Abstract
The authors describe a method for amperometric determination of chloramine-T that is based on the indirect detection of chloramine-T by detecting p-quinone imine (p-QI) that is generated by oxidation of p-aminophenylboronic acid by chloramine-T. p-QI can be detected with excellent selectivity and at low potential by using a glassy carbon electrode. Hence, the method displays attractive features such as high sensitivity, wide detection range and excellent selectivity. The electrode has two linear responses in the 50 nM to 100 μM concentration range and a 6 nM detection limit. Compared to other electrochemical methods, this assay has a detection limit that is better by three orders of magnitude. The relative standard deviation is 3.4% for the determination of 10 μM of the medical chloramine-T sample, and the recovery of a samples containing chloramine-T at a level of 10 μM is 115%.

Highly sensitive electrochemical detection of chloramine-T is achieved based on the reaction of chloramine-T with p-aminophenylboronic acid with a detection limit of 6 nM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chloramine-T, also known as sodium N-chloro-4-methylbenzenesulfonamide, is a byproduct of saccharin fabrication. It is cheap, easy to handle and water-tolerant [1–5]. It plays very important roles in chemical engineering, environmental industries, as well as food and clinical medicine. For example, chloramine-T is disinfectant for external use, and is suitable for the disinfection of tableware, drinking water, vegetables, etc. [6]. It is used as a bleach and oxidative desizing agent in dyeing and printing industry [4]. Chloramine-T has been used for the detection of sulfonamides in pharmaceutical industry and tin [7], the preparation of indicator, and as chlorine reagent in the analysis work of laboratory [1, 3–5]. Obviously, the developments of detection methods for chloramine-T are of broad interest in synthetic chemistry and environmental sciences [1–5].
Many analytical methods for detecting chloramine-T in foodstuff, pharmaceutical, environmental and veterinary samples have been reported. These include cyclic voltammetry [2], differential pulse voltammetry [6], flow injection biamperometry [8], liquid chromatography combined with mass spectrometry [9], spectrophotometry [10]. Either expensive equipments and maintenance and time-consuming sample preparations are necessary or the detection limits are not low enough for most of these methods.
We find that chloramine-T can oxidize p-aminophenylboronic acid to p-QI. Since p-QI is a well-known compound that can be easily measured with excellent sensitivity and selectivity by amperometric method [11–14]. Herein, we report a novel strategy for the detection of chloramine-T at a low potential based on the oxidation of p-aminophenylboronic acid by chloramine-T to generate easily detectable p-QI. Figure 1 shows the detection scheme. Chloramine-T oxidizes p-aminophenylboronic acid to yield p-aminophenol. In contrast to hydrogen peroxide which cannot oxidize p-aminophenol to generate p-QI [15, 16], chloramine-T can further oxidize p-aminophenol to generate p-QI [17–22] because of its stronger oxidation capability. Finally, chloramine-T is measured through the detection of the reduction of p-QI at a low potential. The excellent advantage of this method is high sensitivity, wide linear range, mild reaction conditions and operational simplicity.
Experimental
Chemicals and reagents
p-Aminophenylboronic acid was obtained from Energy Chemical Co., Ltd., Shanghai. (http://www.energy-chemical.com.cn/). Chloramine-T, hydrogen peroxide, sodium chlorate, sodium perchlorate, ammonium sulfate, p-benzoquinone and bromine were purchased from Sinopharm Chemical Reagent Co., Ltd. (http://www.sinoreagent.com/). Chloramine-T medical powder was bought from Tianjin Bo Di Co., Ltd. (http://bdhg.company.lookchem.cn/). Sodium hypochlorite was purchased Aladdin (http://www.aladdin-e.com/). Iodine and potassium iodate were purchased from Beijing Chemical Works. (http://www.pvc123.com/b-beijinghuagong/). Artemisinine was purchased from TCI (Shanghai) Development Co., Ltd. (http://www.tcichemicals.com/zh/cn/). Other chemicals were all of analytical-reagent grade. The double distilled water was used throughout all the electrochemical experiments. All the experiments were carried out at room temperature conditions.
Instruments
A CHI 830B electrochemical Workstation (Shanghai Chenhua, China, http://chi.instrument.com.cn) was employed to carry out electrochemical experiments. The conventional three-electrode cell consisted of glassy carbon working electrode, a gold wire counter electrode and an Ag/AgCl reference electrode (saturated KCl). The working electrode was polished with alumina powder (Al2O3, 0.3 μm, 0.05 μm), sonicated and cleaned with doubly distilled water before measurements.
