphthylidene)-2-aminoethanethiol (HNAET) was synthesized and introduced as the chelating ligand on the surface of AuNPs through the well-known strong Au-S interaction (HNAET-AuNPs) (Scheme 1). The Schiff bases formed on the AuNPs surface may serve as efficient and selective complexing reagents for Al3+ rather than other metal cations [24]. In aqueous media, the affinity of Al3+ toward the functional naphthol group led to an interparticle crosslinking induced aggregation of HNAET–AuNPs and a clear color change from red to blue. Therefore, a colorimetric assay for visual detection of Al3+ was developed. Compared with the use of pentapeptide or mononucleotide modified AuNPs as the Al3+ probe [22, 23], this small molecular ligand-modified AuNPs probe is cheaper and readily achievable. More importantly, the HNAET–AuNPs probe matches the probes in terms of its analytical capacity.
Experimental
Instruments
Transmission electron microscope (TEM) measurements were performed on a JEM-2010 instrument (JEOL Ltd, Japan, www.jeol.cn). Absorption spectra were recorded on a Ultraviolet–visible (UV–Vis) 2550 spectrophotometer (Shimadzu, Kyoto, Japan, www.shi-madzu.com) equipped with a 1 cm quartz cell. Nuclear magnetic resonance (NMR) data of the synthesized ligand were collected using a Agilent-400 MHz NMR instrument (Agilent Technologies Inc., USA, www.agilent.com). Fourier Transform Infrared Spectoscopy (FTIR) spectra were recorded on a Nicolet 5700 Fourier transform spectrometer (Nicolet, USA, www.thermonicolet.com). Mass spectra were obtained with a WatersZQ-4000 ion trap mass spectrometer (Waters Corporation, USA, www.waters.com). The zeta potentials and dynamic light scattering (DLS) of the samples were determined using a Zetasizer (Zetasizer nano zs90, Malvern Instruments, UK, www.malvern.com/en/).
Chemicals
Hydrogen tetrachloroaurate hydrate (HAuCl4·4H2O) was obtained from Sinopharm Group (http://www.sinoreagent.com/). 2-hydroxy-1-naphthaldehyde and 2-aminoethanethiol hydrochloride was purchased from Sigma-Aldrich (http://www.sigmaaldrich.com/). Other chemicals used were of analytical grade or of the highest purity available. All the solutions were prepared using Milli-Q water (Millipore) as the solvent.
Synthesis of 1-(2-aminoethanethiol)-2-naphthol (HNAET)
According to previous reported literature [25], Briefly, to a solution of 2-hydroxy-1-naphthaldehyde (0.378 g, 2 mmol) in ethanol (25.0 mL), 5 mL 2-aminoethanethiol hydrochloride (0.227 g, 2.2 mmol) was added, and a amount of triethylamine was added in to adjust pH 7 ~ 8. The solution was stirred under reflux for 4 h and then allowed to stand at room temperature overnight as shown in Scheme 1a. The precipitate was filtered, washed thoroughly with ethanol and recrystallized with ethanol to give a luminous yellow powder, then dried under reduced pressure to afford compound HNAET in 90 % yield.
1H NMR (400 MHz, DMSO) δ(ppm): 2.98 (t, 1H, SH), 3.14 (t, 2H, CH2), 3.96 (t, 2H, CH2), 6.76 (d, J = 12.0 Hz, 1H, ArH), 7.18 (t, 1H, ArH), 7.40 (t, 1H, ArH), 7.61 (d, J = 8.0 Hz, 1H, ArH), 7.72 (d, J = 8.0 Hz, 1H, ArH), 8.04 (d, J = 8.0 Hz, 1H, ArH), 9.11 (s, 1H, CH = N), 13.96 (s, 1H, OH) (Fig. S1). ESI-MS m/z: [2M-H]+ calcd for C13H13NOS: 461.6; found, 461.1 (Fig. S2). Anal. calcd. for C13H13NOS: C 67.50, H 5.66, N 6.06, found C 67.52, H 4.75, N 6.01.
