Abstract
In this work, an analytical electrochemical method was developed using a glassy carbon electrode (GCE) for the determination of amiloride (AMI). To study the electrochemical behavior of AMI, the cyclic voltammetry and differential pulse voltammetry techniques were used, adding a AMI solution in 0.1 mol L−1 BR buffer solution (pH 2.0) to the electrochemical cell. A reversible AMI oxi-reduction process was revealed in the scan rate of + 0.4 to − 0.1 V. The DPV parameters optimized were the scan rate (10 mV s−1) and amplitude (70 mV). After parameters optimization, the linear working range was from 9.90 to 90.9 μmol L−1, with detection limit of 5.19 μmol L−1 and quantification limit of 17.3 μmol L−1. The validity of the electroanalytical method for the determination of AMI in biological fluids (urine and serum) was demonstrated. The proposed method is advantageous due to its good sensitivity, speed, simplicity, precision, and accuracy towards AMI quantification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amiloride hydrochloride (AMI; N-amino-3,5-diamino-6-chloropyrazine-2-carboxamide) is a diuretic drug that acts by blocking the epithelial sodium channels (ENaC). AMI is also classified as a potassium-sparing diuretic because it prevents the Na+ ions from being reabsorbed, increasing the reabsorption of potassium ions in the distal tubule and collecting duct [1, 2]. AMI is mainly indicated as a first-line treatment for cardiovascular diseases (CVDs). According to the World Health Organization, CVDs account for approximately 17.5 million deaths worldwide per year and are expected to reach 22.2 million deaths by 2030 [3]. However, the use of AMI in combination with another thiazide diuretic demonstrates great efficiency in the prevention of myocardial infarction, stroke, and hypertension control [4], and its chronic use is effective in reducing morbidity and mortality [5, 6].
AMI is also used to treat edema, such as liver cirrhosis and nephrotic syndrome. The use of AMI is prohibited in athletes by the International Olympic Committee because of its ability to dilute the concentration of other substances in the body through urination, as well as its rapid effect in decreasing body weight [1, 2, 7].
Although the efficiency of AMI in treating CVDs has been proven, according to Roush et al. [8], this diuretic is often not prescribed by clinicians due to the lack of studies on its determination after randomized trials. In this context, recent works have described the quantification of AMI in biological fluids by spectrophotometric [7], infrared [9], and chromatographic [10, 11] methods. However, these methods are less sensitive, effective, and accurate, and have a higher operating cost compared to electrochemical techniques [12].
Khorshed et al. [13] developed an electrochemical methodology to determine AMI in pharmaceutical formulations and human urine using differential pulse voltammetry and screen-printed graphite electrodes with a linear working range of 0.33–22.80 μmol L−1 and detection limit of 0.18 μmol L−1. Moraes et al. [14] studied the determination of AMI in pharmaceutical formulations and tap water by square-wave voltammetry using a boron-doped diamond electrode, obtaining a linear working range from 8.70 to 125 μmol L−1 and detection limit of 0.09 μmol L−1. Also using a boron-doped diamond electrode, Pereira et al. [1] performed the AMI determination in pharmaceutical formulations by batch injection analysis with multiple pulse amperometry, defining a linear working range of 3.00–160.00 mg L−1 and detection limit of 0.13 mg L−1. Zayed et al. [15] employed a carbon paste electrode associated with differential pulse voltammetry to obtain a working range of 0.60–4.23 μg mL−1 and a detection limit of 0.26 μg mL−1 for the determination of AMI in pharmaceutical formulations. An electrochemical method employing a Nafion/carbon nanotube-nano-composite film-modified glassy carbon electrode (NAF-CNT-GCE) was developed by Desai [16] for the determination of AMI in pharmaceutical formulations and biological fluids (urine and plasma). The linear working range and detection limit were 0.00907–50.60 μmol L−1 and 0.00702 μmol L−1, respectively. Hamman [17] used a hanging mercury drop electrode and found a linear concentration range from 0.002 to 0.20 μmol L−1 with limit of detection (LOD) and limit of quantification (LOQ) of 0.00019 and 0.00063 μmol L−1, respectively. The determination of AMI was performed in a pharmaceutical formulation and spiked in human serum. The LOD and LOQ of AMI spiked in human serum were 0.00057 and 0.0019 μmol L−1, respectively.