Procedure for the determination of chloramine-T
Chloramine-T was detected by amperometry. Firstly, the working electrode, counter electrode, and reference electrode were immersed in 5 mL of 0.1 M pH 5.5 acetate buffer containing 400 μM p-aminophenylboronic acid. Then the amperometric detection of chloramine-T was performed in stirring solution at the potential of 0 V by the successive addition of given volume of high concentrations of chloramine-T every 30 s. Specifically, 2.5, 2.5, 2.5, 2.5, 10, 10, 10, and 10 μL of 100 μM chloramine-T were injected sequently to the stirring solution for the detection of 50 nM, 100 nM, 150 nM, 200 nM, 400 nM, 600 nM, 800 nM, 1 μM chloramine-T, respectively. And then 1, 2, 2, 5, 5, 5, 5, 5, 5, 5, 5, and 5 μL of 10 mM chloramine-T were injected consecutively to the stirring solution for the detection of 2 μM, 6 μM, 10 μM, 20 μM, 30 μM, 40 μM, 50 μM, 60 μM, 70 μM, 80 μM, 90 μM, and 100 μM of chloramine-T, respectively.
Detection of chloramine-T in pharmaceutical samples
400 μL of 5.0 mM p-aminophenylboronic acid, 10 μL of 5.0 mM medical chloramine-T sample and 40 μL the double distilled water were pipetted into 4.55 L of 0.1 M acetate buffer (pH 5.5) , vortex-mixed and used for amperometry detection. To detect recoveries, a given amount of standard chloramine-T solution was added into the resulting solutions and used for amperometry measurements. The experiments were performed in triplicate.
Detection of chloramine-T in water samples
400 μL of 5.0 mM p-aminophenylboronic acid and 50 μL of tap water were pipetted into 4.55 L of 0.1 M acetate buffer (pH 5.5) , vortex-mixed and used for amperometry detection. To detect recoveries, a given amount of standard chloramine-T solution was added into tap water samples and used for amperometry measurements. The experiments were performed in triplicate. Similarly, the concentration of chloramine-T in lake water samples and the recoveries were determined by amperometric according to the procedures as mentioned above.
Results and discussion
Cyclic voltammograms of p-aminophenylboronic acid in the absence and presence of chloramine-T
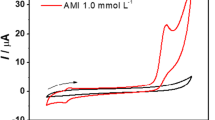
Figure 2a and b shows cyclic voltammograms of 1 mM p-aminophenylboronic acid and 10 μM chloramine-T in 0.1 M pH 5.0 acetate buffer solutions, respectively. No redox peak is observed for either p-aminophenylboronic acid or chloramine-T. A pair of well-defined redox peaks around 0 V appears when chloramine-T is added into p-aminophenylboronic acid solution. The potential differences between cathodic peak and anodic peak were determined to be 33 mV, which shows the good reversible feature of this system. The redox peaks result from p-QI which is generated from the oxidation of p-aminophenylboronic acid by chloramine-T.
Choice of the method and optimization of method
Amperometry, cyclic voltammetry, linear sweep voltammetry, differential pulse voltammetry and square wave voltammetry are frequently used as the electrochemical detection methods. In comparison with other electrochemical methods in which potentials are scanned during measurements, amperometry use a constant potential. By using a suitable potential, it is possible to achieve high selectivity with amperometry. Therefore, amperometry is selected for the detection of chloramine-T.
The following parameters were optimized: (a) Sample pH value; (b) operating potential; (c) reaction time; (d) p-aminophenylboronic acid concentration. Respective data and Figures are given in the Electronic Supporting Material. We found the following experimental conditions to give best results: (a) A sample pH value of 5.5; (b) an operating potential of 0 V; (c) a reaction time of 90 s; (d) a p-aminophenylboronic acid concentration of 400 μM.
Electrochemical detection of chloramine-T
Figure 3a displays a typical current-time plots [23, 24] at the bare glassy carbon electrode at the potential of 0 V upon the successive addition of chloramine-T into 0.1 M (pH = 5.5) acetate buffer solution containing 400 μM p-aminophenylboronic acid. The currents increase with increasing chloramine-T concentrations. As shown in Fig. 3b and c, two linear ranges between chloramine-T concentrations and currents (from 50 nM to 30 μM and from 40 μM to 100 μM) are obtained. The detection limit is 6 nM. A comparison of several analytical parameters of the method with those of previously reported papers is summarized in Table 1. It can be observed that the dynamic range of the method is wider and the detection limit of the method is about three orders of magnitude lower than that of other published electrochemical methods [2, 6, 8]. However, the present method needs the addition of p-aminophenylboronic acid which is more expensive than potassium iodide used in previous studies [8]. Considering its high sensitivity, the present electrochemical approach is a promising detection method for chloramine-T.
