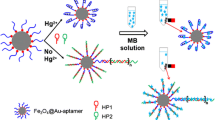
Abstract
The article describes a bienzyme visual system for aptamer-based assay of Hg(II) at nanomolar levels. The detection scheme is based on the finding that Hg(II) ions captured by aptamer-functionalized magnetic beads are capable of inhibiting the enzymatic activity of uricase and thus affect the formation of H2O2 and the blue product, i.e., oxidized tetramethylbenzidine. This strategy allows for a visual detection of Hg(II) at nanomolar levels without additional amplification procedure. Measuring the absorbance at 650 nm, the logarithmic calibration plot is linear in the concentration range of 0.5–50 nM and the limit of detection (LOD) is 0.15 nM. This is as low as the LOD obtained by atomic fluorescence spectrometry (AFS). The ions K+, Mg2+, Na+, Ca2+, Cu2+, Zn2+, Fe3+, Al3+, Co2+, AsO2 −, Ni2+, Cd2+ and Pb2+ do not have a significant effect on color formation. The method was applied to the analysis of (spiked) river water, lake water, mineral water, tap water and certified reference water samples, and the results agreed well with those obtained by AFS or certified values, with recoveries ranging from 97% to 109%. The relative standard deviation for five parallel detections at a 10 nM Hg(II) level is 5.2%.

A bienzyme-based visual aptasensor was fabricated for label-free detection of nanomolar Hg2+ in water samples without any amplification or enrichment procedure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hg2+ can result in DNA mutation, disruption of biological events at the cellular level, damage of the liver and kidney, and even death, and thus it was considered to be an important environmental pollutant. The upper limit of Hg2+ mandated by United States Environmental Protection Agency (EPA) guidelines is 10 nM (2 ppb) in drinking water [1]. Additionally, indirect exposure caused by eating Hg2+-tainted fish or other aquatic products has also been considered as a common route that leads to the toxic effects of Hg2+. Therefore, it is highly desirable to develop a sensitive and selective Hg2+ detection method that can provide simple, practical, and high-throughput routine determination of trace levels of Hg2+ ions in water samples.
Currently, the widely used methods for Hg2+ detection are atomic spectrometry-based approaches, such as atomic absorption spectroscopy, cold vapor generation atomic fluorescence spectrometry (CVG-AFS), and inductively coupled plasma atomic emission spectrometry (ICP-OES) and mass spectrometry (MS) [2]. These methods, although offered the advantages of high accuracy and selectivity, required sophisticated and expensive instrumentation and skilled personnel, which are inappropriate for point-of-use applications. To overcome these drawbacks, much effort has been devoted towards the design of a variety of sensing systems, such as organic chromophores or fluorophores [3, 4], conjugated polymers [5], gold or silver nanoparticles [6–9], upconverting nanoparticles [10], magnetic fluorescence probe [11], etc. for detec-tion of Hg2+ ions. However, most of these methods suffered from limitations such as poor selectivity with interference from closely related metals, insufficient sensitivity, etc.
Aptamer-assay has been considered as a new emerging approach for selective detection of mercury because it can specifically interact with thymine bases to form strong and stable thymine-Hg2+-thymine complexes (T-Hg2+-T) [12]. The high stability of T-Hg2+-T base pair have boosted a large number of fluorescent [13, 14], chemiluminescent [15, 16], electrochemical [17, 18] and colorimetric assays [19, 20]. Among these Hg2+ sensors, colorimetric aptasenosors have attracted particularly much attention for point-of-use applications, since the target recognition event can be determined visually. The reported colorimetric method for Hg2+ detection is mainly based on gold nanoparticles (AuNps), since the color was readily changed by aggregation or deaggregation of AuNps during the target recognition [20–24]. The visual process was can be also realized by inhibition of the G-quadraplex DNAzyme function via T-Hg2+-T [19, 25]. Besides, we also tried to use photocatalytic oxidation of TMB to visual assay of Hg2+ [26]. Although these colorimetric Hg2+ sensors showed the obvious advantage of simplicity, it is difficult for them to distinguish the color change of 10 nM Hg2+ (upper limit of Hg2+ in drinking water) except the use of an extra amplification step [24, 27].
Bienzyme reaction system has attracted much attention because the substrate of the latter enzymatic reaction can be produced on-line by the former enzymatic reaction [28, 29]. By utilizing the efficient bienzyme reaction system, we also have developed ultrasensitive chemiluminescence resonance energy transfer (CRET) biosensor for detection of glucose, cholesterol, and benzylamine [30]. Thus, the use of bienzyme catalytic coloration is also expected to be a promising visual detection scheme for aptamer-assay. We found the coloration of the bienzyme (i.e., uricase and HRP)-TMB system to be inhibited by Hg2+. This effect can be used for aptamer-based assay of Hg2+ at nanomolar levels without an extra amplification (Fig. 1).