Thus, the objective of this work was to develop a simple and direct electroanalytical methodology for the determination of the antihypertensive AMI (in isolation) in human urine and serum using an unmodified glassy carbon electrode.
There are several studies in the literature that deal with the analysis of drugs using different types of carbon electrodes [18,19,20,21,22,23,24,25]. Some of these carbon electrodes show great results in analyzes when they are activated by electrochemical treatment, as the case of the use of electrochemically pretreated carbon electrode for application of uric acid reported by Huang et al. [24]. In addition, boron-doped diamond (BDD) electrodes, with or without electrochemical treatment, are also successfully used for analysis of a wide variety of molecules, including drugs [12]. With the use of the BDD electrode, they can cause a great improvement of analytical signal for drug analysis, when the BDD electrode surface is treated anodically or cathodically, due to the formation of oxygen or hydrogen species on the BDD surface [18, 26]. Treatments of activated carbon surfaces with sulfuric acid solution can cause an improvement in characteristics such as resistance and diffusion in these surfaces for analysis of oxygen-containing functional groups [22].
The great advantage of using carbon unmodified electrodes, such as graphitic carbons and glassy carbon in electroanalysis is due to the fact that the developed methods using these types of electrodes are ease-of-handling, fast, low-cost, shows good electrical conductivity, and high reproducibility [20]. In our work, only the glassy carbon electrode was used without any pre-treatment or preconditioning of the surface of this electrode.
Experimental section
Reagents and solutions
AMI was purchased from Sigma-Aldrich® (St. Louis, USA). A Britton-Robinson stock buffer (BR 0.1 mol L−1) was prepared by mixing equimolar amounts of phosphoric acid (85.0%; Dinamica®, Diadema, Brazil), acetic acid (99.8%; Proquimios®, Rio de Janeiro, Brazil), and boric acid (99.5%; Alphatec®, Macaé, Brazil). pH adjustment was performed using 1.0 mol L−1 sodium hydroxide solution (99.0%; Proquimios®, Rio de Janeiro, Brazil). A 1.0 mmol L−1 stock AMI solution was prepared using 0.0023 g of AMI dissolved in 10 mL of 0.1 mol L−1 BR buffer at pH 1.5. Ascorbic acid and uric acid were purchased from Isofar® (Duque de Caxias, Brazil). Citric acid was purchased from Vetec Química Fina® (Duque de Caxias, Brazil). All chemicals were of analytical grade, and all solutions were prepared with distilled water.
Apparatus
Voltammetric measurements were performed on an Autolab PGSTAT 128 N potentiostat/galvanostat (Metrohm Autolab B.V.®, Utrecht, The Netherlands) controlled by the NOVA® 1.11.0 electrochemical software. The electrochemical cell composed of three electrodes was assembled using a glassy carbon electrode as the working electrode, while the counter electrode was a 1.0 cm2 platinum plate and the reference electrode was the Ag/AgCl electrode 3 mol L−1 KCl. pH measurements were carried out with a Metrohm 827 pH Lab pH meter (Metrohm Autolab B.V®, Utrecht, The Netherlands) calibrated with standard buffer solutions at room temperature.
Analytical procedure
The electrochemical profile of AMI on the glassy carbon electrode was first studied using the cyclic voltammetry technique. Briefly, 10 mL of 0.1 mmol L−1 AMI solution in 0.1 mol L−1 BR buffer solution (pH 2.0) were added to the electrochemical cell, and the AMI electrochemical behavior was investigated at a scan rate of 50 mV s−1 from − 0.1 to + 1.6 V. Then, the following parameters were varied over the potential range from + 0.4 to − 0.1 V: accumulation potentials from 1.2 to 1.6 V, accumulation time from 10 to 80 s, pH from 1.0 to 4.0, and scan rate from 10 to 100 mV s−1.
