Abstract
We report on a flow injection chemiluminescence assay for hydroquinone (HQ) using Fe3O4 magnetic nanoparticles capped with oleic acid and coated with a polymer whose surface was molecularly imprinted for HQ to act as the recognition element. Dispersed in an oil phase, the surface imprinted particles display excellent adsorption capacity (63 mg∙g‾1) and a rapid adsorption rate. Packed into a flow cell, the addition of the reagents hexacyanoferrate(III), luminol and chlorhematin generates chemiluminescence whose intensity is linearly related to the concentration of HQ in the range from 2 × 10−7 to 1.0 × 10−5 mg∙mL‾1, with a detection limit of 7.9 × 10−8 mg∙mL‾1 and a relative standard deviation of 2.3 % at 1.0 × 10−7 mg∙mL‾1 HQ (n = 6). The method was successfully applied to the determination of HQ in spiked water samples.

The flow-injection chemiluminescence assay for hydroquinone uses Fe3O4 magnetic nanoparticles capped with oleic acid and coated with a polymer whose surface was molecularly imprinted for hydroquinone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Hydroquinone (HQ) is scientifically known as 1, 4-dihydroxybenzene. It is widely used as photographic developers, dye, antioxidant and so on [1], contained in cosmetics, medicines, environment, and human diet [2]. Specially, for its certain role of fading sunspots and lightening pigmented areas of the skin, hydroquinone sometimes is overdosed in the bleaching creams, absorbed through the skin and mucous membranes and can produce reactive oxygen species through redox cycle [3], resulting in DNA damage, mutation in cellular transformation and vivo tumourigenesis [4, 5]. Because of the hazards of long-term treatments, the European Committee and China have banned the use of HQ in cosmetics and formulations, and it is available only through prescription by physicians and dermatologists [6]. However, hydroquinone has been being added to some cosmetics illegally by certain manufacturers to increase the bleaching efficacy of the cosmetics [7]. Obviously, it was vital to monitor and control the harmful effects of hydroquinone. A number of methods for the analysis of hydroquinone have been developed, including spectrophotometry [8], liquid chromatography [9], and electrochemical [10]. Those methods can offer accurate determination results. However, some of them need expensive equipments and complex procedures for sample pretreatment, while the other suffers from low selectivity. Hence, an innovative method for rapid, sensitive detection of BPA in various samples is to be developed.
Flow injection chemiluminescence (FI-CL) is known to be a powerful analytical technique that possesses high sensitivity, rapid assay and inexpensive instrumentation in recent research [11, 12]. However, the application and development of this method are suffocated for its poor selectivity. To overcome this difficulty, molecularly imprinted polymer (MIP) which has high specificity recognition and capture capabilities is introduced into CL for improving selectivity [13, 14]. This problem could be overcome when CL is coupled with a selective recognition element MIP.
A novel CL system with core-shell MIP as recognition components has been developed in these years taking advantage of the excellent adsorption ability and rapid binding kinetics of core-shell MIP [15]. The detection results of this method are much more sensitivity and strong anti-interference ability. Surface MIP can obtain a thin film of imprinted polymer grafted onto the surfaces of beads. These nano-structured MIP layer enable complete removal of templates, better site accessibility, reduction of mass transfer resistance effect, and have a well defined shape [16]. Recently, an innovative surface imprinting technique based on magnetic polymer exhibits excellent advantages in many aspects [17], for instance mechanical stability and magnetic separation has received increasing attention. Specially, the oil based magnetic fluid (MF), which with a layer of surfactant adsorbed on the surface and an oil base fluid, has well dispersion in the oil phase.
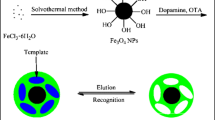
We reported that surface molecularly imprinted polymer was introduced into CL for improving selectivity. Based on this MIP, an innovative MIP-CL system for HQ determination is developed. In the preparation of MIP, OA@Fe3O4 was used as supporters to improve the adsorption capacity and separation. Due to the special binding sites on the OA@Fe3O4-MIP, the HQ could be adsorbed selectively, thus enhance the selectivity of CL analysis. The system was successfully used in determination of HQ in samples. Under optimal conditions, the CL system showed very high sensitivity and selectivity to HQ. Moreover, the reusability of the HQ MIP was studied.