Experimental
Reagents
3,3′,5,5′-Tetramethylbenzidine (TMB), uric acid and urea-formaldehyde magnetic microspheres (10 mg⋅ml-1, 1–2 µm in diameter) were purchased from Aladdin (Shanghai, China, www.aladdin-e.com). Sodium hydroxide, hydrochloric acid, dimethyl sulfoxide (DMSO) and phosphate (KH2PO4) were obtained from Kelong Reagent Co. (Chengdu, China, kelonghg.51pla.com). Uricase and horseradish peroxidase(HRP) were provided by Sangon Biotech (Shanghai, China, www.sangon.com). Mercury standard sample (GSBZ50016–90) was obtained from National Research Center for Standard Materials (Beijing, China, www.ncatn.com ). The Oligonucleotides (5′-NH2-TTCTTTCTTCCCCTTGTTTGTT-3′) for recognition of Hg2+ were also provided by Shanghai Sangon Biotech Co., Ltd. (Shanghai, China, www.sangon.com).
Preparation of aptamer-functionalized magnetic beads
First, 200 μL of magnetic beads (MBs) were diluted to 1 mL in phosphate buffer. 100 μL of 100 μM amine modified Hg2+ aptamer was added to the diluted MBs and vortexed briefly. Ten milligrams of EDC was then added to the MBs/aptamer and vortexed for overnight. The particles were then washed three times using the phosphate buffer, and resuspended in 2 mL of the phosphate buffer. The aptamer-functionalized magnetic beads (AFMBs) were stored at 4 °C prior to use.
Analytical protocol
40 μL of 40 μg⋅mL−1 AFMBs were added to 1.5 mL of Hg2+ standard solutions or samples and incubated for 60 min to recognition of Hg2+; then, the AFMBS were deposited with a magnet and washed twice by phosphate buffer; the Hg2+ ions were desorbed by addition of 100 μL 0.1 M HCl; after 20 min, 330 μL of 0.08 mg⋅mL−1 uricase in phosphate buffer (0.1 M, pH 7.0) were added and the enzymatic activity of uricase was inhibited for 30 min by desorbed Hg2+; 40 μL Uric acid (0.168 mg⋅mL−1 or 1.68 U⋅mL−1) were added to the solution for generation of H2O2 under the catalysis of the uninhibited uricase; the 160 μL of 0.1 M HCl, 40 μL of 0.8 μg⋅mL−1 (or 0.2 U⋅mL−1 ) HRP and 30 μL of 0.1 mg⋅mL−1 TMB were added for coloration. The absorbance was measured at 650 nm. Here, it is worthy of noting that the uricase solution should be freshly prepared daily.
Results and discussion
Design of bienzyme visual aptamer-assay for detection Hg2+
At first, monoenzyme, i.e., HRP was used to catalyze the coloration of TMB. However, the HRP catalytic activity did not changed in the present of Hg2+ (Fig. 2a), and thus was unable to be applied for the visual readout of Hg2+. Further experiments showed that Hg2+ ions in 10 nM concen-tration inhibit the enzymatic activity of uricase. This effect leads to a retarded rate of H2O2 formation in the presence of uricase substrate and slower rate of the chromogenic reaction of TMB as can be seen in Fig. 2a. Thus, the uricase-HRP-TMB system can be used for visual assay of Hg2+. It has been proved that Hg2+ was be readily react with –SH or –NH2 contained compounds [31]. Hence, we infer that Hg2+ inhibited the catalytic activity of uricase via interacting with –SH or –NH2 in amino acid residues, but the specific interaction mechanism remains to be further investigated.
a The inhibition of uricase enzymatic activity by Hg2+; and b the color read-out of bienzyme-based visual assay. Experiment conditions: a Hg2+ concentration, 10 nM; solution pH, 4.5; H2O2 generation time, 20 min; Uric acid amount, 6.72 μg; TMB concentration: 0.01 mg⋅mL−1; and b sample solution pH, 7.0; amount of AFMBs, 40 μg; Hg2+ capture time, 10 min; and other conditions were the same as in a
Figure 2b shows that the Hg2+ ions specifically captured and separated by AFMBs inhibit the coloration of the system; the small amount of Hg2+ ions adsorbed by MBs, in contrast, lead to a much lesser inhibition. This bienzyme-based aptamer assay possessed the advantages of high selectivity (aptamer recognition) and satisfying sensitivity (signal amplified by bienzyme).
