Abstract
The authors describe an electrochemical DNA biosensor based on a glassy carbon electrode modified with gold nanoparticles (AuNPs) and reduced graphene oxide that was functionalized with hemin (hemin-rGO). Capture DNA (a 21-mer) is immobilized on the AuNPs via gold-thiol chemistry. The detection scheme relies on the measurement of the differential pulse voltammetric current change of the oxidation-reduction reaction of hemin before and after DNA hybridization has occurred. The decrease in the voltammetric currents of iron (III) (best measured at −0.3 V vs. Ag/AgCl) is linearly related to the concentration of complementary DNA. The calibration plot covers the 1.0 aM to 0.1 pM concentration range, and the detection limit is as low as 0.14 aM (at a signal-to-noise ratio of 3). The biosensor was successfully applied to the direct determination of complementary DNA in self-prepared samples by using the standard addition method.

An electrochemical DNA biosensor was developed on a platform of gold nanoparticles (AuNPs) and hemin functionalized reduced graphene oxide (hemin-rGO). The principle of DNA detection is based on the voltammetric current change of oxidation-reduction reaction of iron (III) at the core of hemin before and after DNA hybridization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to their potential application in the fields of disease diagnosis, environmental monitoring, and food safety, various DNA sensors, such as optical DNA sensors [1–3], electrochemical DNA sensors [4, 5], and piezoelectric DNA sensors [6], have been developed for their sensitive and selective detection. However, as electrochemical sensors possess advantages of rapid response, high selectivity and sensitivity, and low cost, the construction of electrochemical DNA sensors has been the direct area of research focus [7, 8].
A large variety of nanomaterials have been used in electrochemical DNA sensor construction for improving selectivity, sensitivity, and speed. Among these materials, carbon-based nanomaterials, such as carbon nanotubes [9, 10], carbon spheres [11], graphene nanosheets [12, 13], and gold nanoparticles [14, 15], have been most commonly used in electrochemical DNA detection owing to their unique electronic properties, large surface areas, and ease of modification. Studies show that label-free electrochemical DNA sensors can be conveniently produced by introducing electroactive indicators onto the surfaces of the nanomaterials employed without sacrificing the sensitivity and selectivity of the fabricated biosensors [7, 16]. Lin [16] demonstrated an electrochemical sensor for the highly sensitive detection of natural double-stranded DNA (dsDNA) based on Mo6S9-XIX nanowires attached to the electroactive indicator thionin. The detecting principle of dsDNA was based on a decrease in the voltammetric response of the immobilized thionin, which is attributed to the intercalation of thionin into dsDNA. The biosensor shows good sensitivity and selectivity, and can quantify dsDNA with a detection limit of 0.62 ng mL−1. It can also discriminate dsDNA from various kinds of proteins.
Hemin is an iron porphyrin derivative, which is noted for its ability to mimic the active sites of various enzymes [5, 17, 18]. Based on the redox reaction of iron at the core of hemin, it has been shown to have electrocatalytic abilities towards small molecules including sulfides [19], hydrogen peroxide [20], nitrite [20] and dopamine [21], as well as having telomerase activity [22]. By using the peroxidase-like activity of hemin and the intrinsic discrimination ability of graphene for single-stranded DNA (ssDNA) and dsDNA, several colorimetric DNA biosensors have been exploited based on a hemin-graphene hybrid nanosheet to detect DNA damage [18] and single-nucleotide polymorphism [23]. Studies also showed that in the presence of H2O2, the intrinsic peroxidase-like activity of hemin exhibited different catalytic activity to that of colorimetric substrates such as 3, 3′, 5, 5-tetramethylbenzidine (TMB) or 2, 2′-azinobis-(3-ethylbenzthiazoline)-6-sulphonate (ABTS) when the hybrid nanosheets adsorbed ssDNA or dsDNA [24].