a Current-time response obtained at glassy carbon electrode upon successive addition of different concentrations of chloramine-T under stirring condition, 2 μM, 6 μM, 10 μM, 20 μM, 30 μM, 40 μM, 50 μM, 60 μM, 70 μM, 80 μM, 90 μM, and 100 μM, respectively. (Inset, 50 nM, 100 nM, 150 nM, 200 nM, 400 nM, 600 nM, 800 nM, and 1 μM, respectively.) b Corresponding calibration plot from 50 nM to 30 μM (Inset, enlarged plot from 50 nM to 1 μM ) and (c) Corresponding calibration plot from 40 μM to 100 μM. Applied potential, 0 V; 0.1 M pH 5.5 acetate buffer solutions containing 400 μM p-aminophenylboronic acid
Interference analysis and sample detection
An interference investigation was performed with the solution containing 100 μM of species, including hydrogen peroxide, sodium hypochlorite, sodium chlorate, sodium perchlorate, bromine, iodine, potassium iodate, ammonium sulfate, artemisinin and p-benzoquinone. As shown in Fig. 4, a remarkable signal increment was observed in the presence of chloramine-T. In contrast, the currents of hydrogen peroxide, sodium hypochlorite, sodium chlorate, sodium perchlorate, bromine, iodine, potassium iodate, ammonium sulfate, artemisinin and p-benzoquinone are nearly negligible except the current of p-benzoquinone. The above results suggest the excellent selectivity of our method for the determination of chloramine-T. Some compounds investigated react with p-aminophenylboronic acid, but do not generate p-quinone imine, and some may not react with p-aminophenylboronic acid (e.g. sodium perchlorate), which may result in good selectivity.
Selectivity for the detection of chloramine-T against different interfering species. a hydrogen peroxide, b sodium hypochlorite, c sodium chlorate, d sodium perchlorate, e bromine, f iodine, g potassium iodate, h ammonium sulfate, i artemisinin, j p-benzoquinone, k chloramine-T. The concentrations of all species are 100 μM. Applied potential, 0 V; 0.1 M pH 5.5 acetate buffer solutions containing 400 μM p-aminophenylboronic acid
To test its feasibility for practical applications, the present method was used to detect chloramine-T recoveries in commercially available pharmaceutical samples, lake water samples and tap water samples (in Table 2). Favorable recoveries are obtained. The results show that this study offers a promising method for the measurement of chloramine-T in real samples.
Conclusion
A novel amperometric method based on the oxidation of p-aminophenylboronic acid by chloramine-T was developed for the determination of chloramine-T. The method is more sensitive than other electrochemical analysis methods by about three orders of magnitude. The method does not need the modification of electrode. The simple method displays satisfactory analytical performance such as outstanding sensitivity, excellent selectivity, wide linear range and low detection limit. This method may be extended to detect other oxidants.
References
Manjunatha AS, Dakshayani S, Vaz N, Puttaswamy (2015) Oxidative conversion of anilines to azobenzenes with alkaline chloramine-T. Korean J Chem Eng 33(2):697–706
Sen S, Chattopadhyay S, Sarkar P (2015) Electrochemical sensing of tea polyphenols by chloramine-T modified electrodes: a new approach. J Electrochem Soc 163(3):B49–B55
Agnihotri G (2005) Chloramine-T (sodium N-chloro- p – toluenesulfonamide). Synlett (18):2857–2858
Vinod KN, Puttaswamy, Ninge Gowda KN (2009) Mechanistic aspects for the oxidation of sunset yellow dye by chloramine-T in presence of perchloric acid and in sodium hydroxide medium catalyzed by Os(VIII): a spectrophotometric kinetic approach. Inorg Chim Acta 362:2044–2051
Sukhdev AS, Puttaswamy (2012) Kinetic and mechanistic investigation of S-oxidation of ranitidine hydrochloride with chloramine-T in acid and alkaline media. Prog React Kinet Mec 37(1):42–58 (17)
Baranowska I, Bijak K (2010) Differential pulse voltammetry in analysis of disinfectants — 2-mercaptobenzothiazole, 4-chloro-3-methylphenol, triclosan, chloramine-T. Open Chem 8(6):1266–1272
Krishnamurthy N, Pullarao Y, Satyanarayana V (1974) Cacotheline as redox indicator in the volumetric determination of tin(II), titanium(III) and ascorbic acid with chloramine T. Fresenius. Z Anal Chem 272(5):367–367
Catalá Icardo M (2000) Flow injection biamperometric determination of chloramine-T in environmental, pharmaceutical and veterinary samples. Anal Chim Acta 407:187–192