Parameters affecting visual assay of Hg2+
The following parameters were optimized: (a) time for H2O2 generation; (b) sample pH value; (c) amount of AFMB; (d) time for capturing Hg2+ and (e) inhibition time by Hg2+. Respective data and Figures are given in the Electronic Supporting Material (Figs. S1–S5). We found the following experimental conditions to give best results: (a) 30 min for H2O2 generation; (b) sample pH value of 7.0; (c) 40 μg of AFMB; (d) 60 min for capturing Hg2+; and 30 min for inhibition time by Hg2+.
Analytical performance of aptamer-assay for detection of Hg2+
A series of Hg2+ standard solutions were captured by AFMBs, and then detected by bienzyme-TMB coloration system. The aptamer-assay system permitted color discrimination with a minimal concentration of 2.5 nM (inset of Fig. 3). It should be noted that the toxic level for Hg2+ defined by the US Environmental Protection Agency in drinkable water is below 10 nM. Hence, the resultant color change enables a differentiation between target-containing and target-free samples via visual inspection. Further using spectrophotometry, Fig. 3 shows that the absorbance increases linearly with the logarithm of Hg2+ concentration in the range of 0.5–50 nM, and the limit of detection (3σ) can be calculated to be 0.15 nM (about 0.03 ng⋅mL−1). Table 1 shows that this approach is much more sensitive than the reported colorimetric aptamer-assay without an amplification/enrichment procedure and even comparable to AFS methods. Besides, this assay eliminates the tedious procedure of labeling. The reproducibility was also examined using 10 nM of Hg2+, and the relative standard deviation (RSD) for five parallel detections was 5.2%.
The visual performance and linearity of bienzyme-AFMBs system for detection of Hg2+. Experiment conditions: H2O2 generation time, 20 min; Uric acid amount, 6.72 μg; TMB concentration, 0.01 mg⋅mL−1; sample solution pH, 7.0; amount of AFMBs, 40 μg; and Hg2+ capture time, 60 min; analytical wavelength, 650 nm
Interference study
The specificity of the assay was investigated by using other metal ions in place of Hg2+. The potentially interfering ions such as K+, Mg2+, Na+, Ca2+, Cu2+, Zn2+, Fe3+, Al3+, Co2+, AsO2 −, Ni2+, Cd2+ and Pb2+ were used at concentrations of 1000 nM, and the concentration of Hg2+ was chosen to be 10 nM (100 times lower than the interfering ions). Hg2+ (10 nM) led to an obvious absorbance decrease (more than 50%), while other metallic ions (1000 nM) had no significant effects (Fig. 4 and Fig. S6). It demonstrated good specificity of this bienzyme-based assay, which has more selective for the recently reported aptamer-assay for Hg2+ detection [34].
The specificity of the bienzyme-AFMBs system. The experimental conditions were the same as Fig. 3
Sample analysis
To estimate its real application, this assay was applied for analysis of various water samples, i.e., river water, lake water, mineral water, tap water and certified water (GSBZ50016–90) samples, and the color change for these samples were shown in Fig. 5. The Hg2+ concentrations of mineral, tap, lake and river waters were found to be <10 nM (the toxic level for Hg2+ defined by the EPA in drinkable water) and the Hg2+ concentration in certified water sample (GSBZ50016–90) was higher than 10 nM (Table 2). The results coincided with those obtained by AFS or certified value.
The pictures of analyzing water samples by the visual assay. The experimental conditions were the same as Fig. 3
Using spectrometry, a more quantitative analysis of Hg2+can be made, and the results were in good agreement with those obtained by AFS or certified value (Table S1). The recoveries for the river water, lake water, mineral water, tap water were in the range of 97–109% (Table S2). These results indicated that this system might be a promising tool for fast and convenient detection of Hg2+ in water samples.
Conclusion
We have developed a bienzyme aptamer-assay for ultrasensitive visual detection of Hg2+ in water samples. The efficient inhibition of uricase activity by Hg2+ provided the assay with high sensitivity, allowing detection of Hg2+ at nanomolar level without an extra amplification procedure. The capture of Hg2+ by AFMBs contributed greatly to the high specificity of the system. As a result, 100-fold of potential coexisting metal ions did not yield obvious interference. Simplicity, high sensitivity and selectivity were the main benefits of our assay. By using aptamers selective for other metal ion, this detection scheme may be applied to ions such as Pb2+, Ag+ and the like. Therefore, the bienzyme-AFMBs assay was an appealing tool for fast detection of metal ion pollutants in water samples.