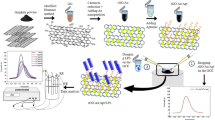
In order to improve the sensitivity of electrochemical DNA sensors, hemin has commonly been used as a label for signal amplification [25]. Inspired by Lin’s strategy [16], we fabricated a electrochemical biosensor by exploiting the redox reaction of iron at the core of hemin. The electrochemical transducing interface was formed on gold nanoparticles (AuNPs) and a hemin functionalized reduced graphene oxide (hemin-rGO) modified glassy carbon electrode (GCE). A decrease in the voltammetric response current of hemin was induced by dsDNA due to the hybridization of the immobilized probe DNA (pDNA) with complementary DNA (cDNA), which blocked the redox reaction of hemin (Fig. 1). Thus, a simple, sensitive, and selective sensing platform was constructed for the detection of cDNA.
Experimental
Reagents
Graphite oxide was purchased from Nanjing Jicang Nano Technology Co., Ltd. (Nanjing, China, http://www.graphenes.net/com/jcnano). Hemin was from Sigma-Aldrich Co. (USA, http://sigmaaldrich.bioon.com.cn). Chloroauric acid (HAuCl4·4H2O) and hydrazine hydrate were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China, http://www.sinoreagent.com). Sodium dodecyl sulfate (SDS), tris-(hydroxymethyl) aminomethane (Tris base) and ethylene diamine tetraacetic acid (EDTA) were ordered from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China, http://solarbio.en.alibaba.com). Mica discs (PELCO®, 9.9 mm diameter) were from Ted Pella, Inc. (www.tedpella.com).
All of the synthetic oligonucleotide sequences were purchased from Sangon Biotech Co., Ltd. (Shanghai, China, http://www.sangon.com/), and their sequences are listed as follows:
-
pDNA, (S1): 5′-SH-TAC GTT ATC AGA CTG ATG ATG A-3′.
-
cDNA, (S2): 5′-ATG CAA TAG TCT GAC TAC TAC T-3′.
-
One-base mismatched sequences (Mis1): 5′-ATG TAA TAG TCT GAC TAC TAC T-3′.
-
Two-base mismatched sequences (Mis2): 5′-ATG TAA TGG TCT GAC TAC TAC T-3′.
-
Three-base mismatched sequences (Mis3): 5′-ATG TAA TGG TCT GTC TAC TAC T-3′.
All oligonucleotide stock solutions were prepared with TE buffer solution (10 mM Tris-HCl, 1.0 mM EDTA, pH 8.0) and stored in a refrigerator. The buffers employed were as follows: hybridization buffer solution (10 mM Tris-HCl, 1.0 mM EDTA, 50 mM NaCl, pH 7.4) and electrochemical test solution (0.1 M phosphate buffer, pH 7.4). All other chemicals were of analytical grade and used as received. Phosphate buffer was prepared by mixing stock solutions of Na2HPO4 and NaH2PO4. Double-distilled water was used throughout this study.
Synthesis of hemin-rGO
Graphene oxide was obtained by ultrasonication of graphite oxide (1.5 mg mL−1) for 12 h. After centrifugation, the unexfoliated graphite oxide was discarded and the graphene oxide suspension was dialyzed, centrifuged, and dried in vacuum. Hemin-rGO was prepared according to Wei’s method [18] with slight modifications. Briefly, graphene oxide suspension (10 mL, 0.5 mg mL−1) was mixed with hemin (10 mL, 0.5 mg mL−1 dissolved with 0.1 M NaOH) under stirring, followed by the addition of ammonia (100 μL, 28 %) solution slowly. When hydrazine (20 μL, 25 %) was added, the mixture was left under vigorous stirring for 30 min, and was then allowed to react at 60 °C for 24 h. The black dispersion was centrifuged and the supernatant was discarded. The sediment was washed several times, and hemin-rGO was obtained by drying under vacuum at room temperature. For comparison, the rGO was also prepared in accordance with the same process except for the hemin, which was not added.