Synthesis of N-(2-hydroxynaphthylidene)-2-aminoethanethiol capped AuNPs (HNAET–AuNPs)
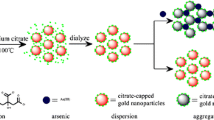
Citrate-capped AuNPs were prepared according to Frens’ method [26]. All glassware were soaked in aqua regia and carefully cleaned before use. Briefly, 100 mL of 10 mM HAuCl4 was taken into a round-bottomed flask and then boiled under vigorous stirring for 20 min. To this, 38.8 mM of trisodium citrate (10 mL) was added rapidly into the reaction flask and the mixture was stirred for another 15 min. The color of the solution changed from pale yellow to wine red, suggesting the formation of AuNPs. In order to remove excess sodium citrate, the citrate-reduced AuNPs were centrifuged for 15 min (1160×g) and then dissolved with Millipore-Q water. The concentration of the AuNPs was calculated according to Beer’s law by using an extinction coefficient of ca. 108 M−1 cm−1 at 520 nm [27] and found to be 15 nM. To obtain HNAET-AuNPs, ethanol solution of HNAET (20 μL, 0.1 mM) was added into 10 mL AuNPs to let stand for ligand exchange reaction for 3 h to allow the modification of HNAET on the AuNPs surface at room temperature as shown in Scheme 1b. The resulting AuNPs solution was used for the colorimetric assay of Al3+ in real samples.
Signal response of HNAET-AuNPs probe to Al3+
A stock solution of aluminum nitrate (1 mM) was prepared by dissolving aluminum nitrate in deionized water for the further use. For the detection of Al3+ by using the HNAET-AuNPs, 1.5 mL pH 6.0 HNAET-AuNPs (3 nM) aqueous solution was spiked with 0.5 mL Al3+ solutions to produce final concentrations in the range of 1.0–31 μM and the color of the solution changed from wine-red to purple then blue. The mixtures were then reacted at room temperature for 30 s and transferred for absorbance and photograph collection. The absorbance ratios of A620/A520 versus Al3+ concentrations were used for calibration. Furthermore, we also evaluated the stability of AuNPs before and after functionalization. It was noticed that citrate modified AuNPs are stable for 2 months and well agreed with the literature [28]. At the same time, HNAET-AuNPs are stable for 3–4 weeks under nitrogen; after that there is a slight color change due to hydrogen bonding formation between HNAET-AuNPs. However, the rapid color change of HNAET-AuNPs solution (red to blue) was attributed to the HNAET-AuNPs aggregation induced by Al3+ ion (1.0–31 μM).
Results and discussion
Characterization of the HNAET–AuNPs
Gold nanoparticles were prepared through the citrate-mediated reduction of HAuCl4. 1-(2-aminoethanethiol)-2-naphthol (HNAET) was added into the AuNPs solution as the capping agent. Figure 1A shows the UV–vis spectra of bare AuNPs and HNAET-AuNPs. It should be noted that the characteristic surface plasmon resonance (SPR) peak (520 nm) of AuNPs was slightly red shifted and the peak intensity was little decreased after functionalization of the HNAET molecules onto the surface of the AuNPs. We also investigated the batch-to-batch reproducibility when making HNAET-AuNPs, as shown in Fig. S3. The RSD is 2.32 % calculated by the absorbance at 520 nm, demonstrating good reproducibility of our approach for fabricating HNAET-AuNPs.