References
US Environmental Protection Agency (2001) Fact Sheet Mercury Update: Impact on Fish Advisories. http://www.epa.gov/ostwater/fish/mercury.html
Gao Y, Shi Z, Long Z, Wu P, Zheng C, Hou X (2012) Determination and speciation of mercury in environmental and biological samples by analytical atomic spectrometry. Microchem J 103:1–14
Metivier R, Leray I, Valeur B (2004) Lead and mercury sensing by calixarene-based fluoroionophores bearing two or four dansyl fluorophores. Chemistry 10:4480–4490
Coronado E, Galan-Mascaros JR, Marti-Gastaldo C, Palomares E, Durrant JR, Vilar R, Gratzel M, Nazeeruddin MK (2005) Reversible colorimetric probes for mercury sensing. J Am Chem Soc 127:12351–12356
Shi HF, Liu SJ, Sun HB, Xu WJ, An ZF, Chen J, Sun S, Lu XM, Zhao Q, Huang W (2010) Simple conjugated polymers with on-chain phosphorescent iridium(III) complexes: toward ratiometric chemodosimeters for detecting trace amounts of mercury(II). Chemistry 16:12158–12167
Huang CC, Chang HT (2007) Parameters for selective colorimetric sensing of mercury(II) in aqueous solutions using mercaptopropionic acid-modified gold nanoparticles. Chem Commun (Camb) 1215–1217
Lin CY, Yu CJ, Lin YH, Tseng WL (2010) Colorimetric sensing of silver(I) and mercury(II) ions based on an assembly of tween 20-stabilized gold nanoparticles. Anal Chem 82:6830–6837
Li L, Gui L, Li W (2015) A colorimetric silver nanoparticle-based assay for Hg(II) using lysine as a particle-linking reagent. Microchim Acta 182:1977–1981
Chen Y, Yao L, Deng Y, Pan D, Ogabiela E, Cao J, Adeloju SB, Chen W (2015) Rapid and ultrasensitive colorimetric detection of mercury(II) by chemically initiated aggregation of gold nanoparticles. Microchim Acta 182:1–8
Zayakhuu G, Huy BT, Chung JW, Lee YI (2015) Selective detection of Hg2+ ion using Upconversion luminescent nanoparticles. Bull Kor Chem Soc 36:1307–1308
Wu J, Jiang W, Deng A, Shen Y, Tian R (2016) Facile synthesis of magnetic fluorescence probe for recyclable displacement detection of Hg2+ in aqueous solutions and living cells. Sensor Actuat B-Chem 234:691–702
Lee JS, Han MS, Mirkin CA (2007) Colorimetric detection of mercuric ion (Hg2+) in aqueous media using DNA-functionalized gold nanoparticles. Angew Chem Int Ed 46:4093–4096
Li M, Zhou X, Ding W, Guo S, Wu N (2013) Fluorescent aptamer-functionalized graphene oxide biosensor for label-free detection of mercury(II). Biosens Bioelectron 41:889–893
Xu JP, Song ZG, Fang Y, Mei J, Jia L, Qin AJ, Sun JZ, Ji J, Tang BZ (2010) Label-free fluorescence detection of mercury(II) and glutathione based on Hg2+ −DNA complexes stimulating aggregation-induced emission of a tetraphenylethene derivative. Analyst 135:3002–3007
Cai S, Lao K, Lau C, Lu J (2011) “Turn-on” chemiluminescence sensor for the highly selective and ultrasensitive detection of Hg2+ ions based on Interstrand cooperative coordination and catalytic formation of gold nanoparticles. Anal Chem 83:9702–9708
Liu F, Wang S, Zhang M, Wang Y, Ge S, Yu J, Yan M (2014) Aptamer based test stripe for ultrasensitive detection of mercury(II) using a phenylene-ethynylene reagent on nanoporous silver as a chemiluminescence reagent. Microchim Acta 181:663–670
Huang RF, Liu HX, Gai QQ, Liu GJ, Wei Z (2015) A facile and sensitive electrochemiluminescence biosensor for Hg2+ analysis based on a dual-function oligonucleotide probe. Biosens Bioelectron 71:194–199
Tang J, Huang Y, Zhang C, Liu H, Tang D (2016) DNA-based electrochemical determination of mercury(II) by exploiting the catalytic formation of gold amalgam and of silver nanoparticles. Microchim Acta 183:1805–1812
Li T, Li B, Wang E, Dong S (2009) G-quadruplex-based DNAzyme for sensitive mercury detection with the naked eye. Chem Commun 3551–3553