Experimental
Materials and reagents
Hydroquinone was purchased from Shanghai Chemical Reagent Company (Shanghai, China); ethylene glycol dimethacrylate (EGDMA, AR) was supplied from Aladdin Reagent Co., Ltd.; Methacrylic acid (MAA, AR), NH4Fe(SO4)2 · 12H2O, Fe(NH4)2(SO4)2 · 6H2O and were purchased from Tianjin Chemical Reagent Co., Ltd.; 2,2-azobisisobutyronitrile (AIBN, AR), oleic acid (OA,CP), absolute ethanol (AR) and methylbenzene (AR) were purchased from Sinopharm Chemical Reagent Co. Ltd. (China) and used directly without any further purification.
The EGDMA was distilled to remove inhibitors. AIBN is re-crystallized prior to its use.
Apparatus
The IFFM-E flow injection CL analyzer (Xi’an Remex Electronic Instrument High-Tech Ltd., China) is equipped with an automatic injection system and a detection system. Polytetrafluoroethylene tube (0.8 mm i.d.) is used to connect all of the components in the flow system. The tube filling with MIP positioned on the FI-CL analyzer. A valve is used to change the flow direction. The CL signal is analyzed with a computer. The schematic diagram of the MIP-CL system used is shown in Fig. 1.
Preparation of OA-coated Fe3O4 nanoparticles
The oleic acid Fe3O4 particles were prepared by co-precipitation method, according to a synthesis route described by Yang et al. [18]. Typically, NH4Fe(SO4)2 · 12H2O (0.045 mol) and Fe(NH4)2(SO4)2 · 6H2O (0.03 mol) were dissolved under nitrogen protection into 50 mL of ultrapure water. The intermixed solution was heated to 40 °C while stirring vigorously. Then, 25 mL of absolute NH4OH was added into the solution. The resulting suspension was vigorously stirred for 3 min and then 2 mL of OA was added into the suspension. After reacting for 30 min, 50 mL of methylbenzene was added and the suspension was quickly heated to 90 °C. After curing reaction for 4 h, the black precipitates were extracted to the supernatant oil phase. The organic phase magnetic fluid was collected with the help of a magnet and washed repeatedly with deionized water.
Preparation of OA@ Fe3O4-MIP particles of HQ
HQ (0.2 mmol) and MAA (0.8 mmol) were dissolved in acetonitrile (30 mL). The intermixed solution was shaken in a water bath at room temperature for 12 h. And then, 4 mL of homemade organic phase magnetic fluid was added into the system with shaking for another 3 h. Furthermore, 4 mmol of EGDMA and 60 mg of AIBN were added into the system and the mixture was treated with the ultrasonic wave in a water bath for 0.5 h. After sealing, shaking, and purging, the polymerization reaction was induced under nitrogen protection at 65 °C, reacting for 24 h. After polymerization, the obtained products were washed three times with chloroform to remove the unreacted reagents and separated by the external magnetic field. Then the template molecule (HQ) was removed by Soxhlet extraction using methanol-acetic acid (9:1, v/v) as eluent, and the eluent was replaced every 8 h. The template had been removed completely when no HQ was detected in eluent by an UV–vis spectrophotometer at 221 nm. The obtained products (MIPs) were dried at 40 °C under vacuum for 12 h. For comparison, NIP was prepared and processed in the same way, but in the absence of any template.
Characterization
The phase purity and crystal structure of the obtained samples were examined by X-ray diffraction (XRD) using D8 Advance X-ray diffraction (Bruker AXS Company, Germany) equipped with Cu-KR radiation (λ) 1.5406 (Å), employing a scanning rate of 0.02° s−1 in the 2θ range from 10 to 80°. Fourier transform infrared (FTIR) spectra were collected by FTIR spectrometer (Nicolet Nexus 470) with the KBr method. The morphology and microstructure were identified by transmission electron microscopy (TEM, Philips Tecnai-12).
