Abstract
A rapid and sensitive aptamer-based assay is described for kanamycin, a veterinary antibiotic with neurotoxic side effects. It is based on a novel FRET pair consisting of fluorescent carbon dots and layered MoS2. This donor-acceptor pair (operated at excitation/emission wavelengths of 380/440 nm) shows fluorescence recovery efficiencies reaching 93 %. By taking advantages of aptamer-induced fluorescence quenching and recovery, kanamycin can be quantified in the of 4–25 μM concentration range, with a detection limit of 1.1 μM. The method displays good specificity and was applied to the determination of kanamycin in spiked milk where it gave recoveries ranging from 85 % to 102 %, demonstrating that the method serves as a promising tool for the rapid detection of kanamycin in milk and other animal-derived foodstuff.

A fluorometric aptasensor was developed for the determination of kanamycin. It is based on a novel FRET pair of carbon dots and layered MoS2. The fluorescence recovery efficiency reached 93 % with a good sensitivity, specificity and recoveries in spiked milk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kanamycin, as an aminoglycoside bacteriocidal antibiotic, has been widely used in veterinary medicine to treat mastitis, ovinia, pneumonia, bacillary diarrhea [1] and other infections. However, the overuse of kanamycin and other antibiotics have put great challenge to human health and the environment safety worldwide. Typically, adverse reaction of kanamycin includes tinnitus and loss of hearing, toxicity to kidneys, respiratory failure and allergic reactions [2]. In particularly, the drug may go into milk with lactation after diseased cattle are treated with excessive kanamycin, thus causing the traditional natural gift no longer safe and reliable. Therefore many countries and regions have set maximum residue limit of kanamycin in milk, in the EU is 150 μg · kg−1 and in China is 200 μg · kg−1. Varieties of methods have been developed to control and detect kanamycin in animal-derived food. The most traditional antibiotics instrumental analytical methods by chromatogram and mass spectroscopy have obvious superiority in quantitative analysis or qualitative identification but require large and expensive facilities, laborious sample preparation and professionals to operate, which is difficult to meet the current demand of large consumption. Motivated by overcoming these drawbacks, abundant attempts have been made to develop rapid and facile method for kanamycin detection including colorimetric method [3], fluorescent probe [4] and electrochemical biosensors [5], which have provided good solutions in some aspects. Among them, fluorescent aptasensor is considered to be competitive alternative techniques for the facile, ultrasensitive, reliable and specific detection of antibiotics residues.
Aptamers are artificially selected oligonucleotide or peptide molecules, which can specifically bind to the target by folding into three-dimensional structures [6]. In principle, aptamers can bind to any given target, including but not limited to drugs [7], toxins [8], metal ions [9], proteins [10], and even whole cells [11] with high affinity and specificity. Compared to natural receptors such as antibodies and enzymes, the selected aptamers can be synthesized with high reproducibility and purity which ensure them cost effective. Furthermore, they are small in size, water soluble, easy to modify and have no immunogenicity [12, 13]. Due to the advantages mentioned, aptamers are now widely used in developing different detection platforms, especially fluorescent nanomaterial enveloped aptasensors [14].
Fluorescent resonance energy transfer (FRET) is a mechanism describing energy transfer between the donor chromophore and the acceptor chromophore through nonradiative dipole–dipole coupling, which is recognized as a sensitive and reliable analytical technique and has been widely used in biological assays [15], Generally, FRET probes consist of a fluorophore (energy donor) and a quencher (energy acceptor) to form a fluorescent resonance energy transfer pair, in which the distance-dependent fluorescence quenching is closely coupled with biomolecular recognition events. To improve FRET efficiency, great efforts have been spent to find the perfect donor-acceptor pair. The energy donors generally include organic fluorescent dyes [16], semiconductor quantum dots (QDs) [17, 18], upconversion fluorescence nanoparticles (UCNPs) [8] and carbon dots [19]. Among these fluorescent materials, carbon dots have significant advantages due to their strongly fluorescent, photochemically stability, nontoxicity, extensive sources, and low cost. As for the energy acceptor, in the past reports, gold nanoparticles (AuNPs) [9, 20] and carbon nanotubes [21] are used as typical energy acceptor to quench the fluorescence before reaching designated target. Research on graphene highlights the world of two-dimensional (2D) nanomaterials. Extensive attention has been focused on the graphene oxide (GO) due to the remarkable optical, electrical, photophysical properties, which have made it a universal quencher. Besides GO, layered MoS2 nanosheets, whose 2D layer structure analogous to graphene, has gained great interest in sensing [22], batteries and electrocatalysis [23]. It was also proved of high fluorescence quenching ability and demonstrated to be a promising candidate to form FRET pair [22, 24]. The large surface area of MoS2 sheets serves as an excellent reaction platform. It also can adsorb single-stranded DNA (ssDNA) via the van der Waals force which further expands the application of MoS2 in bioanalysis [24, 25].