The characterization of decorated AuNPs was also studied in more details by using FTIR spectroscopy. FTIR spectrum of HNAET-AuNPs shows similar peaks characteristic to HNAET (Fig. 1B). It can be seen that for HNAET (Fig. 1B -a), -OH and -CH2 stretches at 3000–3500 and 2913 cm−1, respectively, and that the strongest absorption peak at 1629 cm−1 belongs to C = N stretching mode. These peaks can also be observed in the spectrum of HNAET-AuNPs (Fig. 1B -b). It can be noticed that the sulfhydryl group (-SH) stretching and bending modes were not observed at 2500–2600 cm−1 in the spectrum of HNAET, presumably due to the presence of the strongest absorption peak of C = N stretching. Besides, zeta potential of the AuNPs became less negative from −33.15 to −24.85 mV after functionalization, also indicating that some of citrates on the surface of the AuNPs were replaced by HNAET via ligand exchange because HNAET is nearly neutral. These results revealed that the HNAET molecules were successfully attached onto the surface of the AuNPs via Au–S bond. In addition, in Fig. 2B -a, the HNAET-AuNPs are well dispersed and from their size distribution (Fig. S4) according to the TEM image, the mean size of AuNPs is 12.4 ± 0.03 nm (100 particles counted).
The analyses of the ligand loading on surfaces of the AuNPs was measured by comparing the intensities at ca. 485 nm of supernatant solution of HNAET-AuNPs and pure HNAET solution before functionalization (Fig. S5). It is found that approximately 53 % of HNAET was bound onto the AuNPs, from which we can calculate the amount of ligands to be about 35 HNAET molecules per AuNP. We also investigated the long-term stability of HNAET-AuNPs under air and under nitrogen day to day, as shown in Fig. S6, ESM. It can be seen that HNAET-AuNPs showed higher stability under nitrogen than that under air since the later aggregated slightly after several days, which can be judged from the absorbance at 620 nm. Nevertheless, in view of fast response time to Al3+ (ca. 10 s), as demonstrated below, the relatively high stability of the AuNPs under air for about several days can absolutely fulfill the requirements in practical applications.
Detection mechanism for Al3+
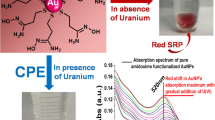
As show in Fig. 2A, for the initially well-dispersed HNAET-AuNPs, upon the addition of Al3+ (20 μM), the absorbance at 520 nm decreased greatly and a new absorption band at 620 nm appeared obviously. Accordingly, the color immediately changed from red to blue (inset in Fig. 2A). These changes were considered to be attributed to the aggregation of HNAET–AuNPs, which was also observed by TEM images (Fig. 2B-b). Furthermore, a gradual aggregation process of HNAET-AuNPs was also monitored by changes in the hydrodynamic diameter from particles to aggregates measured via DLS upon the addition of Al3+ at different concentrations (Fig. S7), in which the mean sizes of HNAET-AuNPs became larger gradually with the increase of Al3+ concentration.
Based on the above phenomenon, we suppose a possible mechanism to elucidate it. It is known that HNAET is a thiol-containing derivative of naphthol. After its functionalization onto AuNPs through Au-S bond, -OH and N atoms in C = N are effectively available to form a stable complex with Al3+, which eventually induced the aggregation of HNAET-AuNPs. Thus, a fast and efficient interparticle crosslinking of the HNAET–AuNPs occurred in the presence of Al3+. Scheme 1b summarizes the procedure for the working mechanism of the HNAET–AuNPs probe for Al3+.
Optimization of reaction conditions
It is significant to choose optimal reaction conditions that determine enough aggregation of AuNPs, so the following parameters were optimized: (a) HNAET concentration; (b) sample pH value; (c) reaction time. Respective data and Figures are given in Fig. S8 and S9 in the Electronic Supporting Material. The following experimental conditions were found to give best results: the HNAET concentration at 2 × 10−7 M was chosen as the optimal ligand concentration, the optimal pH range for detecting by HNAET-AuNPs is 4–6, and reaction time was set at 10 s.