MIP and NIP particles rebinding performance
MIPs and NIPs (20 mg) were dispersed in eight centrifuge tubes respectively, in which there were 10 mL of HQ of various concentrations (0.05 to 1.2 mg∙mL‾1). These centrifuge tubes were performed in a water bath at 30 °C with oscillation for 10 min. Then, MIPs and NIPs were isolated by an external magnetic field and the remained HQ in solution was measured by UV–vis spectrophotometer at 221 nm. The experimental data was presented as the adsorption capacity (Q (mg∙g‾1)) per unit mass (g) of the nanoparticles, and calculated from Eq. (1):
c 0 (mg∙mL‾1) is the initial concentration of HQ solution, c e (mg∙mL‾1) is the HQ concentration of the supernatant solution, V (mL) is the volume of the initial solution and m (g) is the mass of MIPs or NIPs.
Procedure for determination of HQ
The schematic for the CL system was shown in Fig. 1 and the determination could be summarized in four steps as follows, showing in Fig. 2:
-
Step 1:
recognize and adsorb HQ. The pump 2 was started only and the valve was positioned on the left. HQ sample solution was delivered to flow through MIPs column, and HQ in the sample solution was adsorbed selectively.
-
Step 2:
washout the not adsorption materials. Plug line into the water and start the pump 2 only for 2 min.
-
Step 3:
chemiluminescence detect of the adsorption hydroquinone. The pump 1 was started while the pump 2 was stopped and the valve position was changed to right. The merged stream of K3Fe(CN)6, luminol and chlorhematin was flowed to react with the HQ in MIP tube to produce relative light intensity.
-
Step 4:
purify the MIPs tube. The pump 2 was started to deliver the water until no relative light intensity was detected.
Results and discussion
Characterization of OA@ Fe3O4 and MIP particles
The structural properties of synthesized MIPs were analysed by XRD. As can be observed in Fig. 3, the XRD patterns of the synthesized MIPs displayed several relatively strong reflection peaks in the 2θ region of 20 ~ 80°, which is quite similar to those of Fe3O4. The discernible six diffraction peaks (2θ = 30°, 35°, 43°, 53°, 57° and 63°) match well with the database of magnetite in JCPDS (JCPDS Card: 19–629) file. The result shows that the structure of Fe3O4 has been not damaged in the MIP preparation process.
Figure 4 showed the FTIR spectra of OA@ Fe3O4 and MIP. The absorption bands at 595 cm−1 was attributed to Fe-O stretch vibrations which confirm the existence of Fe3O4 and the band at 775 cm−1 was attributed to the rocking vibration of –(CH2)n-groups of OA in the two samples. In addition, on the spectrum (a) of MIP, the peaks of 1636, 1258, 957 cm−1 exhibited the presence of the –COOH. And it was clear that the bands at 1722, 1164 cm−1 which ware refer to the -COOR of ethylene glycol dimethacrylate (EGDMA). All those indicated that the polymerization reaction completed successfully and the functional group (−COOH) of the functional monomer was retained.
Representative SEM images of the obtained OA@ Fe3O4 and MIPs were shown in Fig. 5. The images suggested that Fe3O4 and MIP particles present a more regular morphological feature. The diameters of MIPs were approximately 500 nm and Fe3O4 was about 200 nm. So, the thickness of imprinting layer was estimated to be about 150 nm. The features that nano-sized particles and thin imprinting layer of MIPs mentioned above could increase the adsorption capacity of polymers and improve the mass transfer rate for releasing and rebinding the HQ [19].
MIPs and NIPs rebinding performance
The adsorption results of MIPs and NIPs were calculated from Eq. (1) and plotted according to the binding isotherm, showing in Fig. 6. It was directly perceived through the senses that the adsorptive capacity of MIPs toward to HQ was much higher than that of NIPs. The results demonstrated that the MIPs was suitable for using in the CL system.
To estimate the maximum binding properties of the MIP and NIP, the data were further processed with Scatchard analysis according to the Scatchard equation, Eq. (2) [20].