Herein, in order to better monitor antibiotics residues and guarantee safety of animal derived products, we propose a sensitive and highly efficient fluorescent method for kanamycin in milk by utilizing aptamer spatial conformation induced FRET between layered MoS2 and carbon dots. In this system, the carbon dots, with strong and stable fluorescence, and the layered MoS2 nanosheets are chosen to form a novel donor-acceptor pair, while the singles-stranded DNA aptamer serves as a bridge in the distance-dependent energy transfer process. From another point of view, the kanamycin aptamer act as an antibiotic recognizer and catcher, with the carbon dots labeled to release fluorescent signals, while the layered MoS2 nanosheets provide a platform for aptasensor adsorption and desorption. The sensitivity, specificity and the efficiency of the fluorescent aptasensor are evaluated. The application of the developed method for rapid and sensitive detection of kanamycin in spiked sample is carried as well. We thus proposed a new approach for kanamycin residue detection in milk and provide possible options for improving the current situation of antibiotics abuse.
Experimental
Materials and instrumentation
Bulk MoS2 was purchased from Sigma (http://www.sigmaaldrich.com). Kanamycin and other drugs were from Dr. Ehrenstorfer Gmbh (http://ehrenstorfergmbh.lookchem.com). DNA aptamer and other DNA probes were purchased from Sangon Biotech (http://www.sangon.com). All reagents were at least of analytical reagent grade. Ultrapure water, obtained from a water purification system, was used in the whole experiment. Sonic exfoliation was carried on Scientz JY92-IIDN ultrasonic cell disrupter system. Transmission electron microscopy (TEM) measurements were performed with Hitachi HT7700 electron microscopy at 80 kV. Field emission scanning electron microscopy (SEM) image was taken by a Hitachi S-4800 electron microscope. The UV-visible absorption spectra were recorded on a Shimadzu UV-2550 spectrophotometer. Fluorescence spectra were performed on Perkin Elmer LS55 fluorescence spectrophotometer. The UPLC-MS-MS confirmatory test was achieved on Waters Acqurity/Quattro Premier XE.
Preparation of layered MoS2 nanosheets
Bulk MoS2 must be exfoliated into single or few layered films before engaged into FRET system. In this report, liquid-phase exfoliation of MoS2 is carried out following previous report [26, 27] with necessary modifications. Generally, 5 mg · mL−1 bulk MoS2 and 1.5 mg · mL−1 sodium cholate were dispersed in water and then sonicated at room temperature (25 °C) by a point probe (flathead sonic tip) for 20 h with a power output of 315 W, followed by centrifugation at 15,000 rpm (20627 rcf) for 15 min. After centrifugation, the supernatant the dispersions were abandoned and the MoS2 nanosheets were redispersed in ultrapure water. The washing process was repeated for other two times to remove surfactant sodium cholate completely. The obtained yellow-green MoS2 nanosheets suspension was kept at 4 °C for storage.
Preparation of aptamer based carbon dots
Fluorescent carbon dots were synthesized by a facile one-step hydrothermal method according to a previous report [28]. In brief, a mixture of 0.2 g sodium citrate, 1.5 g NH4HCO3 and 10 mL water was sealed into Teflon equipped stainless steel autoclave and then heated to 180 °C for 4 h. The preparation was terminated by cooling the reaction mixture down to room temperature. The resultant nanoparticles were purified by dialysis (500 Da, molecular weight cutoff) for about 48 h in dark for further experiments.