Selectivity of HNAET-AuNPs toward Al3+
To investigate the analytical application of the HNAET-AuNPs toward various metal ions in aqueous solution, the UV–vis spectra of the synthesized HNAET-AuNPs were measured in the presence of metal ions such as Ag+, Cd2+, Co2+, Al3+, Cr3+, Cu2+, Fe3+, Hg2+, Mg2+, Mn2+, Ni2+, Pb2+, Ba2+, and Zn2+. Figure 3 shows the effect of the metal ions on the appearance of HNAET-AuNPs in solution. Al3+ was the only ion that caused a remarkable peak shift of AuNPs from 520 to 620 nm. This red shift was directly observed as a color change from red to blue. The other metal ions did not influence the absorption spectra, indicating that no aggregation occurred. We reasoned that, as demonstrated above, strong and specific binding of Al3+ between the naphthol on HNAET-AuNPs induced the aggregation of the HNAET-AuNPs in which the naphthol functions as a metal ion chelator (Scheme 1b).
In order to study the influence of other metal ions on Al3+ binding to HNAET-AuNPs, competitive experiments were also carried out with Al3+ (20 μM) in the presence of other metal ions, namely Ag+, Cd2+, Co2+, Al3+, Cr3+, Cu2+, Fe3+, Hg2+, Mg2+, Mn2+, Ni2+, Pb2+, Ba2+, and Zn2+ (Fig. 4). The plasmonic absorption shift caused by the mixture of Al3+ and another metal ion was similar to that caused solely by Al3+. This result clearly indicates that our method was free from interference from other metal ions, which should be noted for practical application to Al3+ assays in real samples.
Quantification of Al3+ by using HNAET-AuNPs as the colorimetric probe
The potential of the HNAET-Au NPs based UV–vis spectrometric method was demonstrated for the quantification of Al3+ in aqueous samples as a model analytical problem. At the optimized conditions, various concentrations of Al3+ were added into an HNAET-AuNPs solution and their UV-visible spectra were measured. Figure 5 shows that the color of the AuNPs in solution gradually changes from red via purple to blue on increasing the concentration of Al3+ which can be observed with bare eyes. Accordingly, the SPR peak of AuNPs at 520 nm decreased gradually, along with an increase of the SPR peak at 620 nm. Furthermore, a calibration curve was constructed between the absorbance ratio (A620/A520) and Al3+ concentration ranging from 9 to 23 μM (y = 0.0743x − 0.452, R 2 = 0.990) (Fig. S10), which can be used for the quantification of Al3+. The detection limit (LOD) is 0.287 μM, which is much lower than the level (7.4 μM) of drinking water defined by the World Health Organization [29].
In addition, we also compared the sensitivity of the present method with the reported nanoparticle-based UV–vis and fluorescence methods for detection of Al3+ (Table 1). It indicates that the present method showed higher sensitivity than the nanoparticle-based UV–vis [30–32] and fluorescence [33–35] methods. Therefore, the present analytical system exploits the advantages of HNAET-AuNPs for the detection of Al3+ by using UV–vis spectrometry.
Application to the analysis of Al3+ in real samples
The present HNAET-gold nanoparticle-based colorimetric assay has been applied to detect Al3+ in aluminium pan water and urine samples. For this, aluminium pan water samples were obtained from distilled water boiled in aluminium pan. Human urine samples were collected randomly from healthy people and diluted 100-fold with ultrapure water. The above real samples spiked with certain amounts of Al3+ were determined according to the standard curve, and the analytical results are shown in Table 2. The recovery values ranged from 90 to 97 %, with the relative standard deviation (RSD) lower than 2.5 %. It suggests that the present method may be used in the detection of Al3+ in water quality monitoring and in urine sample testing.
Conclusions
In summary, a simple, cost-effective colorimetric assay for Al3+ based on the HNAET modified AuNPs is developed via ligand exchange. It is demonstrated that high affinity of Al3+ toward the N atom and the –OH of the Schiff base induces the aggregation of HNAET–AuNPs accompanied by distinct solution color change. Absorbance varies linearly with the Al3+ concentration in the range from 9 to 23 μM, and the detection limit is 0.29 μM. Because this method can address intrinsic limitations of small molecule-based optical sensors, such as complex synthetic procedures, low-sensitivity and poor water compatibility, it shows great potential in selective quantification of Al3+ in routine laboratory practice or rapid on-site assay. Additionally, further study is going on to avoid non-aqueous phase organic synthesis leading to a more environmentally-friendly colorimetric assay.