Xi H, Ma Q, Wang C (2011) Determination of chloramine T in cosmetics by ultrasound-assisted Hydrolyzation-LC and validation by LC-MS/MS. J Instrumental Anal 30(8):907–911
Leggett DJ, Chen NH, Mahadevappa DS (1982) Flow injection analysis of aromatic sulphonyl Haloamines. Fresenius Z Anal Chem 311:687–690
Zhao W, Chen R, Dai P, Li X, Xu J, Chen H (2014) A general strategy for photoelectrochemical immunoassay using an enzyme label combined with a CdS quantum dot/TiO(2) nanoparticle composite electrode. Anal Chem 86(23):11513–11516
Park S, Kim J, Ock H, Dutta G, Seo J, Shin EC, Yang H (2015) Sensitive electrochemical detection of vaccinia virus in a solution containing a high concentration of L-ascorbic acid. Analyst 140(16):5481–5487
Selvaraju T, Das J, Han SW, Yang H (2008) Ultrasensitive electrochemical immunosensing using magnetic beads and gold nanocatalysts. Biosens Bioelectron 23(7):932–938
Amene Amani SK, Davood N (2012) Electrochemical oxidation of 4-(piperazin-1-yl)phenols in the presence of indole derivatives: the unique regioselectivity in the synthesis of highly conjugated bisindolyl- p-quinone derivatives. J Electroanal Chem 670:36–41
Liang F, Hu L, Li Y, Majeed S, Li H, Cai H, Yang X, Xu G (2013) Low-potential determination of hydrogen peroxide, uric acid and uricase based on highly selective oxidation of p-hydroxyphenylboronic acid by hydrogen peroxide. Sensor Actuat B-Chem 178:144–148
Hu L, Han S, Liu Z, Parveen S, Yuan Y, Xu G (2011) A versatile strategy for electrochemical detection of hydrogen peroxide as well as related enzymes and substrates based on selective hydrogen peroxide-mediated boronate deprotection. Electrochem Commun 13(12):1536–1538
La M, Feng Y, Yang C, Chen C (2014) Performances of p-aminophenol redox cycling by thiols and Tris(2-carboxyethyl)phosphine on Cysteamine- and 3- Mercaptopropionic acid-covered gold electrodes. Int J Electrochem Sci 9:6985–6992
Jagotamoy D, Kyungmin J, Jae Wook L, Yang H (2007) Electrochemical Immunosensor using p-aminophenol redox cycling by hydrazine combined with a low background current. Anal Chem 79:2790–2796
Xia N, Ma F, Zhao F, He Q, Du J, Li S, Chen J, Liu L (2013) Comparing the performances of electrochemical sensors using p-aminophenol redox cycling by different reductants on gold electrodes modified with self-assembled monolayers. Electrochim Acta 109:348–354
Tang J, Tang D, Su B, Huang J, Qiu B, Chen G (2011) Enzyme-free electrochemical immunoassay with catalytic reduction of p-nitrophenol and recycling of p-aminophenol using gold nanoparticles-coated carbon nanotubes as nanocatalysts. Biosens Bioelectron 26(7):3219–3226
Tang J, Tang D, Niessner R, Knopp D (2011) A novel strategy for ultra-sensitive electrochemical immunoassay of biomarkers by coupling multifunctional iridium oxide (IrO(x)) nanospheres with catalytic recycling of self-produced reactants. Anal Bioanal Chem 400(7):2041–2051
Walter A, Wu J, Flechsig GU, Haake DA, Wang J (2011) Redox cycling amplified electrochemical detection of DNA hybridization: application to pathogen E. coli bacterial RNA. Anal Chem Acta 689(1):29–33
Jia FF, Zhong H, Li XR, Zhu FX, Liu GQ, Cheng ZP, Guo LP (2015) Research on novel nonenzymatic ECL sensor using Au-HS/SO3H-PMO (et) nanocomposites for glucose detection. J Electroanal Chem 758:93–99
Zhu S, Fan L, Liu X, Shi L, Li H, Han S, Xu G (2008) Determination of concentrated hydrogen peroxide at single-walled carbon nanohorn paste electrode. Electrochem Commun 10(5):695–698
Acknowledgements
This project was kindly supported by the National Natural Science Foundation of China (No. 21475123), Jilin Provincial Science and Technology Department (No. 2015020403YY), Changchun city technology bureau (No. 14KG046), the Chinese Academy of Sciences (CAS)-the Academy of Sciences for the Developing World (TWAS) President’s Fellowship Programme, CAS-TWAS Postgraduate Fellowship and CAS President’s International Fellowship Initiative (PIFI).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 73 kb)
Rights and permissions
About this article
Cite this article
Hui, P., Gao, W., Nsabimana, J. et al. Amperometric detection of chloramine-T based on its reaction with p-aminophenylboronic acid. Microchim Acta 184, 687–691 (2017). https://doi.org/10.1007/s00604-016-2060-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-2060-8