In Eq. (2), Q is the amount of hydroquinone bound to the polymers at equilibrium; c e is the free hydroquinone concentration at equilibrium; Kd is the dissociation constant and Q max is the apparent maximum binding amount. The values of Kd and the Q max can be calculated from the slope and intercept of the linear line plotted in Q/c e versus Q. The Scatchard plot for MIPs is not a single linear curve, but consisted of two linear parts with different slopes. It indicated two kinds of binding sites existed in MIPs. The linear regression equation for the left part of the curve was Q/c e = 33.959−1.264Q (r = 0.9518) and the right part of the curve Q/c e = 14.69−0.204Q (r = 0.9708). The Q max was calculated to be 63.05 mg · g‾1. The binding of hydroquinone to NIPs was also analyzed by Scatchard method. The linear regression equation was Q/c e = 4.058−0.490 Q (r = 0.9801). The maximum adsorption was 15.28 mg · g‾1. The maximum adsorption of MIP is 4.12 times of NIPs.
Choice of materials
The optimization of MIP-CL reagents was important. In order to choose the optimum concentrations for the MIP-CL experiment, a series of concentration of each reagent and pump speed were examined. Considering the factors such as sensitivity, stability and MIP-CL intensity (ΔI = I 0–I is the luminous intensity reduction), the optimal experimental conditions were that luminol at concentration of 7 × 10−5 mol∙L‾1, Hemin at 7.0 × 10−6 g∙mL‾1, K3Fe(CN)6 at 5.0 × 10−4 mol∙L‾1, NaOH at 0.1 mol∙L‾1, pump 1 speed at 30 r min‾1 and adsorption time was 2 min. These concentrations were used for the further work.
The analytical performance of the system
Under the optimum conditions, the analytical performance of the method was obtained. The relative CL intensity (ΔI) was linear to concentration (c) of hydroquinone from 2 × 10−7 to 1.0 × 10−5 mg∙mL‾1. The regression equation was ΔI =114.0 + 11.0 × 107 · c (mol∙L‾1) (R = 0.997).
The numerical values of the analytical performance were obtained as following: the detection limit was 7.9 × 10−8 mg∙mL‾1 (calculated as 3σ/S) and relative standard deviation was 2.3 % at 1.0 × 10−7 mg∙mL‾1 of HQ (n = 6). The results showed that the CL system using MIPs as recognition material exhibited high sensitivity and accuracy.
Interferences study
To evaluate the selectivity of this system, the influences of some potentially interfering inorganic and organic with HQ were investigated. The tolerable limit of potentially interfering ions was taken as a relative error not greater than ±5 % in the recovery at a concentration of 4.0 × 10−6 mg∙mL‾1 standard solution. The results indicated that the MIP-CL intensity of progesterone was not affected by the foreign species compared with CL which were shown in Table 1.
Application of MIP-CL system
In order to evaluate the applicability and reliability of the method, it was applied to determine the content of HQ in drugs of hydroquinone cream samples.
0.2504 g of hydroquinone cream samples were dispersed in 50 mL of methanol aqueous solution (1:4, v:v) and extracted by ultrasonic wave (power:400 W) for 30 min, then the turbid solution was filtered by 0.45 nm organic membrane filter and only the liquid passed into the container. 25 mL of the liquid was pipetted into 100 mL volumetric flask and bring to volume by methanol aqueous solution. The getting sample reserve liquid was diluted 10, 000 times with double distilled water for analysis. At the same time recovery was estimated by addition of known amounts of HQ to the samples. Sample 2 was prepared under the same procedure. All the analysis results were shown in Table 2. The percentage content of HQ in sample was 2.14 % and 2.09 %, while the amount of it determined by HPLC was 2.10 %. The recoveries for different concentration varied from 99 to 105 %. These results showed that the system had good accuracy for the determination of HQ in hydroquinone cream samples.
Conclusions
A MIP-CL system fabricated by using the MIPs as recognition element was described and the high selectivity and sensitivity was obtained. Due to the special binding sites on the surface of molecularly imprinted polymeric microspheres, the hydroquinone could be absorbed selectively, which improved the selectivity and sensitivity of the CL analysis. The CL system was applied to determinate the hydroquinone in samples directly without sample purification step. Since the MIPs could get rid of the interferences effectively. The CL system provided a sensitive and fast method for determination of quercetin with satisfactory results.