Step-by-step modification of GCE with hemin-rGO and AuNPs
The GCE was pretreated by polishing with 0.3 and 0.05 μm Al2O3 slurry in turn, followed by successive sonication in 1:1 HNO3, in ethanol and then in water. It was then allowed to dry with nitrogen. The hemin-rGO solution (5 μL, 0.5 mg mL−1) was poured on the pretreated GCE and dried at room temperature for 2 h. The hemin-rGO modified GCE (hemin-rGO/GCE) was then rinsed with water to thoroughly remove the unabsorbed hemin-rGO. The hemin modified GCE (hemin/GCE) and rGO modified GCE (rGO/GCE) were also fabricated according to similar procedures when performing electrochemical characterization for comparison.
The hemin-rGO/GCE was then subjected to electrochemical deposition for 300 s in 2.0 mg mL−1 HAuCl4 and 0.1 mol L−1 NaNO3 solution at a potential of −200 mV. After the deposition of AuNPs of ~70 nm (data not shown) on hemin-rGO/GCE, the modified electrode consisted of AuNP/hemin-rGO/GCE.
Capture DNA immobilization and hybridization
The thiolated pDNA was covalently linked onto the surface of the AuNP/hemin-rGO modified electrode via thiol-Au interaction. Briefly, AuNP/hemin-rGO/GCE was immersed in 50 μL of 10 μM pDNA and allowed to react at room temperature for 12 h. After immersion in Tris-HCl solution for 5.0 min and washing with 0.1 % SDS solution continually for another 5.0 min to remove the unbound pDNA, the electrode was defined as S1/AuNP/hemin-rGO/GCE. The hybridization process was performed by immersing the S1/AuNP/hemin-rGO/GCE in hybridization buffer containing various concentrations of cDNA for 90 min at 37 °C. The unhybridized cDNA on the electrode was then removed by immersing in Tris-HCl solution for 5.0 min and rinsing with 0.1 % SDS solution for another 5.0 min. The resulting modified electrode was denoted S2/S1/AuNP/hemin-rGO/GCE.
Apparatus and measurements
Field emission transmission electron microscopy (TEM) images were obtained with JEM-2100F (JEOL, Japan, http://www.jeol.co.jp) at an acceleration voltage of 200 kV. X-ray photoelectron spectroscopy (XPS) analysis was performed on an ESCALAB250 X-ray photoelectron spectrometer (Thermo Fisher Scientific, USA, www.thermofisher.com). Two microliters of GO, rGO and hemin-rGO solutions were poured on mica discs and dried at room temperature before TEM and XPS analysis, respectively. The UV-vis absorption spectra were recorded on a UV-4802 spectrophotometer (Unico Shanghai Instrument Co., Ltd. Shanghai, China, http://www.unicosh.com.cn).
All electrochemical measurements were carried out on a CHI 660D electrochemical workstation (Shanghai Chenhua Instruments, China, www.chinstruments.com) at room temperature with a standard three-electrode configuration. A platinum wire provided the counter electrode and an Ag/AgCl electrode acted as the reference. The modified GCE (3 mm in diameter) was used as the working electrode. All the solutions were saturated with N2 during the electrochemical measurements. Electrochemical characterizations were conducted using the cyclic voltammetry method in phosphate buffer (0.1 M, pH 7.4) at a scan rate of 100 mV s−1. Differential pulse voltammetry (DPV) was used to detect the DNA hybridization in a pH 7.4 phosphate buffer. The pulse amplitude was set to be 50 mV, and the potential range was from 0.0 V to minus 0.6 V. The reduction peak current of hemin was recorded. The concentration of cDNA was quantified as the relationship between the current decrement of hemin (ΔI = I ds-DNA - I ss-DNA) and the logarithm of cDNA concentration.