Amino-modified aptamer and mismatched DNA probes were labeled on carbon dots by amidation reaction between the carboxyl group of carbon dots and side amino group of DNA probes. In brief, DNA was dissolved in 0.02 M Tris–HCl buffer (pH 7.4, containing 100 mM NaCl and 5 mM KCl) for 0.1 M stock solution. EDCI and NHS were added into carbon dots solution under stirring to activate carboxyl groups for 2 h before DNA was added. The reaction solution was stirred overnight to allow the reaction to proceed to completion. Excessive EDCI and NHS were removed by dialysis. The product was finally obtained for the following reaction.
General procedures for sensing and specificity test
For the general procedures of kanamycin sensing, the carbon dots were excited at 380 nm initially and the emission spectra was recorded. Then 0.15 mg · mL−1 MoS2 was added to the carbon dots to induce aptamer assembly and fluorescent quenching. After FRET reaction reached equilibrium, different concentration of kanamycin standard solution was added to the system resulting in fluorescent recovery. The quenching and recovery efficiency were calculated respectively.
In order to verify the selectivity of the assay, varieties of antibiotics were used in recovery part for comparison to kanamycin. Additionally, five different strands of DNA with 1 to 5 wrong bases (underlined) were labeled on the carbon dots for FRET experiments to test the specificity of aptamer towards kanamycin. The sequences of amino-modified kanamycin aptamer and sequence-changed DNA probes are as follows:
-
Kanamycin Aptamer: 5′-NH2-C6-TGGGGGTTGAGGCTAAGCCGA-3′
-
DNA-1 (one base changed): 5′-NH2-C6-TGGGGGATGAGGCTAAGCCGA-3′
-
DNA-2 (two bases changed): 5′-NH2-C6-TGGGGGATGAAGCTAAGCCGA-3′
-
DNA-3 (three bases changed): 5′-NH2-C6-TGGGGGATGAAGCTAAGCAGA-3′
-
DNA-4 (four bases changed): 5′-NH2-C6-TGGGGGATGAAGCTAAGAAGA-3′
-
DNA-5 (five bases changed): 5′-NH2-C6-TGGGGGAAGAAGCTAAGAAGA-3′
Preparation of standard milk based matrix
In real sample determinations, confirmed kanamycin-negtive raw milk was spiked with kanamycin at different concentration levels. Each spiked samples was halved for fluorescent determination and UPLC-MS-MS respectively. For UPLC-MS-MS detection, sample was pretreated follow the national standard of China [29] to ensure accuracy of mass spectrometric method. For the fluorescent determination, milk samples should be treated as described to remove protein and fat. Briefly, 10 mL 5 % phosphoric acid was added into 3.0 mL spiked milk samples in a centrifuge tube to extract the drug, and then 1.2 mL trichloroacetic acid was added. After thorough vortex, the mixtures were centrifuged at 10,000 rpm (9168 rcf) for 10 min, and the supernatant was collected and passed through a Waters Oasis MCX column to obtain a clear fraction. Finally, the solution was filtered using 0.22 μm filters before determination.
Results and discussions
Sensing strategy of aptamer-based FRET
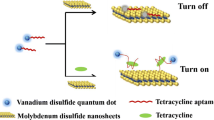
The general sensing strategy of kanamycin contains mainly two steps of quenching and recovery with the fluorescent probe “on-off-on” as shown in Scheme 1. Aptamer labeled carbon dots are assembled onto the surface of MoS2 nanosheets via the van der Waals force between nucleobases of single-stranded DNA and the plane of MoS2 nanosheets, thus quenching the fluorescence of the carbon dots due to energy transition. In the second part, with the addition of kanamycin, DNA aptamer with single chain structure labeled on carbon dots, is inclinable to separate from the surface of MoS2 nanosheets due to the strong binding specificity between the aptamer and target. Accordingly, the carbon dots are set free and escape into the solution which will give rise to a fluorescence recovery. In the whole sensing system, the aptamer acts as fluorescence switch which can connect together or depart the FRET fluorophore and quencher through specific recognition towards kanamycin. As a result, the fluorescence recovery is expected to provide a quantitative readout of the target, to reflect the concentration of kanamycin. The novel combination of the FRET pair of different nanomaterials with the competitive binding of aptamer makes it favorable for sensitive and selective detection of kanamycin.