Afterward, the AMI quantification by differential pulse voltammetry (DPV) was examined. The DPV parameters to be optimized were the scan rate ranging from 1 to 10 mV s−1 and amplitude from 10 to 100 mV. The linearity of the method was evaluated on three different days by the addition of ten AMI solution volumes with linear working range from 9.9 to 90.9 μmol L−1. An analytical curve was constructed, and the linear correlation coefficient was determined by linear regression. The 3σ/b ratio was used to determine the LOD, whereas the 10σ/b ratio was used to determine the LOQ, where b is the slope of the analytical curve and σ is the standard deviation of the white current averages. The accuracy of the method was assessed by inter and intraday repeatability voltammograms.
After optimization of the experimental parameters, the AMI differential pulse voltammograms were recorded to quantify the drug in human serum and urine.
Determination of AMI in human serum and urine
The developed voltammetric method was tested for AMI determination in human serum and urine. Samples of serum (1 mL) and human urine (10 mL) from a volunteer were collected and stored at a temperature of approximately 4 °C. The urine samples were fortified with the addition of 4760 μl, 6540 μl, and 9090 μl aliquots of the standard 1.0 mmol L−1 stock AMI solution, resulting the final AMI concentrations of 47.6 μmol L−1, 65.4 μmol L−1, and 90.9 μmol L−1, respectively. A 1000 μL aliquot of each AMI solution at the obtained concentrations was added to 10-ml volumetric flasks and the final volume was completed with 0.1 mol L−1 BR buffer (pH 1.5). These solutions were added separately to the electrochemical cell, and the AMI concentrations were determined by the standard addition method. For the determination of AMI in serum, the serum was pipetted into a 10-mL volumetric flask and the final volume was completed with 0.1 mol L−1 BR buffer solution (pH 1.5). Next, 1000, 2000, and 3000 μL aliquots of a standard stock AMI solution (30 mmol L−1) were added to the diluted serum to give final AMI concentrations of 30.0, 54.5, and 75.0 μmol L−1 for further electrochemical analysis.
Results
Cyclic voltammetry
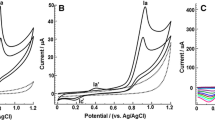
The cyclic voltammogram of the 0.1 mmol L−1 AMI solution is shown in Fig. 1. An irreversible oxidation peak at 1.30 V was observed, as described in the literature [16]. Furthermore, a reversible redox process dependent on oxidation at 1.30 V was found, with anodic peak (Epa) and cathodic peak (Epc) potentials at 0.15 V and 0.12 V, respectively. This process has not been reported in the literature yet, and it was chosen to develop the electrochemical methodology for AMI determination.
Because the reversible AMI oxidation process depends on the oxidation occurring at 1.30 V, as shown in the Fig. 2a, an accumulation potential (Eacc) of 1.40 V, accumulation time (tacc) of 30 s, and the direction of the potential scan were applied and evaluated as shown in the Fig. 2b.
Cyclic voltammograms of amiloride 1.0 mmol L−1 prepared in BR 0.1 mol L−1 (pH 2.0). Working electrode: glassy carbon. Auxiliary electrode: platinum. Reference electrode: Ag/AgCl. v = 50 mV s−1. a Scan rate: − 0.1 a 0.6 V. b Anodic scan rate: − 0.1 V to 0.4 V. Cathodic scan rate: 0.4 to − 0.1 V. Eacc: 1.4 V. tacc: 30 s
The cathodic scan direction was selected for further analysis because it presented higher values of peak oxidation and reduction currents. Then, to optimize the cyclic voltammetry parameters, the Eacc values were varied in the range from 1.20 to 1.60 V, keeping the accumulation time (tacc) at 30 s. The highest values of Ipa and Ipc were found at Eacc = 1.35 V. For the study of the tacc accumulation time in the range from 10 to 80 s, at the previously defined accumulation potential (Eacc), it was observed that the peak currents Ipa and Ipc increased with the increasing accumulation time up to 40 s, which was chosen for the further tests.