Results and discussion
Characterization of hemin-rGO
The morphology of hemin-rGO was characterized by TEM. As shown in Fig. 2a, b and c, the nanosheet of GO, rGO and hemin-rGO are single-layered and wrinkled with random folds and rolled edges, respectively. These nanosheets of GO, rGO and hemin-rGO have a lot of open graphitic edge planes. The atomic ratio of C to O in hemin-rGO was calculated to be 5.896 (Fig. 2f), which was much higher than that in GO of 2.254 (Fig. 2d). This increase in hemin-rGO was due to the removal of oxygen by chemical reduction [26]. However, the ratio was a little bit lower than that in rGO of 6.255 (Fig. 2e), which might be due to the incorporation of hemin, which itself contains an oxygen atom. The introduction of hemin into the rGO matrix was also evidenced by the presence of the Fe 2p band at 710.79 eV (Fe 2p3/2) and 725.20 eV (Fe 2p1/2) (Fig. 2g). It was reported that the binding energy of Fe 2p in hemin was 712.5 (Fe 2p3/2) and 726.3 eV (Fe 2p1/2) [27]. A 2.3 eV shift in binding energy was estimated, suggesting a strong interaction between hemin and the rGO nanosheet. Moreover, the molar ratio of N in hemin-rGO was 5.81 %, which was higher than those of 4.08 % in GO and 1.85 % in rGO. This suggested the existence of an interaction between hemin and the rGO nanosheet, which made hemin stabilize on the surface of the rGO nanosheet.
The UV-vis spectra of graphene oxide, hemin-rGO and hemin are shown in Fig. 3A. The maximum absorption at 233 nm of graphene oxide, attributed to the π-π* transition of aromatic C = C bonds, was redshifted to 267 nm in hemin-rGO, indicating the formation of rGO and the restoration of sp2 structure in rGO [26, 28, 29]. The spectrum of the hemin solution contained a strong peak from about 300 nm to 450 nm, attributed to the Soret band of hemin, along with weak peaks from 500 to 700 nm assigned to the Q-bands of hemin [30]. The hemin-rGO exhibited broad absorption at 267 nm which is presumably related to the presence of reduced graphene oxide. A strong absorption around 410 nm also emerged, which is attributable to the Soret band of hemin. These indicate the interactions between graphene oxide and and confirms the attachment of hemin molecules on the rGO. In addition, the black suspension of hemin-rGO (Fig. 3A, inset II) was stable enough to be stored for up to two months.
A UV-vis spectra of graphene oxide (a), hemin-rGO (b), and hemin (c). Inset: photographs of graphene oxide (a) and hemin-rGO (b); B Cyclic voltammograms of a bare GCE (a), hemin modified GCE (b), rGO modified GCE (c), and hemin-rGO modified GCE (d). Solution: N2-saturated 0.1 M pH 7.4 phosphate buffer, scan rate: 50 mV s−1
The hemin-rGO modified GCE gave a pair of well-defined redox peaks with a formal potential of −0.281 V in phosphate buffer solution when performing cyclic voltammogram characterization (Fig. 3B, curve d). These peaks should be ascribed to the electron transfer of iron at the core of hemin [30]. By contrast, no obvious peaks were observed on a bare GCE (Fig. 3B, curve a) and rGO modified GCE (Fig. 3B, curve c). Only a pair of weak and asymmetrical peaks were present on a hemin modified GCE (Fig. 3B, curve b). These not only indicate that hemin was attached to the rGO and retained its electrochemical activity, but also demonstrate that the enhancement of the voltammetric responses were resulted from the large surface area and good conductivity of rGO.