Characterization of MoS2 nanosheets and carbon dots
After liquid-phase exfoliation and centrifugation remove of excessive surfactant, the layered MoS2 nanosheets suspension was obtained to be use. The inset of Fig. 1a shows photograph of 2 mg⋅mL−1 MoS2 nanosheets dispersion whose color appears to be yellow-green. The Ultraviolet–visible spectroscopy (UV–vis) absorption spectrum is used to demonstrate that bulk MoS2 has been successfully thinned to single or a few layers. MoS2 nanosheets, consisting of one to several layers, possess two characteristic absorption peaks at approximately 610 and 670 nm [30, 31]. As marked in Fig. 1a, these absorption peaks located at 600–700 nm regions can be observed, implying the presence of 2H polytype of MoS2 nanosheets [26, 32]. The further characterization of MoS2 nanosheets was carried out using scanning electron microscope (SEM, Fig. 1b) and transmission electron microscope (TEM, Fig. 1c and d) techniques. It can be observed from the SEM and TEM images that the plane size of MoS2 nanosheets ranges from 100 nm to 200 nm. A higher magnification TEM image (Fig. 1d) can provide the detail of layered MoS2 nanosheets. The bulk MoS2 has been successfully exfoliated into single-layered or few-layered thus providing large surface area for biosorption and energy transition reaction.
Prepared carbon dots were characterized by spectroscopy and transmission electron microscope. TEM characterize the morphology and dispersibility of the carbon dots. It can be observed from Fig. 2a that the carbon dots are well distributed in aqueous solution and uniformed in size. As shown in Fig. 2b, there is a significant absorption band at 340 nm in UV–vis spectroscopy which corresponds to the optimum fluorescence excitation wavelength owing to the n − π* transition of C═O or C─OH in carboxyl groups on carbon dots. The same figure also displayed the fluorescence emission spectra centered at 440 nm where 340 nm was selected as the excitation wavelength. The strong fluorescence of carbon dots makes it available to act as an energy donor in FRET while abundant carboxyl groups make it convenient for DNA labeling to construct an aptasensor.
Aptamer based FRET analysis of kanamycin
In order to investigate the quenching ability of layered MoS2 towards carbon dots and acquire the best FRET sensing for kanamycin, the analysis of kanamycin, including quenching and recovery process is recorded by fluorescence spectrophotometer on the basis of the above-mentioned detection strategy. The results are shown in Fig. 3. By adding equal volume of solutions containing 10, 20, 30, 50, 80, 120 μL of 0.15 mg⋅mL−1 MoS2, the fluorescent intensity of carbon dots experienced a decreasing process as shown in Fig. 3a. During this process, DNA aptamers labeled on the carbon dots were absorbed on the surface of MoS2 nanosheets, thus shortening the distance between the FRET donor and acceptor and inducing energy transition. When quenching reached equilibrium, the intensity of the spectra peak at 440 nm were read and the quenching efficiency E was calculated according to the formula below, where F0 and F are the fluorescence intensities in the absence and the presence of MoS2 input:
The ultimate quenching efficiency of layered MoS2 nanosheets reached 81.26 % when the final MoS2 concentration was 45 μg · mL−1. Considering both the quencher concentration and quenching efficiency, the quenching ability of layered MoS2 is comparable with and even better than that of GO and AuNPs [20, 33]. The result indicated that layered MoS2 nanosheets can serve as a good fluorescent quencher towards carbon dots.
In the recovery process, based on the maximum of quenching, with joining of different concentration of target kanamycin, the fluorescent intensity went up gradually, indicating the release of carbon dots. As illustrated in Fig. 3b, the more kanamycin entered the system, the more DNA labeled carbon dots dropped off from MoS2 nanosheets, which resulted in a gradual fluorescence recovery. In the same way with the quenching efficiency, the recovery efficiency E’ was calculated following another formula with a correction factor α, which is set to normalize and eliminate the effects of different unit of fluorescence intensity:
According to the formula, with the increase of kanamycin concentration, the recovery efficiency raised up to 92.83 % ultimately confirming that the layered MoS2–carbon dots FRET pair an efficient and sensitive combination. Inset of Fig. 3b displays that a good linear correlation exists between the recovery efficiency and kanamycin concentration in the range of 4–25 μM, with the linear regression equation to be E′ = 3.468 x − 0.482, R 2 = 0.9956. The limit of quantity supposed to be 4 μM while the limit of detection (LOD), calculated by 3σ/s criterion, was estimated to be 1.1 μM. σ is standard deviation of a blank and s represents the slope of the calibration curve (n = 6).