References
Kepp KP (2012) Bioinorganic chemistry of Alzheimer’s disease. Chem Rev 112:5193–5239
Mendez-Aèlvarez E, Soto-Otero R, Hermida-Ameijeiras A, Lopez-Real AM, Labandeira-Garc JL (2001) Effects of aluminum and zinc on the oxidative stress caused by 6-hydroxydopamine autoxidation: relevance for the pathogenesis of Parkinson’s disease. Biochim Biophys Acta 1586:155–168
Wills MR, Savory J (1983) Aluminium poisoning: dialysis encephalopathy, osteomalacia, and anaemia. Lancet 2:29–34
Krejpcio Z, Wójciak RW (2002) The influence of Al3+ ions on pepsin and trypsin activity in vitro. Pol J Environ Stud 11:251–254
Frankowski M, Zioła-Frankowska A, Siepak J (2010) New method for speciation analysis of aluminium fluoride complexes by HPLC–FAAS hyphenated technique. Talanta 80:2120–2126
Chen B, Zeng Y, Hu B (2010) Study on speciation of aluminum in human serum using zwitterionic bile acid derivative dynamically coated C18 column HPLC separation with UV and on-line ICP-MS detection. Talanta 81:180–186
Wang H, Yu Z, Wang Z, Hao H, Chen Y, Wan P (2011) Preparation of a preplated bismuth film on Pt electrode and its application for determination of trace aluminum(III) by adsorptive stripping voltammetry. Electroanalysis 23:1095–1099
Arduini M, Felluga F, Mancin F, Rossi P, Tecilla P, Tonellato U, Valentinuzzi N (2003) Aluminium fluorescence detection with a FRET amplified chemosensor. Chem Commun 13:1606–1607
Maity D, Govindaraju T (2010) Pyrrolidine constrained bipyridyl-dansyl click fluoroionophore as selective Al3+ sensor. Chem Commun 46:4499–4501
Kim S, Noh JY, Kim KY, Kim JH, Kang HK, Nam SW, Kim SH, Park S, Kim C, Kim J (2012) Salicylimine-based fluorescent chemosensor for aluminum ions and application to bioimaging. Inorg Chem 51:3597–3602
Elghanian R, Storhoff JJ, Mucic RC, Letsinger RL, Mirkin CA (1997) Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 277:1078–1081
Liu J, Lu Y (2006) Preparation of aptamer-linked gold nanoparticle purple aggregates for colorimetric sensing of analytes. Nat Protoc 1:246–252
Lee JS, Ulmann PA, Han MS, Mirkin CA (2008) A DNA–gold nanoparticle-based colorimetric competition assay for the detection of cysteine. Nano Lett 8:529–533
Saha K, Agasti SS, Kim C, Li X, Rotello VM (2012) Gold nanoparticles in chemical and biological sensing. Chem Rev 112:2739–2779
Sung YM, Wu SP (2014) Colorimetric detection of Cd(II) ions based on di-(1H-pyrrol-2-yl) methanethione functionalized gold nanoparticles. Sensors Actuators B 201:86–91
Chai F, Wang CG, Wang TT, Li L, Su ZM (2010) Colorimetric detection of Pb2+ using glutathione functionalized gold nanoparticles. ACS Appl Mater Interfaces 2:1466–1470
Chen GH, Chen WY, Yen YC, Wang CW, Chang HT, Chen CF (2014) Detection of mercury(II) ions using colorimetric gold nanoparticles on paper-based analytical devices. Anal Chem 86:6843–6849
Mehta VN, Kumar MA, Kailasa SK (2013) Colorimetric detection of copper in water samples using dopamine dithiocarbamate-functionalized Au nanoparticles. Ind Eng Chem Res 52:4414–4420
Lee YF, Nan FH, Chen MJ, Wu HY, Ho CW, Chen YY, Huang CC (2012) Detection and removal of mercury and lead ions by using gold nanoparticle-based gel membrane. Anal Methods 4:1709–1717
Liu R, Chen ZP, Wang SS, Qu CL, Chen LX, Wang Z (2013) Colorimetric sensing of copper(II) based on catalytic etching of gold nanoparticles. Talanta 112:37–42