Regarding the influence of pH on the electrochemical AMI response in BR 0.1 mol L−1 buffer solution in the pH range from 1.0 to 4.0, it was observed that the highest values of Ipa and Ipc were obtained at pH 1.5, so this value was used in the additional experiments. Also, it was found that the anodic (Epa) and cathode (Epc) peak potentials became less positive as pH was increased, suggesting the H+ ions play a role in the AMI oxi-reduction reaction.
Also, it was found that the anodic (Epa) and cathode (Epc) peak potentials became less positive as pH was increased, suggesting the H+ ions play a role in the AMI oxi-reduction reaction. From the slop value of − 0.036 V pH −1 obtained from the Epa vs. pH and Epc vs. pH plots, the number of protons was estimated being equal to 1. Also, the number of electrons (n) involved in the oxi-reduction reaction was calculated considering the difference between Epa and Epc, shown in Fig. 2. This difference was approximately 40 mV, providing an electron number close to 1.
According to the results obtained, a possible oxi-reduction mechanism for AMI was proposed. In this mechanism, the AMI (structure I) is oxidized at 1.30 V to form the species shown in structure II. This result is consistent with the mechanism proposed in the work of Desai et al. [16]. Subsequently, it was proposed that the species II formed is protonated and then reduced to form a tertiary radical, relatively stable, in the potential of 0.12 V (species III), which is reoxidized in 0.15 V. Proposed mechanism is shown in Fig. 3.
The influence of scan rate variation was studied over the range from 10 to 100 mV s−1, as shown in Fig. 4a. Also, from the study of scan rate variation, it was possible to verify that there is a linear increase of the Ipa and Ipc currents with the increase of the square root of the scan rate (Fig. 4b). This result is consistent with to a reversible electrochemical process. Moreover, this linear plot of Ip vs. v1/2 for a reversible system reveals the possibility of a diffusive process. To prove this, a graph of log Ip vs. log v was plotted (Fig. 4c) showing that the slope of the log curves Ipa vs. log v and log Ipc vs. log v present values of 0.36 and 0.37, respectively. According to the literature [27], the theoretical value of 0.5 is the expected for an ideal diffusive process. However, the deviation from the theoretical value in this work can be justified due to the occurrence of a chemical step followed by an electrochemical step during the oxi-reduction process of AMI as showed in Fig. 3.
Differential pulse voltammetry
The pulse velocity (v) was studied in the scan rate from 1 to 10 mV s−1 and, according to the results, the value of 10 mV s−1 was chosen because it presented a well-defined peak, constant peak current, and maximum intensity.
The peak current increased linearly with the increasing pulse amplitude up to 70 mV without displacement and decreased signal resolution. This is amplitude value was set for further analysis.
Validation of the method
After optimization of the DPV parameters for the AMI determination, the AMI concentration was varied from 9.90 to 90.9 μmol L−1 under the conditions previously established for the construction of the analytical curve (inserted in Fig. 5).
In the analytical curve shown in the Fig. 5, it is possible to observe that there is a linear relation between the AMI concentration and the peak current intensity in the studied interval, this relation being expressed by the equation Ip (μA) = − 0.06 ± 0.02 [AMI]/μmol L−1 + 0.0120 ± 0.0005 (μA) with the correlation coefficient (R) equal to 0.99.
Using the value of b, which is the angular coefficient of the analytical curve, the LOD and LOQ values were calculated to be 5.19 μmol L−1 and 17.3 μmol L−1, respectively. These results demonstrate the good sensitivity of the proposed method.
The linear working range and LOD of electroanalytical method proposed here were close to those of other previously reported electroanalytical techniques (Table 1). This demonstrates that the glassy carbon electrode/DPV method can be effectively used to quantify AMI in human urine and serum.