With large surface area and unique properties, two-dimensional nanomaterials, have attracted long-lasting interest. Layered molybdenum disulfide (MoS2) is one of the typical 2D materials whose loose stacked layers are comprised of covalently bonded Mo atoms and S atoms, in the stoichiometry MoS2. Previous researchers have found that MoS2 nanosheets are able to quench light of fluorescent dye [22, 24]. And this experiment reveals that MoS2 can also quench the fluorescent from other nanomaterials like carbon dots and form a highly efficient FRET pair at the same time. Compared to aptasensor made of fluorescent dye, carbon dots provide strong and stable fluorescence which can improve the sensitivity and efficiency [34]. This novel fluorescence “on-off-on” aptasensor based on the match of carbon dots and layered MoS2 nanosheets can not only give out strong fluorescent signal in the absence of kanamycin but also changes sensitively with target addition thus providing possible quantitive sensing for kanamycin analysis.
Specificity evaluation of the assay
As an important aspect to evaluate the performance of a new assay, it is necessary to explore the selectivity towards the target. In livestock breeding, famers usually use more than one kind of drugs to treat diseased animals. This can result in multiple residues in animal products. Therefore, target-specificity of the method should be confirmed so that potential interferences from other drugs can be eliminated. The target-specificity is estimated via the recovery efficiency E’ in the presence of various antibiotics in comparison with kanamycin. Typically, other aminoglycoside antibiotics such as streptomycin (STR), neomycin (NEO) and gentamycin (GEN) are tested, as well as other categories of veterinary antibiotics and drugs (sulfadimethoxine (SUL), ampicillin (AMP), clenbuterol (CLEN), metronidazole (MTR), and nitrofurantion (NIT)). The concentrations of the antibiotics and drugs are set to be 300 μM, which is ten times of kanamycin and the results are presented in Fig. 4a. All tested drugs showed little response in the recovery demonstrating negligible effects on the signal for the detection of kanamycin.
One of the key factors that affect the specificity of the assay is the selectivity of the aptamer. In a similar way, result of aptamer sequence-specific analysis is shown in Fig. 4b. For comparison, DNA probes used in the experiment have 1–5 bases different from the kanamycin aptamer. It can be found that the fluorescent recovery efficiency dropped sharply to approximately 55 % when only 1 base of aptamer changed, and the efficiency fell below 10 % when more than three bases changed. The result demonstrates that the aptamer sequence is favorable under the present system, and can avoid potential interferences from other DNA fragments effectively. Thus both the target-specific and sequence-specific analysis showed excellent performance. This reduces false positive results and warrants the accuracy of the assay.
Analysis of kanamycin in spiked samples
Having achieved satisfactory sensitivity and specificity in standard solutions, it is necessary to explore matrices influence and pretreatment effect in practical. To validate the reliability of the method, the fluorescent aptasensor and UPLC-MS-MS methods were used to determine the contents of kanamycin in raw milk, respectively. A standard addition method was adopted in this study. Generally, confirmed kanamycin-negtive samples were spike in four different levels (2, 4, 10 and 20 μM) and halved for two methods. After treated severally according to the procedure described in Section 2, the determination results are shown in Table 1. The results showed that both the two methods were available to detect kanamycin residues in milk samples in the four concentration levels with the coefficient of variation less than 10 %. Recovery test showed that and the fluorescent aptasensor exhibit better recovery rate, probably due to its simple pretreatment. The results of UPLC-MS-MS validation indicate that when compared to instrumental analysis, electrochemical aptasensor or immunoassay, the fluorescence aptasensor has the advantages of easy sample treatment, short detection time and low requirement of professional skills. These make it a competitive candidate in analytical method.