Wang Y, Yang F, Yang X (2010) Colorimetric biosensing of mercury(II) ion using unmodified gold nanoparticle probes and thrombin-binding aptamer. Biosens Bioelectron 25:1994–1998
Xia F, Zuo X, Yang R, Xiao Y, Kang D, Vallee-Belisle A, Gong X, Yuen JD, Hsu BB, Heeger AJ, Plaxco KW (2010) Colorimetric detection of DNA, small molecules, proteins, and ions using unmodified gold nanoparticles and conjugated polyelectrolytes. Proc Natl Acad Sci U S A 107:10837–10841
Li L, Li B, Qi Y, Jin Y (2009) Label-free aptamer-based colorimetric detection of mercury ions in aqueous media using unmodified gold nanoparticles as colorimetric probe. Anal Bioanal Chem 393:2051–2057
He Y, Zhang X, Zeng K, Zhang S, Baloda M, Gurung AS, Liu G (2011) Visual detection of Hg(2)(+) in aqueous solution using gold nanoparticles and thymine-rich hairpin DNA probes. Biosens Bioelectron 26:4464–4470
Chen J, Zho S, Wen J (2014) Disposable strip biosensor for visual detection of Hg(2+) based on Hg(2+)-triggered toehold binding and exonuclease III-assisted signal amplification. Anal Chem 86:3108–3114
Li T, Dong S, Wang E (2009) Label-free colorimetric detection of aqueous mercury ion (Hg2+) using Hg2+ −modulated G-quadruplex-based DNAzymes. Anal Chem 81:2144–2149
Zhang X, Huang C, Xu S, Chen J, Zeng Y, Wu P, Hou X (2015) Photocatalytic oxidation of TMB with the double stranded DNA–SYBR green I complex for label-free and universal colorimetric bioassay. Chem Commun 51:14465–14468
Wang L, Liu F, Sui N, Liu M, Yu WW (2016) A colorimetric assay for Hg(II) based on the use of a magnetic aptamer and a hybridization chain reaction. Microchim Acta. doi:10.1007/s00604-016-1932-2
Rasmussen M, Ritzman RE, Lee I, Pollack AJ, Scherson D (2012) An implantable biofuel cell for a live insect. J Am Chem Soc 134:1458–1460
Xu S, Qi H, Zhou S, Zhang X, Zhang C (2014) Mediatorless amperometric bienzyme glucose biosensor based on horseradish peroxidase and glucose oxidase cross-linked to multiwall carbon nanotubes. Microchim Acta 181:535–541
Xu S, Li X, Li C, Li J, Zhang X, Wu P, Hou X (2016) In situ generation and consumption of H2O2 by bienzyme-quantum dots Bioconjugates for improved chemiluminescence resonance energy transfer. Anal Chem 88:6418–6424
Hadavifar M, Bahramifar N, Younesi H, Qin L (2014) Adsorption of mercury ions from synthetic and real wastewater aqueous solution by functionalized multi-walled carbon nanotube with both amino and thiolated groups. Chem Eng J 237:217–228
Liu CW, Hsieh YT, Huang CC, Lin ZH, Chang HT (2008) Detection of mercury(II) based on Hg2+ −DNA complexes inducing the aggregation of gold nanoparticles. Chem Commun (Camb) 2242–2244
Zhang R, Peng M, Zheng C, Xu K, Hou X (2016) Application of flow injection–green chemical vapor generation–atomic fluorescence spectrometry to ultrasensitive mercury speciation analysis of water and biological samples. Microchem J 127:62–67
Hu TY, Yan X, Na WD, Su XG (2016) Aptamer-based aggregation assay for mercury(II) using gold nanoparticles and fluorescent CdTe quantum dots. Microchim Acta 183:2131–2137
Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (Nos. 21475013 and 21305009), China Postdoctoral Science Foundation (Nos. 2015 M570773 and 2016 T90840), and the scientific research and innovation team in University of Sichuan Provincial Department of Education (15TD0009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 1110 kb)
Rights and permissions
About this article
Cite this article
Zhang, R., Deng, L., Zhu, P. et al. Bienzyme-based visual and spectrophotometric aptamer assay for quantitation of nanomolar levels of mercury(II). Microchim Acta 184, 541–546 (2017). https://doi.org/10.1007/s00604-016-2033-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-2033-y