Guo Y, Wang Z, Qu W, Shao H, Jiang X (2011) Colorimetric detection of mercury, lead and copper ions simultaneously using protein-functionalized gold nanoparticles. Biosens Bioelectron 26:4064–4069
Li XK, Wang J, Sun LL, Wang ZX (2010) Gold nanoparticle-based colorimetric assay for selective detection of aluminium cation on living cellular surfaces. Chem Commun 46:988–990
Zhang M, Liu YQ, Ye BC (2012) Mononucleotide-modified metal nanoparticles: an efficient colorimetric probe for selective and sensitive detection of aluminum(III) on living cellular surface. Chem Eur J 18:2507–2513
Chang YJ, Hung PJ, Wan CF, Wu AT (2014) A highly selective fluorescence turn-on and reversible sensor for Al3+ ion. Inorg Chem Commun 39:122–125
Liu X, Lin Q, Wei TB, Zhang YM (2014) A highly selective colorimetric chemosensor for detection of nickel ions in aqueous solution. New J Chem 38:1418–1423
Frens G (1973) Controlled nucleation for the regulation of particle size in monodisperse gold suspensions. Nature 241:20–22
Huang CC, Chang HT (2007) Parameters for selective colorimetric sensing of mercury(II) in aqueous solutions using mercaptopropionic acid-modified gold nanoparticles. Chem Commun 75:1215–1217
Brewer SH, Glomm WR, Johnson MC, Knag MK, Franzen S (2005) Probing BSA binding to citrate-coated gold nanoparticles and surface. Langmuir 21:9303–9307
Guidelines for Drinking Water Quality (2004) World Health Organization, three edn. Geneva, 397, pp 301–311
Chen S, Fang YM, Xiao Q, Li J, Li SB, Chen HJ, Sun JJ, Yang HH (2012) Rapid visual detection of aluminium ion using citrate capped gold nanoparticles. Analyst 137:2021–2023
Xue DS, Wang HY, Zhang YB (2014) Specific and sensitive colorimetric detection of Al3+ using 5-mercaptomethyltetrazole capped gold nanoparticles in aqueous solution. Talanta 119:306–311
Yang NN, Gao YX, Zhang YJ, Shen ZY, Wu AG (2014) A new rapid colorimetric detection method of Al3+ with high sensitivity and excellent selectivity based on a new mechanism of aggregation of smaller etched silver nanoparticles. Talanta 122:272–277
Mu XY, Qi L, Qiao J, Ma HM (2014) One-pot synthesis of tyrosine-stabilized fluorescent gold nanoclusters and their application as turn-on sensors for Al3+ ions and turn-off sensors for Fe3+ ions. Anal Methods 6:6445–6451
Zhou TY, Lin LP, Rong MC, Jiang YQ, Chen X (2013) Silver–gold alloy nanoclusters as a fluorescence-enhanced probe for aluminum ion sensing. Anal Chem 85:9839–9844
Zou Y, Yan FY, Dai LF, Luo YM, Fu Y, Yang N, Wun JY, Chen L (2014) High photoluminescent carbon nanodots and quercetin-Al3+ construct a ratiometric fluorescent sensing system. Carbon 77:1148–1156
Acknowledgements
We gratefully acknowledge the financial support by Natural Science Foundation of China (no. 21365014, 21505067), Jiangxi Province Natural Science Foundation (JXNSF no. 20132BAB203011), and Doctoral Start-up Funding of Nanchang University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 787 kb)
Rights and permissions
About this article
Cite this article
Huang, P., Li, J., Liu, X. et al. Colorimetric determination of aluminum(III) based on the aggregation of Schiff base-functionalized gold nanoparticles. Microchim Acta 183, 863–869 (2016). https://doi.org/10.1007/s00604-015-1734-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1734-y