Conclusion
In summary, a novel fluorescent aptasensor for the rapid and selective detection of kanamycin residue in milk was developed. Taking advantages of aptamer spatial conformation induced FRET quenching and recovery, kanamycin concentration can be measured by fluorescence recovery efficiency. The method exhibits low detection limit, satisfactory specificity and short analyzing time. Besides, the accuracy of the assay in milk sample was confirmed by mass spectrometric analysis, indicating potential application in real sample analysis. Particularly, the study demonstrated that layered MoS2 an efficient fluorescent quencher towards carbon dots. As a typical practice of the combination of FRET and aptasensor, the detection scheme exhibits advantages that make it attractive for applications to other food safety risk factors.
References
Jin Y, Jang WJ, Han CH, Lee MH (2006) Development of immunoassays for the detection of kanamycin in veterinary fields. J Vet Sci 7(2):111–117
U.S. National Library of Medicine http://dailymed.nlm.nih.gov/
Song KM, Cho M, Jo H, Min K, Jeon SH, Kim T, Han MS, Ku JK, Ban C (2011) Gold nanoparticle-based colorimetric detection of kanamycin using a DNA aptamer. Anal Biochem 415(2):175–181
Xing YP, Liu C, Zhou XH, Shi HC (2015) Label-free detection of kanamycin based on a G-quadruplex DNA aptamer-based fluorescent intercalator displacement assay. Sci Rep 5:8125
Zhu Y, Chandra P, Song KM, Ban C, Shim YB (2012) Label-free detection of kanamycin based on the aptamer-functionalized conducting polymer/gold nanocomposite. Biosens Bioelectron 36(1):29–34
Tombelli S, Minunni M, Mascini M (2007) Aptamers-based assays for diagnostics, environmental and food analysis. Biomol Eng 24(2):191–200
Liu J, Guan Z, Lv Z, Jiang X, Yang S, Chen A (2014) Improving sensitivity of gold nanoparticle based fluorescence quenching and colorimetric aptasensor by using water resuspended gold nanoparticle. Biosens Bioelectron 52:265–270
Wu S, Duan N, Ma X, Xia Y, Wang H, Wang Z, Zhang Q (2012) Multiplexed fluorescence resonance energy transfer aptasensor between upconversion nanoparticles and graphene oxide for the simultaneous determination of mycotoxins. Anal Chem 84(14):6263–6270
Tianyu H, Xu Y, Weidan N, Xingguang S (2016) Aptamer-based aggregation assay for mercury (II) using gold nanoparticles and fluorescent CdTe quantum dots. Microchim Acta 183(7):2131–2137
Wang GL, Hu XL, Wu XM, Dong YM, Li ZJ (2016) Fluorescent aptamer-based assay for thrombin with large signal amplification using peroxidase mimetics. Microchim Acta 183(2):765–771
Yi Z, Li XY, Gao Q, Tang LJ, Chu X (2013) Aptamer-aided target capturing with biocatalytic metal deposition: an electrochemical platform for sensitive detection of cancer cells. Analyst 138(7):2032–2037
Tan W, Donovan MJ, Jiang J (2013) Aptamers from cell-based selection for bioanalytical applications. Chem Rev 113(4):2842–2862
Song S, Wang L, Li J, Fan C, Zhao J (2008) Aptamer-based biosensors. TrAC Trends Anal Chem 27(2):108–117
Chen T, Shukoor MI, Chen Y, Yuan Q, Zhu Z, Zhao Z, Basri G, Tan W (2011) Aptamer-conjugated nanomaterials for bioanalysis and biotechnology applications. Nanoscale 3(2):546–556
Zhang C, Yuan Y, Zhang S, Wang Y, Liu Z (2011) Biosensing platform based on fluorescence resonance energy transfer from upconverting nanocrystals to graphene oxide. Angew Chem Int Ed 50(30):6851–6854
Li W, Yang X, Wang K, Tan W, Li H, Ma C (2008) FRET-based aptamer probe for rapid angiogenin detection. Talanta 75(3):770–774
Shi L, De Paoli V, Rosenzweig N, Rosenzweig Z (2006) Synthesis and application of quantum dots FRET-based protease sensors. J Am Chem Soc 128(32):10378–10379