Electrochemical characterization of the construction process of the biosensor
DPV was conducted to monitor the construction process of the DNA sensor. Figure 4 shows DPV’s of the various GCE in 0.1 M pH 7.4 N2-saturated phosphate buffer. No peak was found on the bare GCE, while a distinct reduction peak can be observed on the hemin-rGO-modified GCE. The peak is ascribed to the reduction of Fe(III) in hemin (Fig. 4 curve b). When the same solution was measured with the AuNP/hemin-rGO/GCE electrode, the reduction peak was decreased (Fig. 4 curve c), which may be due to the electrostatic repulsion of the negatively charged AuNP’s toward the Fe(III) in hemin. After the pDNA was assembled onto the electrode surface to form S1/AuNP/hemin-rGO/GCE, the further electrostatic repulsion of the negatively charged phosphate backbones of S1 was taken into account [31], and therefore a further decrease in DPV response was observed (Fig. 4 curve d). The value of the reduction peak of hemin decreased gradually as cDNA (1⋅10−15 M) were assembled onto the surface of hemin-rGO stepwise (Fig. 4 curve e), resulting from the blockage of electron transfer of hemin. Taken together, these results indicate that the Fe(III) in hemin retained extreme high direct-electron transfer activity. Hemin-rGO makes it suitable for extensive application in the optical or electrochemical biosensing fields.
Electrochemical detection of the complementary DNA
In order to verify the practical use of the DNA sensor, the selectivity of the prepared sensor towards various mismatched sequences was investigated. Figure 5A shows the DPV responses of the S1/AuNP/hemin-rGO/GCE before (curve a) and after hybridization with three-base mismatched sequences of Mis3 (curve b), two-base mismatched sequences of Mis2 (curve c), one-base mismatched sequences of Mis1 (curve d), and the complementary sequences of S2 (curve e). Values of the corresponding peak current on each hybridized electrode are displayed as a histogram in Fig. 5B. It is clear that the signal recorded on the Mis3/S1/AuNP/hemin-rGO/GCE is very close to that on the S1/AuNP/hemin-rGO/GCE. The values of the peak current on the Mis2/S1/AuNP/hemin-rGO/GCE and the Mis1/S1/AuNP/hemin-rGO/GCE decreased by 13.5 % and 15.4 %, respectively. However, when S1/AuNP/hemin-rGO/GCE was hybridized with the perfectly complementary sequences of S2, it was found that the value of the peak current on the S2/S1/AuNP/hemin-rGO/GCE decreased remarkably. These results demonstrate that the method shows high selectivity for the detection of specific sequences of DNA.
A DPVs of S1/AuNP/hemin-rGO/GCE before (a) and after hybridization with the three-base mismatch sequence (b), two-base mismatch sequence (c), one-base mismatch sequence (d), and complementary sequence (e). B Histogram of the corresponding peak current values on different electrodes. The concentrations for all hybridized sequences are 1.0⋅10−13 M
The following parameters were optimized: (a) concentration of pDNA; (b) hybridization buffer pH value; and (c) hybridization time. The relevant data are given in the Electronic Supplementary Material, Fig. S1. We found the following experimental conditions gave the best results: (a) 10 μM pDNA solution; (b) pH of 7.4; and (c) 90 min of hybridization time.
The analytical performance of the DNA sensor was assessed by using various concentrations of complementary sequences of S2 for hybridization under optimal conditions. As shown in Fig. 6A, the values of the DPV peak current were expected to decrease with increasing concentrations of cDNA, suggesting that increasing specific recognition occurred between pDNA and cDNA. The calibration plots show good linear relationships between the decrease in peak currents and the logarithm of the concentrations of cDNA in the range from 1.0⋅10−18 to 1.0⋅10−13 M with a coefficient of 0.998. The detection limit was estimated to be 1.41⋅10−19 M. These results show that the DNA biosensor is a potential method for the sensitive detection of DNA.
The DNA sensor was also applied to samples containing unknown concentrations of cDNA using the standard addition method. The recoveries ranged from 91.1 % to 99.2 %. The good recoveries indicate that the method has good reliability. Moreover, a comparison between our work and some gene sensor methods which imply a signal amplification approach, show very low detection limits, dynamic ranges and LOD’s has been made and the results are listed in Table 1. According to the data listed, we can conclude that the fabricated biosensor shows a wide linear range and a quite low LOD for cDNA, which makes it a potential method for target DNA determination.