Intraday and interday repeatability parameters of our electroanalytical method were analyzed at three display levels present within analytical curve interval (24.4, 47.6, and 90.9 μmol L−1). Comparing the repeated peak currents in the repeatability intraday (n = 10), the standard deviations (RSD) were 0.80%, 0.75%, and 0.42%, respectively. Likewise, the interday repeatability was evaluated by recording the peak currents (n = 5) of the defined concentrations, obtaining the RSD values of 4.86%, 1.37%, and 2.29%, respectively. All RSD values were below 5.0%, confirming the good accuracy of our electrochemical methodology.
In order to analyze the selectivity of the proposed methodology, a study was carried out on substances that are potentially interfering with human urine and serum (citric acid, uric acid, and ascorbic acid). It was noted that the acids did not interfere on the urine analysis. However, interference of ascorbic acid was observed on the quantification of AMI in the serum.
AMI determination in the human urine and serum was performed in triplicate to analyze the accuracy of the method. Addition and recovery tests with the addition of three known amounts of AMI were performed in the presence of these biological fluids.
The results are presented in the Supplementary Information (Tables 2 and 3). The recovery values were 82.3 ± 9.3% to 106.3 ± 10.8% for the serum and 99.2 ± 3.6% to 106.3 ± 4.0% for the urine, proving the good accuracy of the developed method.
Conclusion
This work enabled the development of a new electrochemical methodology based on GCE and differential pulse voltammetry (DPV) for the direct determination of AMI in human urine and serum. The proposed method presented a wide linear working range from 9.90 to 90.9 μmol L−1, low LOD (5.19 μmol L−1), and low LOQ (17.3 μmol L−1), demonstrating good sensitivity, good accuracy (RSD lower than 5.0%), and good accuracy (recovery values close to 100%). This GCE/DPV method is a fast and low-cost alternative for the determination of AMI in human urine and serum.
References
Pereira PF, da Silva WP, Marra MC, Muñoz RAA, Richter EM (2016) A high-throughput BIA-MPA method for the simultaneous determination of amiloride and furosemide. Analytical Methods 8(44):7959–7965
Brunton L, Knollman B, Hilal-Dandan R (2017) Goodman and Gilman’s the pharmacological basis of therapeutics, 13th edition: McGraw-Hill education
Organization WH (2016) Hearts: technical package for cardiovascular disease management in primary health care
Brown MJ, Williams B, Morant SV, Webb DJ, Caulfield MJ, Cruickshank JK, Ford I, McInnes G, Sever P, Salsbury J, Mackenzie IS, Padmanabhan S, MacDonald T, British Hypertension Society’s Prevention and Treatment of Hypertension with Algorithm-based Therapy (PATHWAY) Studies Group (2016) Effect of amiloride, or amiloride plus hydrochlorothiazide, versus hydrochlorothiazide on glucose tolerance and blood pressure (PATHWAY-3): a parallel-group, double-blind randomised phase 4 trial. The Lancet Diabetes & Endocrinology 4(2):136–147
MRC Working Party (1992) Medical Research Council trial of treatment of hypertension in older adults: principal results. BMJ 304(6824):405–412
Brown MJ, Palmer CR, Castaigne A, de Leeuw PW, Mancia G, Rosenthal T et al (2000) Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT). Lancet 356(9227):366–372
Chen W, Xiong Y, Wang W, Wu T, Li L, Kang Q et al (2019) Assembly of a UV-LED induced fluorescence system for rapid determination of amiloride in pharmaceutical tablet and human serum. Talanta 203:77–82
Roush GC, Ernst ME, Kostis JB, Yeasmin S, Sica DA (2016) Dose doubling, relative potency, and dose equivalence of potassium-sparing diuretics affecting blood pressure and serum potassium: systematic review and meta-analyses. J Hypertens 34(1):11–19
Tang J, Li X, Feng Y, Liang B (2016) Simultaneous determination of amiloride and hydrochlorothiazide in a compound tablet by diffuse reflectance spectroscopy and chemometrics. J Appl Spectrosc 83(4):710–716