Duan N, Wu S, Dai S, Miao T, Chen J, Wang Z (2015) Simultaneous detection of pathogenic bacteria using an aptamer based biosensor and dual fluorescence resonance energy transfer from quantum dots to carbon nanoparticles. Microchim Acta 182(5–6):917–923
Yu C, Li X, Zeng F, Zheng F, Wu S (2013) Carbon-dot-based ratiometric fluorescent sensor for detecting hydrogen sulfide in aqueous media and inside live cells. Chem Commun 49(4):403–405
Dai H, Shi Y, WangY SY, Hu J, Ni P, Li Z (2014) A carbon dot based biosensor for melamine detection by fluorescence resonance energy transfer. Sensors Actuators B Chem 202:201–208
Yang R, Jin J, Chen Y, Shao N, Kang H, Xiao Z, Tang Z, Wu Y, Zhu Z, Tan W (2008) Carbon nanotube-quenched fluorescent oligonucleotides: probes that fluoresce upon hybridization. J Am Chem Soc 130(26):8351–8358
Zhu C, Zeng Z, Li H, Li F, Fan C, Zhang H (2013) Single-layer MoS2-based nanoprobes for homogeneous detection of biomolecules. J Am Chem Soc 135(16):5998–6001
Ding J, Zhou Y, Li Y, Guo S, Huang X (2016) MoS2 nanosheet assembling superstructure with a three-dimensional ion accessible site: a new class of bifunctional materials for batteries and electrocatalysis. Chem Mater 28(7):2074–2080
Ge J, Ou EC, Yu RQ, Chu X (2014) A novel aptameric nanobiosensor based on the self-assembled DNA–MoS2 nanosheet architecture for biomolecule detection. J Mater Chem B 2(6):625–628
Singh P, Gupta R, Sinha M, Kumar R, Bhalla V (2016) MoS2 based digital response platform for aptamer based fluorescent detection of pathogens. Microchim Acta 183(4):1501–1506
Wang K, Wang J, Fan J, Lotya M, O’Neill A, Fox D, Feng Y, Zhang X, Jiang B, Zhao Q, Zhang H, Coleman JN, Zhang L, Zhang H (2013) Ultrafast saturable absorption of two-dimensional MoS2 nanosheets. ACS Nano 7(10):9260–9267
Li BL, Luo HQ, Lei JL, Li NB (2014) Hemin-functionalized MoS2 nanosheets: enhanced peroxidase-like catalytic activity with a steady state in aqueous solution. RSC Adv 4(46):24256–24262
Guo Y, Wang Z, Shao H, Jiang X (2013) Hydrothermal synthesis of highly fluorescent carbon nanoparticles from sodium citrate and their use for the detection of mercury ions. Carbon 52:583–589
National Standard of China. Determination of streptomycin, dihydrostreptomycin and kanamycin residues in milk and milk powder–LC-MS-MS method. GB/T 22969–2008
Nicolosi V, Chhowalla M, Kanatzidis MG, Strano MS, Coleman JN (2013) Liquid exfoliation of layered materials. Science 340(6139):1226419
Splendiani A, Sun L, Zhang Y, Li T, Kim J, Chim CY, Galli G, Wang F (2010) Emerging photoluminescence in monolayer MoS2. Nano Lett 10(4):1271–1275
Beal AR, Knights JC, Liang WY (1972) Transmission spectra of some transition metal dichalcogenides. II. Group VIA: trigonal prismatic coordination. J Phys C Solid State Phys 5(24):3540
Cui X, Zhu L, Wu J, Hou Y, Wang P, Wang Z, Yang M (2015) A fluorescent biosensor based on carbon dots-labeled oligodeoxyribonucleotide and graphene oxide for mercury (II) detection. Biosens Bioelectron 63:506–512
Liu C, Lu C, Tang Z, Chen X, Wang G, Sun F (2015) Aptamer-functionalized magnetic nanoparticles for simultaneous fluorometric determination of oxytetracycline and kanamycin. Microchim Acta 182(15–16):2567–2575
Acknowledgments
This work is supported by the National Science & Technology Pillar Program of China (No. 2014BAD13B05) and the Yangling Agricultural Hi-tech Industries Demonstration Zone (2014NY-35).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Wang, Y., Ma, T., Ma, S. et al. Fluorometric determination of the antibiotic kanamycin by aptamer-induced FRET quenching and recovery between MoS2 nanosheets and carbon dots. Microchim Acta 184, 203–210 (2017). https://doi.org/10.1007/s00604-016-2011-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-2011-4