Reproducibility, regeneration, and stability of the biosensor
Fabrication reproducibility was tested by detecting a cDNA of 1.0⋅10−15 M with 3 different S1/AuNP/hemin-rGO/GCEs. The relative standard deviation was estimated to be 4.3 % (Fig. 6B). Regeneration of the electrode surface was carried out by electrochemical reductive desorption of the self-assembled monolayers by applying a constant negative potential (−1.1 V) to the modified Au electrode vs. Ag/AgCl reference electrode in 0.1 M KNO3. After electrochemical detection, an electrochemical reductive desorption method was performed to break the gold–thiol bond and remove all components on the AuNP/hemin-rGO/GCE surface. The results showed that the biosensor possesses about 96 % of its original response current after 2 cycles, but retains about 80 % after 3 cycles (Electronic Supplementary Material, Fig. S2). The stability of the DNA sensor was also investigated. The biosensor retained 95.4 % and 77.0 % of its initial voltammetric response after 7 and 15 days’ storage in a refrigerator at 4 °C, respectively (Electronic Supplementary Material, Fig. S3). These results showed that the current gene biosensor has good fabrication reproducibility, stability, and high regeneration.
Conclusion
We have constructed an electrochemical DNA sensor on a platform of AuNPs and hemin-rGO for detecting cDNA. Detection is based on a decrease in the DPV response of hemin before and after the hybridization of cDNA. This made the specific-DNA recognition operation simple and direct. The detection limit is 1.41⋅10−19 M which is estimated to be about 425 copies of target DNA in 5 mL of sample solution under optimal experimental conditions. The method was successfully applied to the direct determination of complementary DNA in self-prepared samples by using the standard addition method. The biosensor possessed good stability, reproducibility and high regeneration, making this electrochemical transducing interface an ideal platform for fabricating DNA biosensors.
References
Zhao H, Dong J, Zhou F, Li B (2015) G-quadruplex-based homogenous fluorescence platform for ultrasensitive DNA detection through isothermal cycling and cascade signal amplification. Microchim Acta 182:15
Hu K, Zhong T, Huang Y, Chen Z, Zhao S (2015) Graphitic carbon nitride nanosheet-based multicolour fluorescent nanoprobe for multiplexed analysis of DNA. Microchim Acta 182:5
Liu Y, Wei M, Zhang L, Wei W, Zhang Y, Liu S (2015) Evaluation of DNA methyltransferase activity and inhibition via chiroplasmonic assemblies of gold nanoparticles. Chem Comm 51:14350
Liu L, Xiang G, Jiang D, Du C, Liu C, Huang W, Pu X (2016) Electrochemical gene sensor for mycoplasma pneumoniae DNA using dual signal amplification via a Pt@Pd nanowire and horseradish peroxidase. Microchim Acta 183:379
Liang G, Liu X (2015) G-quadruplex based impedimetric 2-hydroxyfluorene biosensor using hemin as a peroxidase enzyme mimic. Microchim Acta 182:13
Zhou L, Lu P, Zhu M, Li B, Yang P, Cai J (2016) Silver nanocluster based sensitivity amplification of a quartz crystal microbalance gene sensor. Microchim Acta 183:881
Wang T, Zhu R, Zhuo J, Zhu Z, Shao Y, Li M (2014) Direct detection of DNA below ppb level based on thionin-functionalized layered MoS2 electrochemical sensors. Anal Chem 86:12064
Ding Y, Wang Q, Gao F, Gao F (2013) Highly sensitive and selective DNA biosensor using a dumbbell-shaped bis-groove binder of bi-acetylferrocene ethylenediamine complex as electrochemical indicator. Electrochim Acta 106:35