Hu Y, Wu H-L, Yin X-L, Gu H-W, Kang C, Xiang S-X, Xia H, Yu RQ (2015) Chemometrics-assisted determination of amiloride and triamterene in biological fluids with overlapped peaks and unknown interferences. Bioanalysis 7(13):1685–1697
Naguib IA, Abdelaleem EA, Draz ME, Zaazaa HE (2015) Development and validation of RP-HPLC method for determination of hydrochlorothiazide, amiloride hydrochloride and related impurities in bulk and pharmaceutical dosage forms. Analytical Chemistry Letters 5(2):85–93
Ferraz BRL, Leite FRF, Batista BL, Malagutti AR (2016) Voltammetric determination of ethionamide in pharmaceutical formulations and human urine using a boron-doped diamond electrode. J Braz Chem Soc 27:677–684
Khorshed AA, Khairy M, Banks CE (2019) Electrochemical determination of antihypertensive drugs by employing costless and portable unmodified screen-printed electrodes. Talanta 198:447–456
Moraes JT et al (2017) Advanced sensing performance towards simultaneous determination of quaternary mixture of antihypertensives using boron-doped diamond electrode. Microchem J 134:173–180
Zayed SIM, Arida HAM (2013) Preparation of carbon paste electrodes and its using in voltammetric determination of amiloride hydrochloride using in the treatment of high blood pressure. Int J Electrochem Sci 8:1340–1348
Desai PB, Srivastava KA (2012) Determination of amiloride at Nafion–CNT-nano-composite film sensor employing adsorptive stripping differential pulse voltammetry. Sensors and Actuators B 169:341–348
Hamman E (2004) Behavior and quantification studies of amiloride drug using cyclic and square-wave adsorptive stripping voltammetry at a mercury electrode. J Pharm Biomed Anal 34:1109–1116
Neufeld AK, O'Mullane AP (2006) Effect of the mediator in feedback mode-based SECM interrogation of indium tin-oxide and boron-doped diamond electrodes. J Solid State Electrochem 10:808–816
Holloway AF et al (2008) The influence of edge-plane defects and oxygen-containing surface groups on the voltammetry of acid-treated, annealed and “super-annealed” multiwalled carbon nanotubes. J Solid State Electrochem 12:1337–1348
Barrière F, Downard AJ (2008) Covalent modification of graphitic carbon substrates by non-electrochemical methods. J Solid State Electrochem 12:1231–1244
Tenent RC, Wipf DO (2009) Local electron transfer rate measurements on modified and unmodified glassy carbon electrodes. J Solid State Electrochem 13:583–590
Liu X et al (2011) Effect of oxygen-containing functional groups on the impedance behavior of activated carbon-based electric double-layer capacitors. J Solid State Electrochem 15:413–419
Thangaraj R, Kumar AS (2013) Simultaneous detection of guanine and adenine in DNA and meat samples using graphitized mesoporous carbon modified electrode. J Solid State Electrochem 17:583–590
Huang D et al (2015) The determination of uric acid in human body fluid samples using glassy carbon electrode activated by a simple electrochemical method. J Solid State Electrochem 19:435–443
Lota G et al (2016) The application of activated carbon modified by ozone treatment for energy storage. J Solid State Electrochem 20:2857–2864
Granger MC, Swain GM (1999) The influence of surface interactions on the reversibility of Ferri/Ferrocyanide at boron-doped diamond thin-film electrodes. J Electrochem Soc 146:4551–4558
Bard AJ, Faulkner LR. Electrochemical methods: fundamentals and applications. 2 ed. N Y
Acknowledgments
The authors thank FINEP for the financial support and CAPES for the scholarship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Nascimento, T.O., Leite, F.R.F., Mourão, H.A.J.L. et al. Development of an electroanalytical methodology using differential pulse voltammetry for amiloride determination. J Solid State Electrochem 24, 1735–1741 (2020). https://doi.org/10.1007/s10008-020-04559-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04559-5