References
Wang H, Chen D, Wei Y, Yu L, Zhang P, Zhao J (2011) A localized surface plasmon resonance light scattering-based sensing of hydroquinone via the formed silver nanoparticles in system. Spectrochim Acta A 79:2012–2016
Duarte-Davidson R, Courage C, Rushton L, Levy L (2001) Benzene in the environment: an assessment of the potential risks to the health of the population. Occup Environ Med 58:2–13
Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ (2000) Role of quinones in toxicology. Chem Res Toxicol 13:135–160
Luo L, Jiang L, Geng C, Cao J, Zhong L (2008) Hydroquinone-induced genotoxicity and oxidative DNA damage in HepG2 cells. Chem Biol Interact 173:1–8
Abernethy DJ, Kleymenova EV, Rose J, Recio L, Faiola B (2004) Human CD3 4+ hematopoietic progenitor cells are sensitive targets for toxicity induced by 1, 4-benzoquinone. Toxicol Sci 79:82–89
Xu G, Li B, Luo X (2013) Carbon nanotube doped poly (3, 4-ethylenedioxythiophene) for the electrocatalytic oxidation and detection of hydroquinone. Sensors Actuators B 176:69–74
Liu W, Li C, Tang L, Tong A, Gu Y, Cai R et al (2013) Nanopore array derived from l-cysteine oxide/gold hybrids: Enhanced sensing platform for hydroquinone and catechol determination. Electrochim Acta 88:15–23
Sirajuddin MI, Bhanger AN, Shah A, Rauf A (2007) Ultra-trace level determination of hydroquinone in waste photographic solutions by UV–vis spectrophotometry. Talanta 72:546–553
Gao WH, Quigley CL (2011) Fast and sensitive high performance liquid chromatography analysis of cosmetic creams for hydroquinone, phenol and six preservatives. J Chromatogr A 1218:4307–4311
Zhang YL, Xiao SX, Xie JL, Yang ZM, Pang PF, Gao YT (2014) Simultaneous electrochemical determination of catechol and hydroquinone based on graphene–TiO2nanocomposite modified glassy carbon electrode. Sensors Actuators B 204:102–108
Waseem A, Yaqoob M, Nabi A (2013) Analytical applications of flow injection chemiluminescence for the determination of pharmaceuticals–a review. Curr Pharm Anal 9:363–395
Wulff G (2013) Fourty years of molecular imprinting in synthetic polymers: origin, features and perspectives. Microchim Acta 180:1359–1370
Lu F, Sun M, Fan L, Qiu H, Li X, Luo C (2012) Flow injection chemiluminescence system based on core–shell magnetic molecularly imprinted nanoparticles for determination of chrysoidine in food samples. Sensors Actuators B 173:591–598
Yu J, Wan F, Zhang C, Yan M, Zhang X, Wang S (2010) Molecularly imprinted polymeric microspheres for determination of bovine serum albumin based on flow injection chemiluminescence system. Biosens Bioelectron 26:632–637
Xie C, Li H, Li S, Gao S (2011) Surface molecular imprinting for chemiluminescence detection of the organophosphate pesticide chlorpyrifos. Microchim Acta 174:311–320
Chen L, Xu S, Li J (2011) Recent advances in molecular imprinting technology: current status, challenges and highlighted applications. Chem Soc Rev 40:2922–2942
Yao GH, Liang RP, Huang CF, Wang Y, Qiu JD (2013) Surface plasmon resonance system based on magnetic molecularly imprinted polymers amplification for pesticide recognition. Anal Chem 85:11944–11951
Xu L, Pan J, Dai J et al (2012) Preparation of thermal-responsive magnetic molecularly imprinted polymers for selective removal of antibiotics from aqueous solution. J Hazard Mater 233–234:48–56
Lu S, Cheng G, Zhang H, Pang X (2006) Preparation and characteristics of Tryptophan-imprinted Fe3O4/P (TRIM) composite microspheres with magnetic susceptibility by inverse emulsion–suspension polymerization. J Appl Polym Sci 99:3241–3250
Ikegami T, Mukawa T, Nariai H, Takeuchi T (2004) Bisphenol A-recognition polymers prepared by covalent molecular imprinting. Anal Chim Acta 504:131–135
Acknowledgments
This work was supported by the Natural Science Foundation of Shandong Province (No. ZR2011BL008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chao, Y., Zhang, X., Liu, L. et al. Determination of hydroquinone by flow injection chemiluminescence and using magnetic surface molecularly imprinted particles. Microchim Acta 182, 943–948 (2015). https://doi.org/10.1007/s00604-014-1415-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1415-2