Dong X, Lu X, Zhang K, Zhang Y (2013) Chronocoulometric DNA biosensor based on a glassy carbon electrode modified with gold nanoparticles, poly(dopamine) and carbon nanotubes. Microchim Acta 180:101
Li J, Lee EC (2015) Carbon nanotube/polymer composite electrodes for flexible, attachable electrochemical DNA sensors. Biosens Bioelectron 71:414
Dong H, Zhu Z, Ju H, Yan F (2012) Triplex signal amplification for electrochemical DNA biosensing by coupling probe-gold nanoparticles-graphene modified electrode with enzyme functionalized carbon sphere as tracer. Biosens Bioelectron 33:228
Li R, Liu L, Bei H, Li Z (2016) Nitrogen-doped multiple graphene aerogel/gold nanostar as the electrochemical sensing platform for ultrasensitive detection of circulating free DNA in human serum. Biosens Bioelectron 79:457
Li B, Pan G, Avent ND, Lowry RB, Madgett TE, Waines PL (2015) Graphene electrode modified with electrochemically reduced graphene oxide for label-free DNA detection. Biosens Bioelectron 72:313
Fang L-X, Cao J-T, Huang K-J (2015) A sensitive electrochemical biosensor for specific DNA sequence detection based on flower-like VS2, graphene and Au nanoparticles signal amplification. J Electroanal Chem 746:1
Liu YL, Fu WL, Li CM, Huang CZ, Li YF (2015) Gold nanoparticles immobilized on metal-organic frameworks with enhanced catalytic performance for DNA detection. Anal Chim Acta 861:55
Lin H, Cheng H, Liu L, Zhu Z, Shao Y, Papakonstantinou P, Mihailovič D, Li M (2011) Thionin attached to a gold electrode modified with self-assembly of Mo6S9-XIX nanowires for amplified electrochemical detection of natural DNA. Biosens Bioelectron 26:1866
Yang Z, Qian J, Yang X, Jiang D, Du X, Wang K, Mao H, Wang K (2015) A facile label-free colorimetric aptasensor for acetamiprid based on the peroxidase-like activity of hemin-functionalized reduced graphene oxide. Biosens Bioelectron 65:39
Wei W, Zhang D, Li H, Yin L, Pu Y, Liu S (2014) Label-free and rapid colorimetric detection of DNA damage based on self-assembly of a hemin-graphene nanocomposite. Microchim Acta 181:1557
Cao X, Gao J, Ye Y, Wang P, Ding S, Ye Y, Sun H (2016) Amperometric determination of sulfide by glassy carbon electrode modified with hemin functionalized reduced graphene oxide. Electroanalysis 28:140
Valentini F, Cristofanelli L, Carbone M, Palleschi G (2012) Glassy carbon electrodes modified with hemin-carbon nanomaterial films for amperometric H2O2 and NO2 − detection. Electrochim Acta 63:37
Zou HL, Li BL, Luo HQ, Li NB (2015) A novel electrochemical biosensor based on hemin functionalized graphene oxide sheets for simultaneous determination of ascorbic acid, dopamine and uric acid. Sensors Actuators B Chem 207:535
Liu Y, Wei M, Liu X, Wei W, Zhao H, Zhang Y, Liu S (2016) Label-free ultrasensitive detection of telomerase activity via multiple telomeric hemin/G-quadruplex triggered polyaniline deposition and a DNA tetrahedron-structure regulated signal. Chem Comm 52:1796
Guo Y, Deng L, Li J, Guo S, Wang E, Dong S (2011) Hemin-graphene hybrid nanosheets with intrinsic peroxidase-like activity for label-free colorimetric detection of single-nucleotide polymorphism. ACS Nano 5:1282
Hu C, Xi Q, Ge J, Luo F-Y, Tang L-J, Jiang J-H, Yu R-Q (2014) Graphene-hemin hybrid nanosheets as a label-free colorimetric platform for DNA and small molecule assays. RSC Adv 4:64252
Li Y, Deng J, Fang L, Yu K, Huang H, Jiang L, Liang W, Zheng J (2015) A novel electrochemical DNA biosensor based on HRP-mimicking hemin/G-quadruplex wrapped GOx nanocomposites as tag for detection of Escherichia coli O157:H7. Biosens Bioelectron 63:1
Oprea R, Peteu SF, Subramanian P, Qi W, Pichonat E, Happy H, Bayachou M, Boukherroub R, Szunerits S (2013) Peroxynitrite activity of hemin-functionalized reduced graphene oxide. Analyst 138:4345
Liang Z-X, Song H-Y, Liao S-J (2011) Hemin: a highly effective electrocatalyst mediating the oxygen reduction reaction. J Phys Chem C 115:2604
Cao X, Xu H, Ding S, Ye Y, Ge X, Yu L (2016) Electrochemical determination of sulfide in fruits using alizarin-reduced graphene oxide nanosheets modified electrode. Food Chem 194:1224
Ye Y, Ding S, Ye Y, Xu H, Cao X, Liu S, Sun H (2015) Enzyme-based sensing of glucose using a glassy carbon electrode modified with a one-pot synthesized nanocomposite consisting of chitosan, reduced graphene oxide and gold nanoparticles. Microchim Acta 182:1783
Guo Y, Li J, Dong S (2011) Hemin functionalized graphene nanosheets-based dual biosensor platforms for hydrogen peroxide and glucose. Sensor Actuat B 160:295
Li S, Qiu W, Zhang X, Ni J, Gao F, Wang Q (2016) A high-performance DNA biosensor based on the assembly of gold nanoparticles on the terminal of hairpin-structured probe DNA. Sensor Actuat B 223:861
Hu Q, Hu W, Kong J, Zhang X (2014) PNA-based DNA assay with attomolar detection limit based on polygalacturonic acid mediated in-situ deposition of metallic silver on a gold electrode. Microchim Acta 182:427
Chen A, Ma S, Zhuo Y, Chai Y, Yuan R (2016) In situ electrochemical generation of electrochemiluminescent silver naonoclusters on target-cycling synchronized rolling circle amplification platform for microRNA detection. Anal Chem 88:3203
Liu H, Bei X, Xia Q, Fu Y, Zhang S, Liu M, Fan K, Zhang M, Yang Y (2015) Enzyme-free electrochemical detection of microRNA-21 using immobilized hairpin probes and a target-triggered hybridization chain reaction amplification strategy. Microchim Acta 183:297
Rasheed PA, Radhakrishnan T, Shihabudeen PK, Sandhyarani N (2016) Reduced graphene oxide-yttria nanocomposite modified electrode for enhancing the sensitivity of electrochemical genosensor. Biosen Bioelectron 83:361
Wan J, Liu X, Zhang Y, Gao Q, Qi H, Zhang C (2015) Sensitive impedimetric detection of microRNAs using a hairpin probe based on DNAzyme-functionalized gold nanoparticle tag-initiated deposition of an insulating film on gold electrode. Sensor Actuat B 213:409
Acknowledgments
The project was supported by the National Natural Science Foundation of China (Grant No. 31401573), Natural Science Foundation of Anhui Province (No. 1508085MC42 and 1508085MC47), Fundamental Research Funds for the Central Universities (No. JZ2015QSJH0217), China Postdoctoral Science Foundation (No. 2016M592050), Science and Technology Program of Guangzhou, China (No. 201604020036), and Open Research Fund of Key Laboratory of Environmental Medicine Engineering, Ministry of Education, Southeast University (2015EME003).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Compliance with ethical standards
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 439 kb)
Rights and permissions
About this article
Cite this article
Ye, Y., Gao, J., Zhuang, H. et al. Electrochemical gene sensor based on a glassy carbon electrode modified with hemin-functionalized reduced graphene oxide and gold nanoparticle-immobilized probe DNA. Microchim Acta 184, 245–252 (2017). https://doi.org/10.1007/s00604-016-1999-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1999-9